Regeneration of Hair Cells from Endogenous Otic Progenitors in the Adult Mammalian Cochlea: Understanding Its Origins and Future Directions
Abstract
:1. Introduction
2. Hearing Loss and Its Pathophysiology
2.1. Age-Related Hearing Loss
2.2. Noise-Induced Hearing Loss
2.3. Ototoxicity
3. Cochlear Development and Its Associated Pathways
3.1. Hair Cell Differentiation
3.1.1. Wnt and Notch Signaling
3.1.2. Sonic Hedgehog (Shh) Signaling
3.1.3. Fibroblast Growth Factor (FGF) Signaling
3.1.4. Bone Morphogenetic Protein (BMP) and Tissue Growth Factor β (TGFβ) Signaling
3.1.5. The Role of Micro-RNAs in Cochlear Development
3.2. Spiral Ganglion Neuron Differentiation
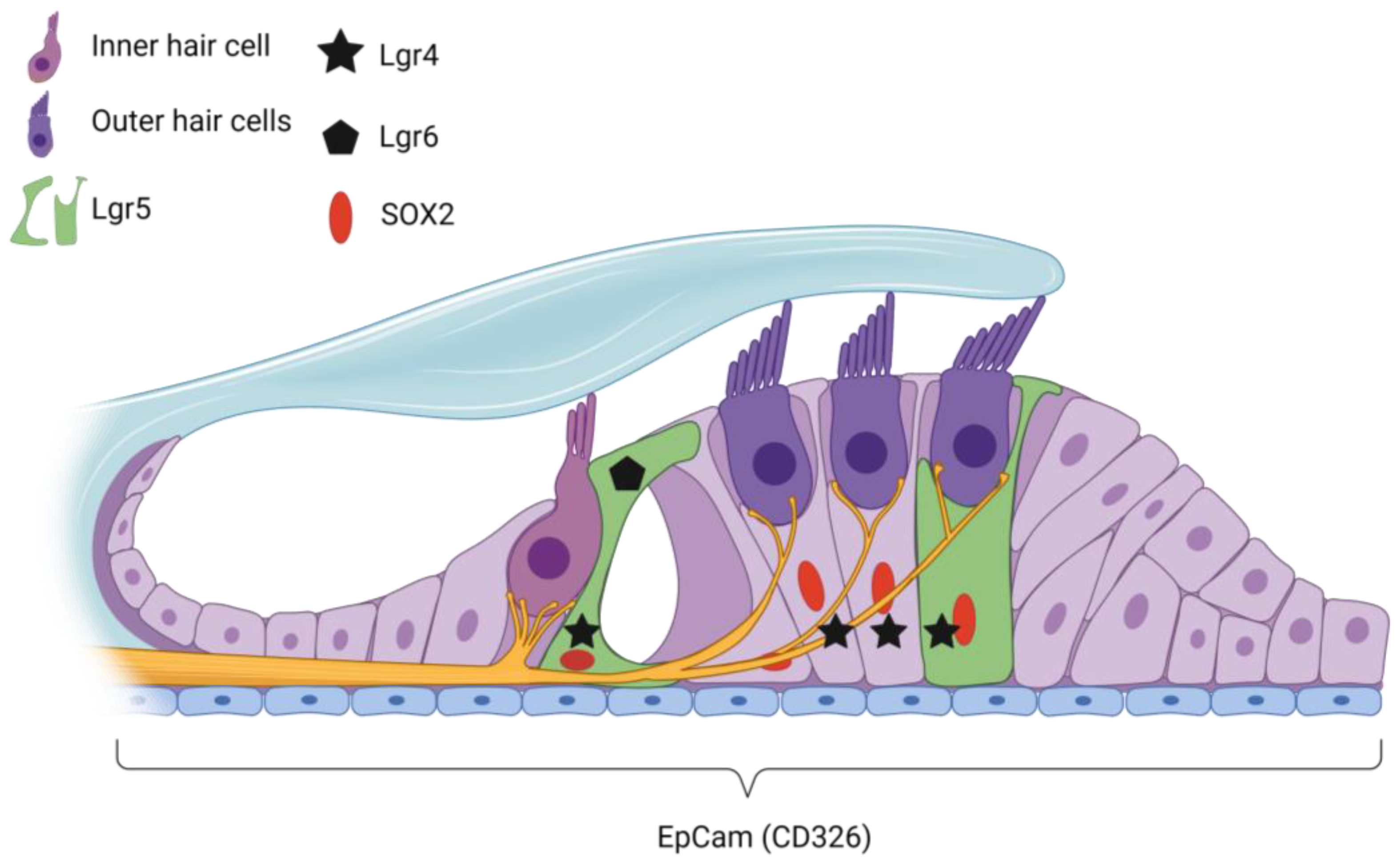
4. Target Cells for Potential Regenerative Treatment
5. Regenerative Potential of the Cochlea
5.1. Non-Mammalian Vertebrates
5.2. Neonatal Mammalian Cochlea
5.3. Adult Mammalian Cochlea
5.4. Epigenetic Barrier to Hair Cell Regeneration in Adult Cochlea
6. Promoting Regeneration & Re-Innervation
6.1. Manipulating a Single Signaling Pathway
6.2. Combined Strategies
6.3. Improving Functional Outcomes: Inner Hair Cell Re-Innervation after Cochlear Trauma
7. Human Inner Ear Regeneration and Clinical Trials Targeting Endogenous Stem Cells
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, B.S.; Tucci, D.L.; Merson, M.H.; O’Donoghue, G.M. Global hearing health care: New findings and perspectives. Lancet 2017, 390, 2503–2515. [Google Scholar] [CrossRef]
- Cosh, S.; Helmer, C.; Delcourt, C.; Robins, T.G.; Tully, P.J. Depression in elderly patients with hearing loss: Current perspectives. Clin. Interv. Aging 2019, 14, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.E.; Kapteyn, T.S.; Kuik, D.J.; Deeg, D.J.H. The Association of Hearing Impairment and Chronic Diseases with Psychosocial Health Status in Older Age. J. Aging Health 2002, 14, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-M.; Zhang, X.; Hoffman, H.J.; Cotch, M.F.; Themann, C.L.; Wilson, M.R. Hearing Impairment Associated with Depression in US Adults, National Health and Nutrition Examination Survey 2005–2010. JAMA Otolaryngol. Neck Surg. 2014, 140, 293–302. [Google Scholar] [CrossRef]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.-L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M.; et al. Hearing Loss and Cognitive Decline in Older Adults. JAMA Intern. Med. 2013, 173, 293–299. [Google Scholar] [CrossRef]
- Loughrey, D.G.; Kelly, M.E.; Kelley, G.A.; Brennan, S.; Lawlor, B.A. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: A systematic review and meta-analysis. JAMA Otolaryngol.–Head Neck Surg. 2018, 144, 115–126. [Google Scholar] [CrossRef]
- Monzani, D.; Galeazzi, G.M.; Genovese, E.; Marrara, A.; Martini, A. Psychological profile and social behaviour of working adults with mild or moderate hearing loss. Acta Otorhinolaryngol. Ital. 2008, 28, 61–66. [Google Scholar] [PubMed]
- Powell, D.S.; Oh, E.S.; Lin, F.R.; Deal, J.A. Hearing Impairment and Cognition in an Aging World. J. Assoc. Res. Otolaryngol. 2021, 22, 387–403. [Google Scholar] [CrossRef]
- Peters, J.P.M.; Wendrich, A.W.; van Eijl, R.H.M.; Rhebergen, K.S.; Versnel, H.; Grolman, W. The Sound of a Cochlear Implant Investigated in Patients with Single-Sided Deafness and a Cochlear Implant. Otol. Neurotol. 2018, 39, 707–714. [Google Scholar] [CrossRef]
- Waissbluth, S. Clinical trials evaluating transtympanic otoprotectants for cisplatin-induced ototoxicity: What do we know so far? Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2413–2422. [Google Scholar] [CrossRef]
- Le, T.N.; Straatman, L.V.; Lea, J.; Westerberg, B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol.—Head Neck Surg. 2017, 46, 41. [Google Scholar] [CrossRef] [PubMed]
- Brignull, H.R.; Raible, D.W.; Stone, J.S. Feathers and fins: Non-mammalian models for hair cell regeneration. Brain Res. 2009, 1277, 12–23. [Google Scholar] [CrossRef]
