SerpinA3K Deficiency Reduces Oxidative Stress in Acute Kidney Injury
Abstract
:1. Introduction
2. Results
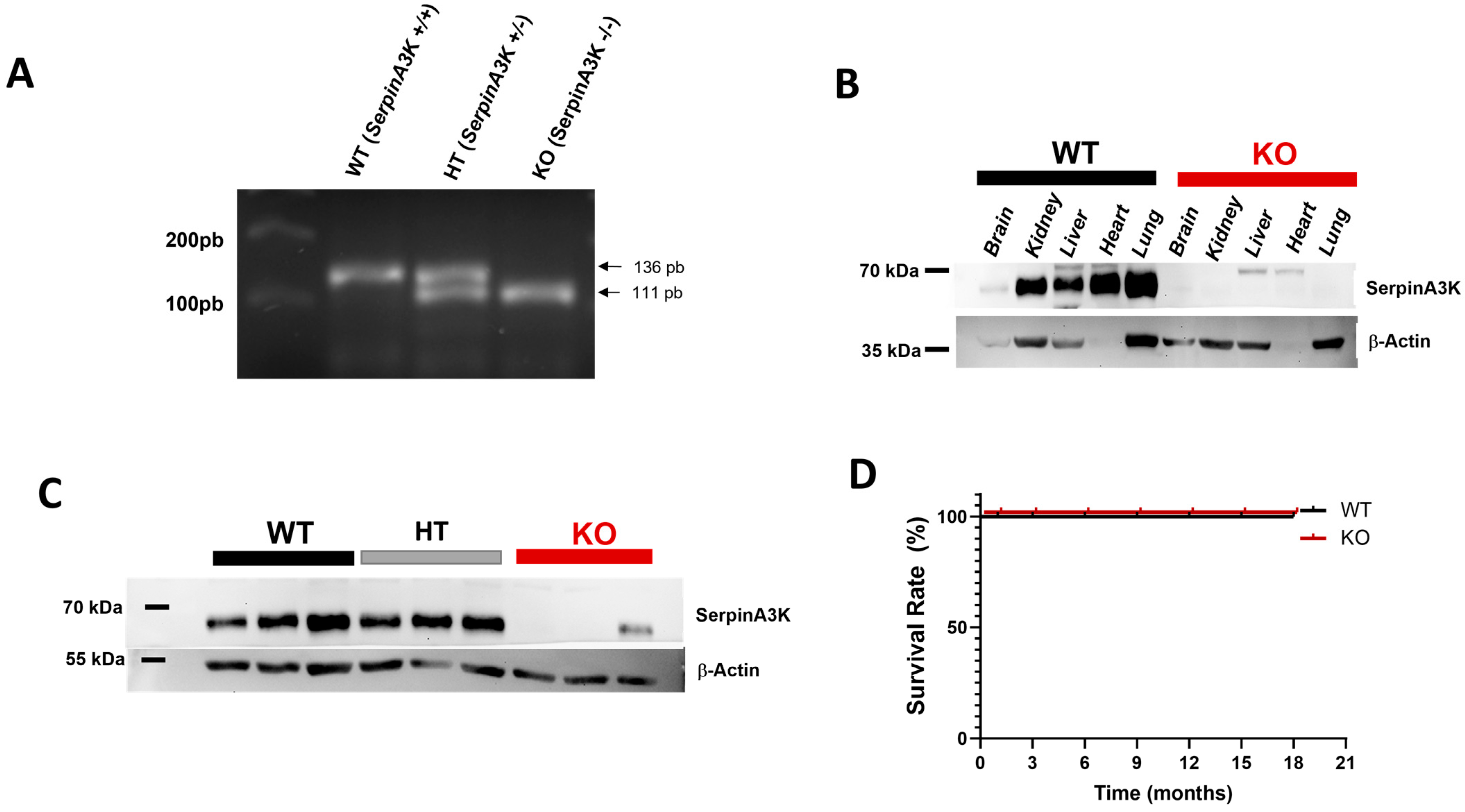
2.1. Characterization of SerpinA3K Deficiency in Mice
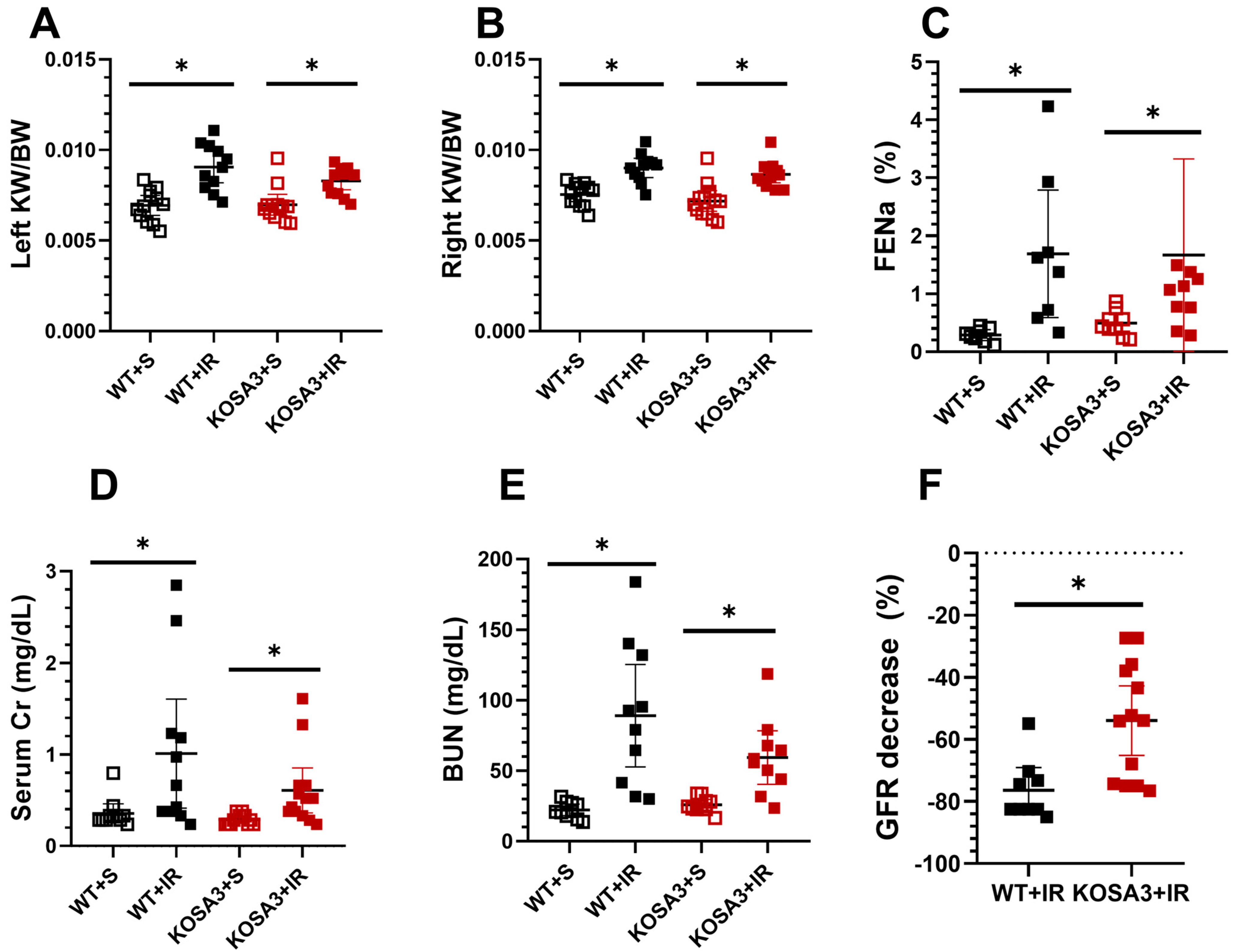
2.2. Impact of SerpinA3K Deficiency during AKI
2.3. Histopathological Evaluation
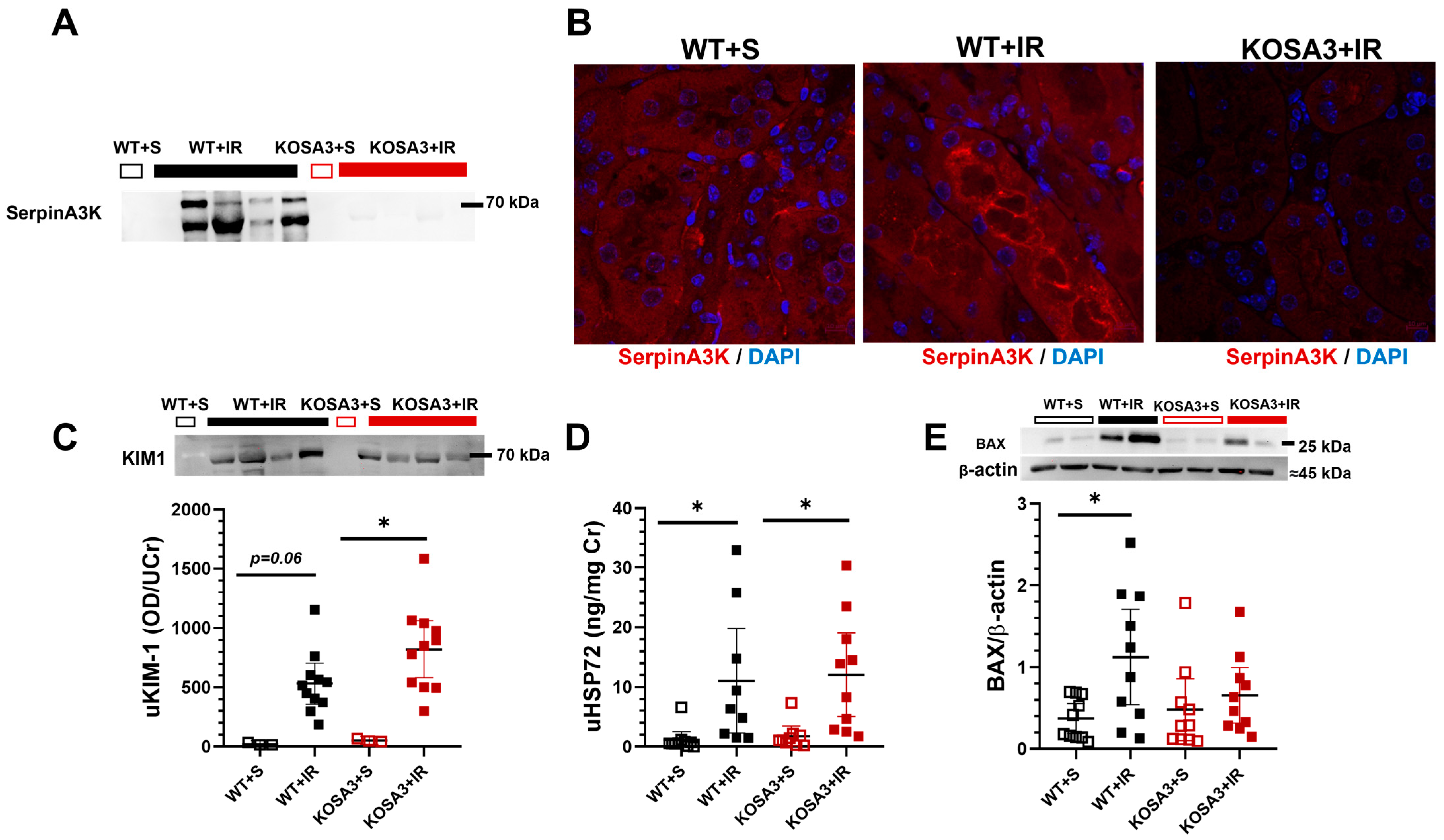
2.4. Renal Location of SerpinA3K and Renal Damage Evaluation of Acute Kidney Injury
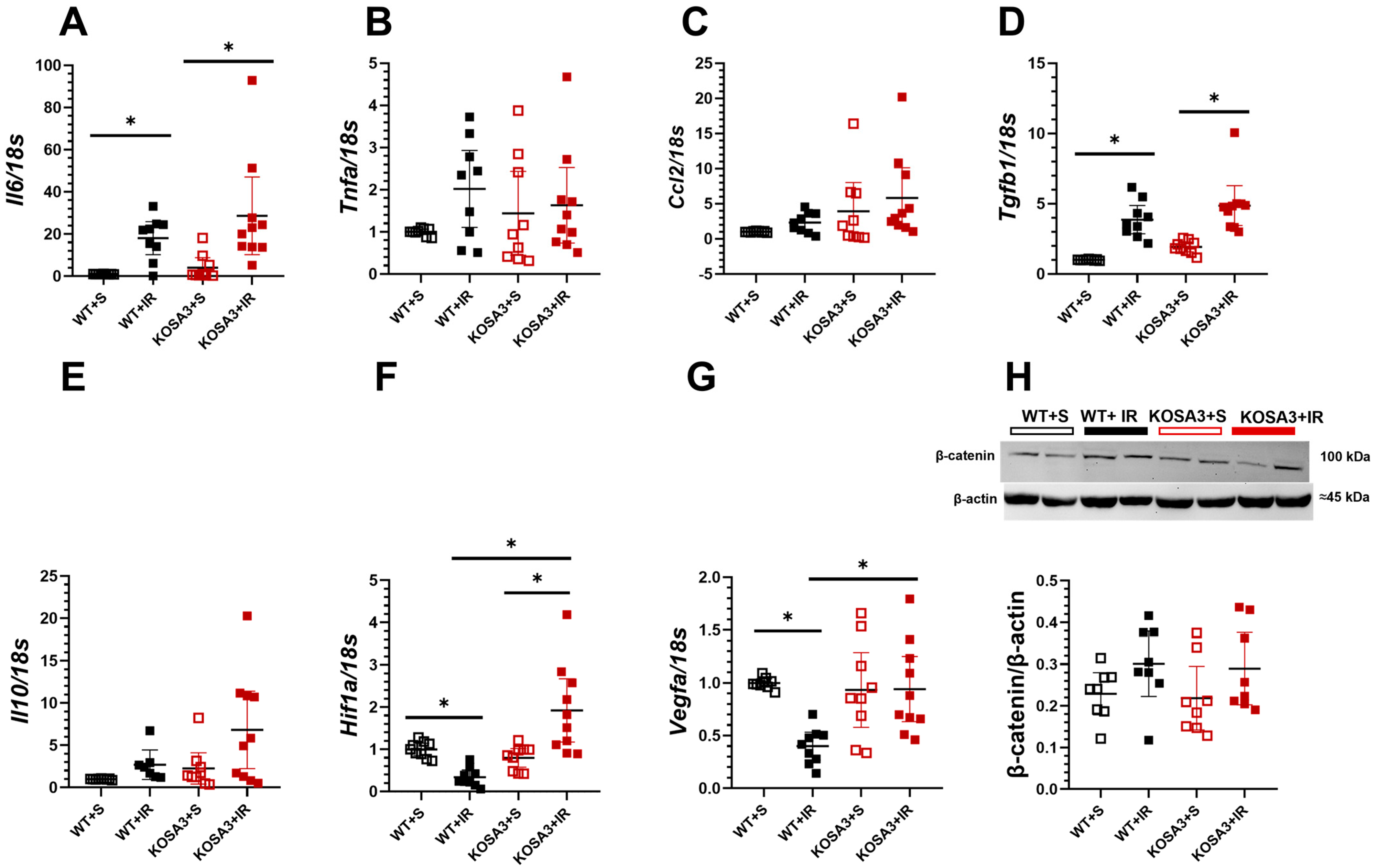
2.5. Inflammatory Response during AKI in SerpinA3K Deficiency
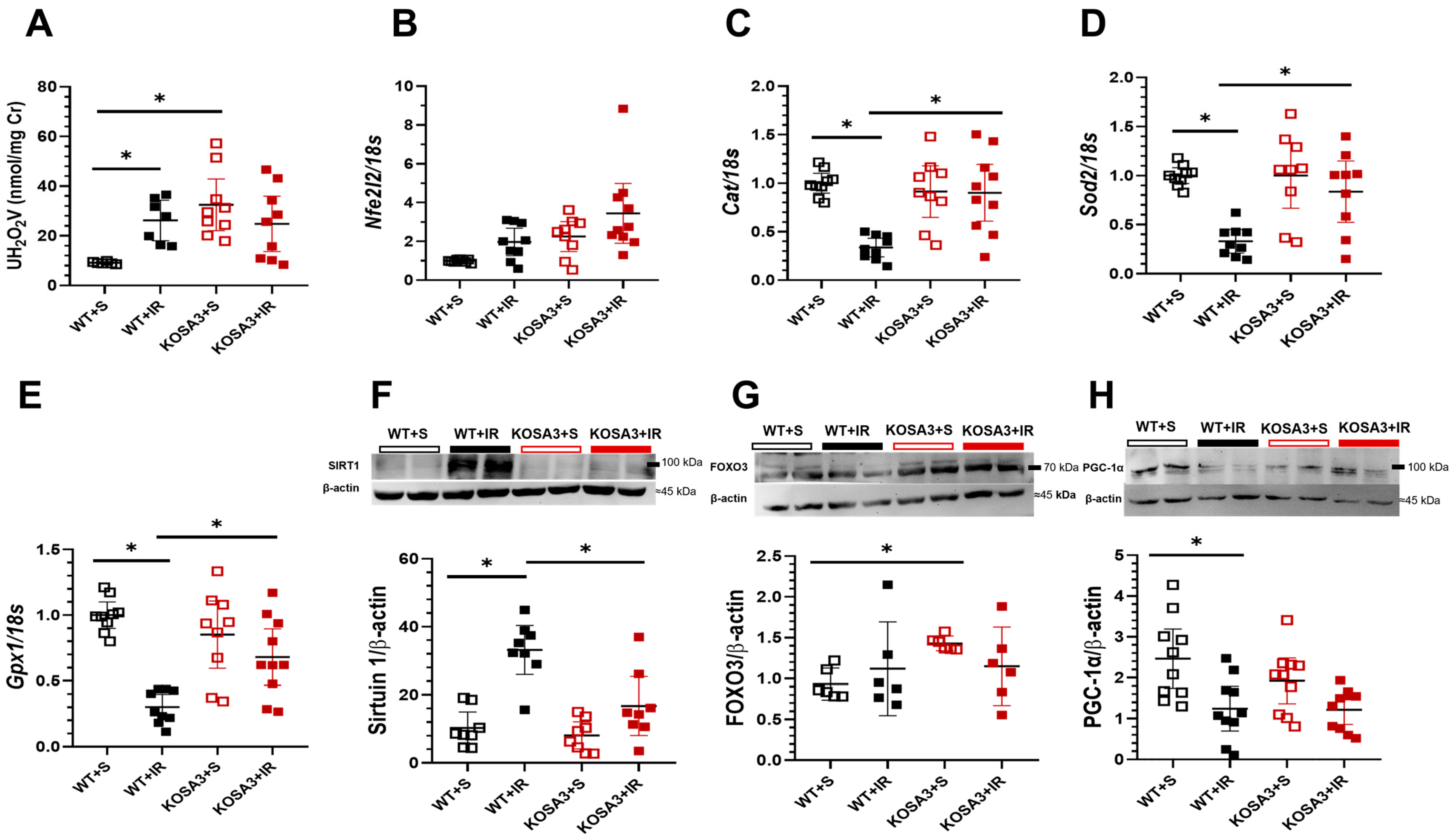
2.6. Response to Oxidative Stress in SerpinA3K Deficient Mice
3. Discussion
4. Material and Methods
4.1. Mouse Kidney Bilateral Renal Ischemia Model
4.2. Glomerular Filtration Rate Measurement
4.3. Histological Assessment of Tubular Injury
4.4. SerpinA3K Immunofluorescence
4.5. Urinary Hydrogen Peroxide Excretion
4.6. Serum and Urine Creatinine
4.7. Serum BUN, Sodium, and Potassium
4.8. Western Blot
4.9. mRNA Levels Assessed by Semiquantitative RT-PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensiv. Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Shemies, R.S.; Nagy, E.; Younis, D.; Sheashaa, H. Renal replacement therapy for critically ill patients with COVID-19-associated acute kidney injury: A review of current knowledge. Ther. Apher. Dial. 2022, 26, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Parikh, C.R.; Himmelfarb, J.; Chinchilli, V.M.; Liu, K.D.; Coca, S.G.; Garg, A.X.; Hsu, C.-Y.; Siew, E.D.; Wurfel, M.M.; et al. A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int. 2021, 99, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Hukriede, N.A.; Soranno, D.E.; Sander, V.; Perreau, T.; Starr, M.C.; Yuen, P.S.T.; Siskind, L.J.; Hutchens, M.P.; Davidson, A.J.; Burmeister, D.M.; et al. Experimental models of acute kidney injury for translational research. Nat. Rev. Nephrol. 2022, 18, 277–293. [Google Scholar] [CrossRef]
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef]
- García-Ortuño, L.E.; Bobadilla, N.A. Integrative view of the mechanisms that induce acute kidney injury and its transition to chronic kidney disease. Rev. De Investig. Clin. 2018, 70, 261–268. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Marquez-Expósito, L.; Rodrigues-Diez, R.; Sanz, A.B.; Guiteras, R.; Doladé, N.; Rubio-Soto, I.; Manonelles, A.; Codina, S.; Ortiz, A.; et al. Molecular Mechanisms of Kidney Injury and Repair. Int. J. Mol. Sci. 2022, 23, 1542. [Google Scholar] [CrossRef]
- Hepokoski, M.; Singh, P. Mitochondria as mediators of systemic inflammation and organ cross talk in acute kidney injury. Am. J. Physiol. Ren. Physiol. 2022, 322, F589–F596. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; Mejía-Vilet, J.M.; Pérez-Villalva, R.; Carrillo-Pérez, D.L.; Marquina-Castillo, B.; Gamba, G.; Bobadilla, N.A. SerpinA3 in the Early Recognition of Acute Kidney Injury to Chronic Kidney Disease (CKD) transition in the rat and its Potentiality in the Recognition of Patients with CKD. Sci. Rep. 2019, 9, 10350. [Google Scholar] [CrossRef]