- Bermingham-McDonogh, O.; Rubel, E.W. Hair cell regeneration: Winging our way towards a sound future. Curr. Opin. Neurobiol. 2003, 13, 119–126. [Google Scholar] [CrossRef]
- Taylor, R.R.; Jagger, D.J.; Forge, A. Defining the Cellular Environment in the Organ of Corti following Extensive Hair Cell Loss: A Basis for Future Sensory Cell Replacement in the Cochlea. PLoS ONE 2012, 7, e30577. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.I.; Cotanche, D.A. Sensory hair cell death and regeneration: Two halves of the same equation. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; Stone, J.S. Development and regeneration of vestibular hair cells in mammals. Semin. Cell Dev. Biol. 2017, 65, 96–105. [Google Scholar] [CrossRef]
- Bramhall, N.F.; Shi, F.; Arnold, K.; Hochedlinger, K.; Edge, A.S. Lgr5-Positive Supporting Cells Generate New Hair Cells in the Postnatal Cochlea. Stem Cell Rep. 2014, 2, 311–322. [Google Scholar] [CrossRef]
- Cox, B.C.; Chai, R.; Lenoir, A.; Liu, Z.; Zhang, L.; Nguyen, D.-H.; Chalasani, K.; Steigelman, K.A.; Fang, J.; Rubel, E.W.; et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 2014, 141, 816–829. [Google Scholar] [CrossRef]
- Chai, R.; Kuo, B.; Wang, T.; Liaw, E.J.; Xia, A.; Jan, T.A.; Liu, Z.; Taketo, M.M.; Oghalai, J.S.; Nusse, R.; et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. USA 2012, 109, 8167–8172. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Ni, W.; Guo, L.; Lu, X.; Liu, L.; Li, W.; Sun, S.; Wang, L.; Li, H. Dynamic expression of Lgr6 in the developing and mature mouse cochlea. Front. Cell. Neurosci. 2015, 9, 165. [Google Scholar] [CrossRef]
- Oesterle, E.C.; Campbell, S.; Taylor, R.R.; Forge, A.; Hume, C.R. Sox2 and Jagged1 Expression in Normal and Drug-Damaged Adult Mouse Inner Ear. J. Assoc. Res. Otolaryngol. 2008, 9, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Kempfle, J.S.; Edge, A.S.B. Wnt-Responsive Lgr5-Expressing Stem Cells Are Hair Cell Progenitors in the Cochlea. J. Neurosci. 2012, 32, 9639–9648. [Google Scholar] [CrossRef]
- Żak, M.; van Oort, T.; Hendriksen, F.G.; Garcia, M.-I.; Vassart, G.; Grolman, W. LGR4 and LGR5 Regulate Hair Cell Differentiation in the Sensory Epithelium of the Developing Mouse Cochlea. Front. Cell. Neurosci. 2016, 10, 186. [Google Scholar] [CrossRef]
- White, P.M. Perspectives on Human Hearing Loss, Cochlear Regeneration, and the Potential for Hearing Restoration Therapies. Brain Sci. 2020, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Singh, A.; Bojrab, D.I.; Sim, N. Insights into the molecular mechanisms regulating mammalian hair cell regeneration. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 400–406. [Google Scholar] [CrossRef]
- WHO. Addressing the Rising Prevalence of Hearing Loss; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Merchant, S.N.; Tsuji, K.; Wall, I.C.; Velázquez-Villaseñor, L.; Glynn, R.J.; Rauch, S.D. Temporal Bone Studies of the Human Peripheral Vestibular System. Normative Vestibular Hair Cell Data. Ann. Otol. Rhinol. Laryngol. 2000, 109, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.M.; O’Malley, J.T.; Burgess, B.J.; Jones, D.D.; Oliveira, C.A.; Santos, F.; Merchant, S.N.; Liberman, L.D.; Liberman, M.C. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear. Res. 2015, 327, 78–88. [Google Scholar] [CrossRef]
- Schuknecht, H.F.; Gacek, M.R. Cochlear Pathology in Presbycusis. Ann. Otol. Rhinol. Laryngol. 1993, 102, 1–16. [Google Scholar] [CrossRef]
- Schuknecht, H.F. Further Observations on the Pathology of Presbycusis. Arch. Otolaryngol. Neck Surg. 1964, 80, 369–382. [Google Scholar] [CrossRef]
- Wu, P.-Z.; O’Malley, J.T.; De Gruttola, V.; Liberman, M.C. Age-Related Hearing Loss Is Dominated by Damage to Inner Ear Sensory Cells, Not the Cellular Battery That Powers Them. J. Neurosci. 2020, 40, 6357–6366. [Google Scholar] [CrossRef]