- Martínez-Rojas, M.; Sánchez-Navarro, A.; Mejía-Vilet, J.M.; Pérez-Villalva, R.; Uribe, N.; Bobadilla, N.A. Urinary serpin-A3 is an early predictor of clinical response to therapy in patients with proliferative lupus nephritis. Am. J. Physiol. Renal. Physiol. 2022, 323, F425–F434. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Navarro, A.; González-Soria, I.; Caldiño-Bohn, R.; Bobadilla, N.A. An integrative view of serpins in health and disease: The contribution of SerpinA3. Am. J. Physiol. Cell Physiol. 2021, 320, C106–C118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, J.-X. SERPINA3K prevents oxidative stress induced necrotic cell death by inhibiting calcium overload. PLoS ONE 2008, 3, e4077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, Y.; Ma, J.-X. Anti-inflammatory and antioxidant effects of SERPINA3K in the retina. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3943–3952. [Google Scholar] [CrossRef]
- Zhang, B.; Abreu, J.G.; Zhou, K.; Chen, Y.; Hu, Y.; Zhou, T.; He, X.; Ma, J.-X. Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proc. Natl. Acad. Sci. USA 2010, 107, 6900–6905. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, K.K.; Ma, J.-X. Inhibition of CTGF overexpression in Diabetic Retinopathy by SERPINA3K. Diabetes 2010, 59, 1809–1816. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Z.; Zhou, T.; Zong, R.; He, H.; Liu, Z.; Ma, J.-X.; Liu, Z.; Zhou, Y. Anti-angiogenic and anti-inflammatory effects of SERPINA3K on corneal injury. PLoS ONE 2011, 6, e016712. [Google Scholar] [CrossRef]