- Fujioka, M.; Okano, H.; Ogawa, K. Inflammatory and immune responses in the cochlea: Potential therapeutic targets for sensorineural hearing loss. Front. Pharmacol. 2014, 5, 287. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, K.O.; Klepper, K.; Saliba, J.; Friedman, R.A. Advances in understanding of presbycusis. J. Neurosci. Res. 2020, 98, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Paplou, V.; Schubert, N.M.A.; Pyott, S.J. Age-Related Changes in the Cochlea and Vestibule: Shared Patterns and Processes. Front. Neurosci. 2021, 15, 680856. [Google Scholar] [CrossRef]
- Keithley, E.M. Pathology and mechanisms of cochlear aging. J. Neurosci. Res. 2020, 98, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.-Y. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Oto-Rhino-Laryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef]
- Robertson, D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hear. Res. 1983, 9, 263–278. [Google Scholar] [CrossRef]
- Shi, L.; Chang, Y.; Li, X.; Aiken, S.; Liu, L.; Wang, J. Cochlear Synaptopathy and Noise-Induced Hidden Hearing Loss. Neural Plast. 2016, 2016, 6143164. [Google Scholar] [CrossRef]
- Moser, T.; Predoehl, F.; Starr, A. Review of Hair Cell Synapse Defects in Sensorineural Hearing Impairment. Otol. Neurotol. 2013, 34, 995–1004. [Google Scholar] [CrossRef]
- Cunningham, L.L.; Tucci, D.L. Restoring Synaptic Connections in the Inner Ear after Noise Damage. N. Engl. J. Med. 2015, 372, 181–182. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Perez, A.; Lin, R.; Sajjadi, A.; Ricci, A.J.; Cheng, A.G. Towards the Prevention of Aminoglycoside-Related Hearing Loss. Front. Cell. Neurosci. 2017, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Cianfrone, G.; Pentangelo, D.; Cianfrone, F.; Mazzei, F.; Turchetta, R.; Orlando, M.P.; Altissimi, G. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: A reasoned and updated guide. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 601–636. [Google Scholar] [PubMed]
- Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen, A.A.; Parfitt, R.; Ciarimboli, G. Drug-induced ototoxicity: Mechanisms, Pharmacogenetics, and protective strategies. Clin. Pharmacol. Ther. 2017, 101, 491–500. [Google Scholar] [CrossRef]
- González-González, S. The role of mitochondrial oxidative stress in hearing loss. Neurol. Disord. Ther. 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Alharazneh, A.; Luk, L.; Huth, M.; Monfared, A.; Steyger, P.S.; Cheng, A.G.; Ricci, A.J. Functional Hair Cell Mechanotransducer Channels Are Required for Aminoglycoside Ototoxicity. PLoS ONE 2011, 6, e22347. [Google Scholar] [CrossRef]
- Nagai, J.; Takano, M. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochem. Pharmacol. 2014, 90, 331–337. [Google Scholar] [CrossRef]
- Steyger, P.S. Cellular Uptake of Aminoglycosides. Volta Rev. 2005, 105, 299–324. [Google Scholar]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front. Cell Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef]
- Gentilin, E.; Simoni, E.; Candito, M.; Cazzador, D.; Astolfi, L. Cisplatin-Induced Ototoxicity: Updates on Molecular Targets. Trends Mol. Med. 2019, 25, 1123–1132. [Google Scholar] [CrossRef]
- Raphael, Y.; Altschuler, R.A. Scar formation after drug-induced cochlear insult. Hear. Res. 1991, 51, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Forge, A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear. Res. 1985, 19, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, T.; Belevich, I.; Kirjavainen, A.; Laos, M.; Brakebusch, C.; Jokitalo, E.; Pirvola, U. How to Bury the Dead: Elimination of Apoptotic Hair Cells from the Hearing Organ of the Mouse. J. Assoc. Res. Otolaryngol. 2014, 15, 975–992. [Google Scholar] [CrossRef] [PubMed]