- Zhou, T.; Zong, R.; Zhang, Z.; Zhu, C.; Pan, F.; Xiao, X.; Liu, Z.; He, H.; Ma, J.-X.; Liu, Z.; et al. SERPINA3K protects against oxidative stress via modulating ROS generation/degradation and KEAP1-NRF2 pathway in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5033–5043. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, L.; Huang, C.; Lin, Z.; Zong, R.; Zhu, C.; Pan, F.; Ma, J.; Liu, Z.; Zhou, Y. Serine proteinase inhibitor SERPINA3K suppresses corneal neovascularization via inhibiting Wnt signaling and VEGF. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4863–4872. [Google Scholar] [CrossRef]
- Zhu, C.; Pan, F.; Ge, L.; Zhou, J.; Chen, L.; Zhou, T.; Zong, R.; Xiao, X.; Dong, N.; Yang, M.; et al. SERPINA3K plays antioxidant roles in cultured pterygial epithelial cells through regulating ROS system. PLoS ONE 2014, 9, e108859. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; Murillo-De-Ozores, A.R.; Pérez-Villalva, R.; Linares, N.; Carbajal-Contreras, H.; Flores, M.E.; Gamba, G.; Castañeda-Bueno, M.; Bobadilla, N.A. Transient response of serpinA3 during cellular stress. FASEB J. 2022, 36, e22190. [Google Scholar] [CrossRef]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT–β-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-H.; Wang, C.; He, Y.-X.; Mao, X.-Y.; Zhang, M.-Z.; Hou, Y.-P.; Li, B. Hypoxia-inducible factor protects against acute kidney injury via the Wnt/b-catenin signaling pathway. Am. J. Physiol. Renal. Physiol. 