- van Loon, M.C.; Ramekers, D.; Agterberg, M.J.; de Groot, J.C.; Grolman, W.; Klis, S.F.; Versnel, H. Spiral ganglion cell morphology in guinea pigs after deafening and neurotrophic treatment. Hear. Res. 2013, 298, 17–26. [Google Scholar] [CrossRef]
- Ramekers, D.; Versnel, H.; Grolman, W.; Klis, S.F. Neurotrophins and their role in the cochlea. Hear. Res. 2012, 288, 19–33. [Google Scholar] [CrossRef]
- Zilberstein, Y.; Liberman, M.C.; Corfas, G. Inner Hair Cells Are Not Required for Survival of Spiral Ganglion Neurons in the Adult Cochlea. J. Neurosci. 2012, 32, 405–410. [Google Scholar] [CrossRef]
- Bae, W.Y.; Kim, L.S.; Hur, D.Y.; Jeong, S.W.; Kim, J.R. Secondary Apoptosis of Spiral Ganglion Cells Induced by Aminoglycoside: Fas–Fas Ligand Signaling Pathway. Laryngoscope 2008, 118, 1659–1668. [Google Scholar] [CrossRef]
- Dodson, H.; Mohuiddin, A. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J. Neurocytol. 2000, 29, 525–537. [Google Scholar] [CrossRef]
- McFadden, S.L.; Ding, D.; Jiang, H.; Salvi, R.J. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004, 997, 40–51. [Google Scholar] [CrossRef]
- Takeno, S.; Wake, M.; Mount, R.J.; Harrison, R.V. Degeneration of Spiral Ganglion Cells in the Chinchilla afterInner H air Cell Loss Induced by Carboplatin. Audiol. Neurotol. 1998, 3, 281–290. [Google Scholar] [CrossRef]
- Havenith, S.; Klis, S.F.L.; Versnel, H.; Grolman, W. A Guinea Pig Model of Selective Severe High-Frequency Hearing Loss. Otol. Neurotol. 2013, 34, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Versnel, H.; Agterberg, M.J.; de Groot, J.C.; Smoorenburg, G.F.; Klis, S.F. Time course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemide. Hear. Res. 2007, 231, 1–12. [Google Scholar] [CrossRef]
- Basile, A.S.; Huang, J.-M.; Xie, C.; Webster, D.; Berlin, C.; Skolnick, P. N-Methyl-D-aspartate antagonists limit aminoglycoside antibiotic–induced hearing loss. Nat. Med. 1996, 2, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Agerman, K.; Ernfors, P.; Canlon, B. Complementary roles of neurotrophin 3 and a N-Methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc. Natl. Acad. Sci. USA 2000, 97, 7597–7602. [Google Scholar] [CrossRef]
- Ruan, Q.; Ao, H.; He, J.; Chen, Z.; Yu, Z.; Zhang, R.; Wang, J.; Yin, S. Topographic and quantitative evaluation of gentamicin-induced damage to peripheral innervation of mouse cochleae. Neurotoxicology 2014, 40, 86–96. [Google Scholar] [CrossRef]
- Sargsyan, L.; Hetrick, A.P.; Gonzalez, J.G.; Leek, M.R.; Martin, G.K.; Li, H. Effects of combined gentamicin and furosemide treatment on cochlear ribbon synapses. Neurotoxicology 2021, 84, 73–83. [Google Scholar] [CrossRef]
- Locher, H.; Frijns, J.H.; van Iperen, L.; de Groot, J.C.; Huisman, M.A.; Lopes, S.M.C.D.S. Neurosensory development and cell fate determination in the human cochlea. Neural Dev. 2013, 8, 20. [Google Scholar] [CrossRef]
- Morsli, H.; Choo, D.; Ryan, A.; Johnson, R.; Wu, D.K. Development of the Mouse Inner Ear and Origin of Its Sensory Organs. J. Neurosci. 1998, 18, 3327–3335. [Google Scholar] [CrossRef]
- Munnamalai, V.; Fekete, D.M. Wnt signaling during cochlear development. Semin. Cell Dev. Biol. 2013, 24, 480–489. [Google Scholar] [CrossRef]
- Bok, J.; Zenczak, C.; Hwang, C.H.; Wu, D.K. Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13869–13874. [Google Scholar] [CrossRef]
- Pechriggl, E.J.; Bitsche, M.; Glueckert, R.; Rask-Andersen, H.; Blumer, M.J.F.; Schrott-Fischer, A.; Fritsch, H. Development of the innervation of the human inner ear. Dev. Neurobiol. 2015, 75, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Delacroix, L.; Malgrange, B. Cochlear afferent innervation development. Hear. Res. 2015, 330, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Driver, E.C.; Kelley, M.W. Development of the cochlea. Development 2020, 147, dev162263. [Google Scholar] [CrossRef] [PubMed]