2022, 322, L611–L624. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.W.-M.; Benzie, I. Hydrogen peroxide in urine as a potential biomarker of whole body oxidative stress. Free. Radic. Res. 2003, 37, 1209–1213. [Google Scholar] [CrossRef]
- Wei, W.; Ma, N.; Fan, X.; Yu, Q.; Ci, X. The role of Nrf2 in acute kidney injury: Novel molecular mechanisms and therapeutic approaches. Free Radic. Biol. Med. 2020, 158, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2022, 19, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Raji-Amirhasani, A.; Khaksari, M.; Mahani, F.D.; Hajializadeh, Z. Activators of SIRT1 in the kidney and protective effects of SIRT1 during acute kidney injury (AKI) (effect of SIRT1 activators on acute kidney injury). Clin. Exp. Nephrol. 2021, 25, 807–821. [Google Scholar] [CrossRef]
- Peterson, W.; Gabbai, F.B.; Myers, R.R.; Mizisin, A.P.; Blantz, R.C. A single nephron model of acute tubular injury: Role of tubuloglomerular feedback. Kidney Int. 1989, 36, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, V.; D’apolito, L.; Sardella, D.; Iervolino, A.; La Manna, G.; Capasso, G.; Frische, S.; Trepiccione, F. Single nephron glomerular filtration rate measured by linescan multiphoton microscopy compared to conventional micropuncture. Pflugers Arch. 2022, 474, 733–741. [Google Scholar] [CrossRef]
- Docherty, N.; Pérez-Barriocanal, F.; Balboa, N.E.; Lopez-Novoa, J.M. Transforming growth factor-β1 (TGF-β1): A potential recovery signal in the post-ischemic kidney. Ren. Fail. 2002, 24, 391–406. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, J.-X. Canonical Wnt signaling in diabetic retinopathy. Vis. Res. 2017, 139, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Tang, T.-T.; Shen, A.-R.; Cao, J.-Y.; Jing, J.; Wang, C.; Zhu, X.-X.; Wen, Y.; Li, Z.-L.; Bin Wang, B.; et al. Tubular epithelial cells-derived small extracellular vesicle-VEGF-A promotes peritubular capillary repair in ischemic kidney injury. NPJ Regen. Med. 2022, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Zhang, M.-Z.; You, L.; Davis, L.S.; Fan, H.; Yang, H.-C.; Fogo, A.B.; Zent, R.; Harris, R.C.; et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Investig. 2010, 120, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; He, J.; Sun, X.; Li, L.; Zhang, X.; Gan, H. Activation of sirtuin1 protects against ischemia/reperfusion-induced acute kidney injury. Biomed. Pharmacother. 2020, 125, 110021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: Their clinical significance and regulation. BioMed Res. Int. 2014, 2014, 925350. [Google Scholar] [CrossRef]
- Li, L.; Kang, H.; Zhang, Q.; D’agati, V.D.; Al-Awqati, Q.; Lin, F. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J. Clin. Investig. 2019, 129, 2374–2389. [Google Scholar] [CrossRef]