- Pavlinkova, G. Molecular Aspects of the Development and Function of Auditory Neurons. Int. J. Mol. Sci. 2021, 22, 131. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, L.V. Early Development of the Spiral Ganglion. In The Primary Auditory Neurons of the Mammalian Cochlea. Springer Handbook of Auditory Research; Dabdoub, A., Fritzsch, B., Popper, A., Fay, R., Eds.; Springer: New York, NY, USA, 2015; Volume 52. [Google Scholar] [CrossRef]
- Waqas, M.; Zhang, S.; He, Z.; Tang, M.; Chai, R. Role of Wnt and Notch signaling in regulating hair cell regeneration in the cochlea. Front. Med. 2016, 10, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Żak, M.; Klis, S.F.; Grolman, W. The Wnt and Notch signalling pathways in the developing cochlea: Formation of hair cells and induction of regenerative potential. Int. J. Dev. Neurosci. 2015, 47, 247–258. [Google Scholar] [CrossRef]
- Riccomagno, M.M.; Takada, S.; Epstein, D.J. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005, 19, 1612–1623. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Yang, J.; Sun, S.; Chai, R.; Chen, Z.-Y.; Li, H. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc. Natl. Acad. Sci. USA 2014, 112, 166–171. [Google Scholar] [CrossRef]
- Jacques, B.E.; Puligilla, C.; Weichert, R.M.; Ferrer-Vaquer, A.; Hadjantonakis, A.-K.; Kelley, M.W.; Dabdoub, A. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development 2012, 139, 4395–4404. [Google Scholar] [CrossRef]
- Chai, R.; Xia, A.; Wang, T.; Jan, T.A.; Hayashi, T.; Bermingham-McDonogh, O.; Cheng, A.G.-L. Dynamic Expression of Lgr5, a Wnt Target Gene, in the Developing and Mature Mouse Cochlea. J. Assoc. Res. Otolaryngol. 2011, 12, 455–469. [Google Scholar] [CrossRef]
- Shi, F.; Cheng, Y.-F.; Wang, X.L.; Edge, A.S. β-Catenin Up-regulates Atoh1 Expression in Neural Progenitor Cells by Interaction with an Atoh1 3′ Enhancer. J. Biol. Chem. 2010, 285, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Owen, T.; Zhang, L.; Zuo, J. Dynamic expression pattern of Sonic hedgehog in developing cochlear spiral ganglion neurons. Dev. Dyn. 2010, 239, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Riccomagno, M.M.; Martinu, L.; Mulheisen, M.; Wu, D.K.; Epstein, D.J. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002, 16, 2365–2378. [Google Scholar] [CrossRef] [PubMed]
- Sapède, D.; Pujades, C. Hedgehog Signaling Governs the Development of Otic Sensory Epithelium and Its Associated Innervation in Zebrafish. J. Neurosci. 2010, 30, 3612–3623. [Google Scholar] [CrossRef]
- Lu, N.; Chen, Y.; Wang, Z.; Chen, G.; Lin, Q.; Chen, Z.-Y.; Li, H. Sonic hedgehog initiates cochlear hair cell regeneration through downregulation of retinoblastoma protein. Biochem. Biophys. Res. Commun. 2013, 430, 700–705. [Google Scholar] [CrossRef]
- Driver, E.C.; Pryor, S.P.; Hill, P.; Turner, J.; Rüther, U.; Biesecker, L.G.; Griffith, A.J.; Kelley, M.W. Hedgehog Signaling Regulates Sensory Cell Formation and Auditory Function in Mice and Humans. J. Neurosci. 2008, 28, 7350–7358. [Google Scholar] [CrossRef]
- Freter, S.; Muta, Y.; Mak, S.-S.; Rinkwitz, S.; Ladher, R. Progressive restriction of otic fate: The role of FGF and Wnt in resolving inner ear potential. Development 2008, 135, 3415–3424. [Google Scholar] [CrossRef]
- Ladher, R.K.; Wright, T.J.; Moon, A.M.; Mansour, S.L.; Schoenwolf, G.C. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005, 19, 603–613. [Google Scholar] [CrossRef]
- Wright, T.J.; Mansour, S.L. Fgf3 and Fgf10 are required for mouse otic placode induction. Development 2003, 130, 3379–3390. [Google Scholar] [CrossRef]
- Hayashi, T.; Ray, C.A.; Younkins, C.; Bermingham-McDonogh, O. Expression patterns of FGF receptors in the developing mammalian cochlea. Dev. Dyn. 2010, 239, 1019–1026. [Google Scholar] [CrossRef]
- Huh, S.-H.; Warchol, M.E.; Ornitz, D.M. Cochlear progenitor number is controlled through mesenchymal FGF receptor signaling. Elife 2015, 4, e05921. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, M.; Huh, S.-H. FGF signaling: Diverse roles during cochlear development. BMB Rep. 2017, 50, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Weir, F.W.; Hatch, J.L.; Muus, J.S.; Wallace, S.A.; Meyer, T.A. Audiologic Outcomes in Ehlers-Danlos Syndrome. Otol. Neurotol. 2016, 37, 748–752. [Google Scholar] [CrossRef]
- Jeon, J.W.; Christensen, J.; Chisholm, J.; Zalewski, C.; Rasooly, M.; Dempsey, C.; Magnani, A.; Frischmeyer-Guerrerio, P.; Brewer, C.C.; Kim, H.J. Audiologic and Otologic Clinical Manifestations of Loeys-Dietz Syndrome: A Heritable Connective Tissue Disorder. Otolaryngol. Neck Surg. 2022, 166, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Corrales, C.E.; Wang, Z.; Zhao, Y.; Wang, Y.; Liu, H.; Heller, S. BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev. Biol. 2005, 5, 16. [Google Scholar] [CrossRef]
- Ma, J.-Y.; You, D.; Li, W.-Y.; Lu, X.-L.; Sun, S.; Li, H.-W. Bone morphogenetic proteins and inner ear development. J. Zhejiang Univ. B 2019, 20, 131–145. [Google Scholar] [CrossRef]
- Pujades, C.; Kamaid, A.; Alsina, B.; Giraldez, F. BMP-signaling regulates the generation of hair-cells. Dev. Biol. 2006, 292, 55–67. [Google Scholar] [CrossRef]
- Ohyama, T.; Basch, M.L.; Mishina, Y.; Lyons, K.M.; Segil, N.; Groves, A.K. BMP Signaling Is Necessary for Patterning the Sensory and Nonsensory Regions of the Developing Mammalian Cochlea. J. Neurosci. 2010, 30, 15044–15051. [Google Scholar] [CrossRef]
- Kolla, L.; Kelly, M.C.; Mann, Z.F.; Anaya-Rocha, A.; Ellis, K.; Lemons, A.; Palermo, A.T.; So, K.S.; Mays, J.C.; Orvis, J.; et al. Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat. Commun. 2020, 11, 2389. [Google Scholar] [CrossRef]
- Koehler, K.R.; Mikosz, A.M.; Molosh, A.I.; Patel, D.; Hashino, E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 2013, 500, 217–221. [Google Scholar] [CrossRef]
- Weston, M.D.; Pierce, M.L.; Jensen-Smith, H.C.; Fritzsch, B.; Rocha-Sanchez, S.; Beisel, K.W.; Soukup, G.A. MicroRNA-183 family expression in hair cell development and requirement of microRNAs for hair cell maintenance and survival. Dev. Dyn. 2011, 240, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.K.; Zhang, K.D.; Fekete, D.M. The Genetics of Hair Cell Development and Regeneration. Annu. Rev. Neurosci. 2013, 36, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, A.; Ackerveken, P.V.D.; Sacheli, R.; Prévot, P.-P.; Thelen, N.; Renauld, J.; Thiry, M.; Delacroix, L.; Nguyen, L.; Malgrange, B. MicroRNA-124 Regulates Cell Specification in the Cochlea through Modulation of Sfrp4/5. Cell Rep. 2015, 13, 31–42. [Google Scholar] [CrossRef]
- Yang, T.; Kersigo, J.; Jahan, I.; Pan, N.; Fritzsch, B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear. Res. 2011, 278, 21–33. [Google Scholar] [CrossRef]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Leung, C.; Tan, S.H.; Barker, N. Recent Advances in Lgr5 + Stem Cell Research. Trends Cell Biol. 2018, 28, 380–391. [Google Scholar] [CrossRef]
- McLean, W.J.; Yin, X.; Lu, L.; Lenz, D.R.; McLean, D.; Langer, R.; Karp, J.M.; Edge, A.S. Clonal Expansion of Lgr5-Positive Cells from Mammalian Cochlea and High-Purity Generation of Sensory Hair Cells. Cell Rep. 2017, 18, 1917–1929. [Google Scholar] [CrossRef]
- Smith-Cortinez, N.; Yadak, R.; Hendriksen, F.G.J.; Sanders, E.; Ramekers, D.; Stokroos, R.J.; Versnel, H.; Straatman, L.V. LGR5-Positive Supporting Cells Survive Ototoxic Trauma in the Adult Mouse Cochlea. Front. Mol. Neurosci. 2021, 14, 729625. [Google Scholar] [CrossRef]
- Wang, T.; Chai, R.; Kim, G.S.; Pham, N.; Jansson, L.; Nguyen, D.-H.; Kuo, B.; May, L.A.; Zuo, J.; Cunningham, L.L.; et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat. Commun. 2015, 6, 6613. [Google Scholar] [CrossRef]