- Chambers, J.M.; Wingert, R.A. PGC-1α in Disease: Recent Renal Insights into a Versatile Metabolic Regulator. Cells 2020, 9, 2234. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; Martínez-Rojas, M.; Albarrán-Godinez, A.; Pérez-Villalva, R.; Auwerx, J.; de la Cruz, A.; Noriega, L.G.; Rosetti, F.; Bobadilla, N.A. Sirtuin 7 Deficiency Reduces Inflammation and Tubular Damage Induced by an Episode of Acute Kidney Injury. Int. J. Mol. Sci. 2022, 23, 2573. [Google Scholar] [CrossRef]
- Schreiber, A.; Shulhevich, Y.; Geraci, S.; Hesser, J.; Stsepankou, D.; Neudecker, S.; Koenig, S.; Heinrich, R.; Hoecklin, F.; Pill, J.; et al. Transcutaneous measurement of renal function in conscious mice measurement of renal function in conscious mice. Am. J. Physiol. Renal. Physiol. 2012, 303, 783–788. [Google Scholar] [CrossRef]
- McLarnon, S.R.; Wilson, K.; Patel, B.; Sun, J.; Sartain, C.L.; Mejias, C.D.; Musall, J.B.; Sullivan, J.C.; Wei, Q.; Chen, J.-K.; et al. Lipopolysaccharide Pretreatment Prevents Medullary Vascular Congestion following Renal Ischemia by Limiting Early Reperfusion of the Medullary Circulation. J. Am. Soc. Nephrol. 2022, 33, 769–785. [Google Scholar] [CrossRef]
- Ortega-Trejo, J.A.; Pérez-Villalva, R.; Sánchez-Navarro, A.; Marquina, B.; Rodríguez-Iturbe, B.; Bobadilla, N.A. Repeated Episodes of Ischemia/Reperfusion Induce Heme-Oxygenase-1 (HO-1) and Anti-Inflammatory Responses and Protects against Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 14573. [Google Scholar] [CrossRef] [PubMed]

| Target Genes | Probe gen Catalog Number |
|---|---|
| Hif1a | Mm00468869_m1 |
| Gpx1 | Mm00656767_g1 |
| Sod2 | Mm01313000_m1 |
| Tnfa | Mm00443258_m1 |