- Basch, M.L.; Ii, R.M.B.; Jen, H.-I.; Semerci, F.; Depreux, F.; Edlund, R.K.; Zhang, H.; Norton, C.R.; Gridley, T.; Cole, S.E.; et al. Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. Elife 2016, 5, e19921. [Google Scholar] [CrossRef]
- Hoa, M.; Olszewski, R.; Linthicum, F.H.; Taukulis, I.; Gu, S.; Detorres, A.; Lopez, I.; Linthicum, F.H., Jr.; Ishiyama, A.; Martin, D.; et al. Characterizing Adult Cochlear Supporting Cell Transcriptional Diversity Using Single-Cell RNA-Seq: Validation in the Adult Mouse and Translational Implications for the Adult Human Cochlea. Front. Mol. Neurosci. 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Dollé, L.; Theise, N.D.; Schmelzer, E.; Boulter, L.; Gires, O.; van Grunsven, L. EpCAM and the biology of hepatic stem/progenitor cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G233–G250. [Google Scholar] [CrossRef] [PubMed]
- Roccio, M.; Perny, M.; Ealy, M.; Widmer, H.R.; Heller, S.; Senn, P. Molecular characterization and prospective isolation of human fetal cochlear hair cell progenitors. Nat. Commun. 2018, 9, 4027. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.S.; Frumm, S.M.; Park, J.S.; Lee, K.; Wong, D.M.; Byrnes, L.; Knox, S.M.; Sneddon, J.B.; Tward, A.D. Development of the Mouse and Human Cochlea at Single Cell Resolution. bioRxiv 2019, 739680. [Google Scholar] [CrossRef]
- Steevens, A.R.; Glatzer, J.C.; Kellogg, C.C.; Low, W.C.; Santi, P.A.; Kiernan, A.E. SOX2 is required for inner ear growth and cochlear nonsensory formation before sensory development. Development 2019, 146, dev170522. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jin, Y.; Chen, J.; Rottier, R.J.; Steel, K.P.; Kiernan, A.E. Ectopic Expression of Activated Notch or SOX2 Reveals Similar and Unique Roles in the Development of the Sensory Cell Progenitors in the Mammalian Inner Ear. J. Neurosci. 2013, 33, 16146–16157. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, A.E.; Cordes, R.; Kopan, R.; Gossler, A.; Gridley, T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 2005, 132, 4353–4362. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Pelling, A.L.; Leung, K.K.H.; Tang, A.S.P.; Bell, D.M.; Tease, C.; Lovell-Badge, R.; Steel, K.P.; Cheah, K.S.E. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005, 434, 1031–1035. [Google Scholar] [CrossRef]
- Oesterle, E.C.; Campbell, S. Supporting Cell Characteristics in Long-deafened Aged Mouse Ears. J. Assoc. Res. Otolaryngol. 2009, 10, 525–544. [Google Scholar] [CrossRef]
- Cheng, C.; Guo, L.; Lu, L.; Xu, X.; Zhang, S.; Gao, J.; Waqas, M.; Zhu, C.; Chen, Y.; Zhang, X.; et al. Characterization of the Transcriptomes of Lgr5+ Hair Cell Progenitors and Lgr5- Supporting Cells in the Mouse Cochlea. Front. Mol. Neurosci. 2017, 10, 122. [Google Scholar] [CrossRef]
- Neves, J.; Uchikawa, M.; Bigas, A.; Giraldez, F. The Prosensory Function of Sox2 in the Chicken Inner Ear Relies on the Direct Regulation of Atoh1. PLoS ONE 2012, 7, e30871. [Google Scholar] [CrossRef] [PubMed]
- Benkafadar, N.; Janesick, A.; Scheibinger, M.; Ling, A.H.; Jan, T.A.; Heller, S. Transcriptomic characterization of dying hair cells in the avian cochlea. Cell Rep. 2021, 34, 108902. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.; Scheibinger, M.; Benkafadar, N.; Kirti, S.; Ellwanger, D.C.; Heller, S. Cell-type identity of the avian cochlea. Cell Rep. 2021, 34, 108900. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, J.; Jin, R.; Bai, H.; Zhang, M.; Yang, S.; Zhang, X.; Zhang, X.; Han, Z.; Zeng, S. Transcriptomic analysis of chicken cochleae after gentamicin damage and the involvement of four signaling pathways (Notch, FGF, Wnt and BMP) in hair cell regeneration. Hear. Res. 2018, 361, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, M.; Kita, T.; Yamamoto, R.; Yamamoto, N.; Okano, T.; Omori, K.; Sakamoto, S.; Nakagawa, T. Initiation of Supporting Cell Activation for Hair Cell Regeneration in the Avian Auditory Epithelium: An Explant Culture Model. Front. Cell. Neurosci. 