| Il6 | Mm00446190_m1 |
| Tfgb1 | Mm01178820_m1 |
| Il10 | Mm01288386_m1 |
| Ccl2 | Rn00580555_m1 |
| Nfe2l2 | Rn00582415_m1 |
| Vegfa | Rn01511602_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Soria, I.; Soto-Valadez, A.D.; Martínez-Rojas, M.A.; Ortega-Trejo, J.A.; Pérez-Villalva, R.; Gamba, G.; Sánchez-Navarro, A.; Bobadilla, N.A. SerpinA3K Deficiency Reduces Oxidative Stress in Acute Kidney Injury. Int. J. Mol. Sci. 2023, 24, 7815. https://doi.org/10.3390/ijms24097815
González-Soria I, Soto-Valadez AD, Martínez-Rojas MA, Ortega-Trejo JA, Pérez-Villalva R, Gamba G, Sánchez-Navarro A, Bobadilla NA. SerpinA3K Deficiency Reduces Oxidative Stress in Acute Kidney Injury. International Journal of Molecular Sciences. 2023; 24(9):7815. https://doi.org/10.3390/ijms24097815
Chicago/Turabian StyleGonzález-Soria, Isaac, Axel D. Soto-Valadez, Miguel Angel Martínez-Rojas, Juan Antonio Ortega-Trejo, Rosalba Pérez-Villalva, Gerardo Gamba, Andrea Sánchez-Navarro, and Norma A. Bobadilla. 2023. "SerpinA3K Deficiency Reduces Oxidative Stress in Acute Kidney Injury" International Journal of Molecular Sciences 24, no. 9: 7815. https://doi.org/10.3390/ijms24097815
APA StyleGonzález-Soria, I., Soto-Valadez, A. D., Martínez-Rojas, M. A., Ortega-Trejo, J. A., Pérez-Villalva, R., Gamba, G., Sánchez-Navarro, A., & Bobadilla, N. A. (2023). SerpinA3K Deficiency Reduces Oxidative Stress in Acute Kidney Injury. International Journal of Molecular Sciences, 24(9), 7815. https://doi.org/10.3390/ijms24097815