2020, 14, 583994. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yang, S.; Xi, C.; Wang, X.; Xu, J.; Weng, M.; Zhao, R.; Jiang, L.; Gao, X.; Bing, J.; et al. Signaling pathways (Notch, Wnt, Bmp and Fgf) have additive effects on hair cell regeneration in the chick basilar papilla after streptomycin injury in vitro: Additive Effects of Signaling Pathways on Hair Cell Regeneration. Hear. Res. 2021, 401, 108161. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.S.; Scheibinger, M.; Benkafadar, N.; Kirti, S.; Heller, S. Avian auditory hair cell regeneration is accompanied by JAK/STAT-dependent expression of immune-related genes in supporting cells. Development 2022, 149, dev200113. [Google Scholar] [CrossRef]
- Lush, M.E.; Piotrowski, T. Sensory hair cell regeneration in the zebrafish lateral line. Dev. Dyn. 2014, 243, 1187–1202. [Google Scholar] [CrossRef]
- Kniss, J.S.; Jiang, L.; Piotrowski, T. Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr. Opin. Genet. Dev. 2016, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Romero-Carvajal, A.; Acedo, J.N.; Jiang, L.; Kozlovskaja-Gumbrienė, A.; Alexander, R.; Li, H.; Piotrowski, T. Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways. Dev. Cell 2015, 34, 267–282. [Google Scholar] [CrossRef]
- Head, J.R.; Gacioch, L.; Pennisi, M.; Meyers, J.R. Activation of canonical Wnt/β-catenin signaling stimulates proliferation in neuromasts in the zebrafish posterior lateral line. Dev. Dyn. 2013, 242, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Jacques, B.E.; Iv, W.H.M.; Uribe, P.M.; Yatteau, A.; Asuncion, J.D.; Resendiz, G.; Matsui, J.I.; Dabdoub, A. The role of Wnt/β-catenin signaling in proliferation and regeneration of the developing basilar papilla and lateral line. Dev. Neurobiol. 2014, 74, 438–456. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Tran, N.T.; Diaz, D.C.; Tsai, Y.-Y.; Acedo, J.N.; Lush, M.E.; Piotrowski, T. Single-cell transcriptome analysis reveals three sequential phases of gene expression during zebrafish sensory hair cell regeneration. Dev. Cell 2022, 57, 799–819.e6. [Google Scholar] [CrossRef] [PubMed]
- Lush, M.E.; Diaz, D.C.; Koenecke, N.; Baek, S.; Boldt, H.; Peter, M.K.S.; Gaitan-Escudero, T.; Romero-Carvajal, A.; Busch-Nentwich, E.M.; Perera, A.G.; et al. scRNA-Seq reveals distinct stem cell populations that drive hair cell regeneration after loss of Fgf and Notch signaling. Elife 2019, 8, e44431. [Google Scholar] [CrossRef]
- Jiang, L.; Romero-Carvajal, A.; Haug, J.S.; Seidel, C.W.; Piotrowski, T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc. Natl. Acad. Sci. USA 2014, 111, E1383–E1392. [Google Scholar] [CrossRef]
- Steiner, A.B.; Kim, T.; Cabot, V.; Hudspeth, A.J. Dynamic gene expression by putative hair-cell progenitors during regeneration in the zebrafish lateral line. Proc. Natl. Acad. Sci. USA 2014, 111, E1393–E1401. [Google Scholar] [CrossRef]
- Son, E.J.; Ma, J.-H.; Ankamreddy, H.; Shin, J.-O.; Choi, J.Y.; Wu, D.K.; Bok, J. Conserved role of Sonic Hedgehog in tonotopic organization of the avian basilar papilla and mammalian cochlea. Proc. Natl. Acad. Sci. USA 2015, 112, 3746–3751. [Google Scholar] [CrossRef]
- Liu, Z.; Dearman, J.A.; Cox, B.C.; Walters, B.J.; Zhang, L.; Ayrault, O.; Zindy, F.; Gan, L.; Roussel, M.F.; Zuo, J. Age-Dependent In Vivo Conversion of Mouse Cochlear Pillar and Deiters’ Cells to Immature Hair Cells by Atoh1 Ectopic Expression. J. Neurosci. 2012, 32, 6600–6610. [Google Scholar] [CrossRef]
- Kelly, M.C.; Chang, Q.; Pan, A.; Lin, X.; Chen, P. Atoh1 Directs the Formation of Sensory Mosaics and Induces Cell Proliferation in the Postnatal Mammalian Cochlea In Vivo. J. Neurosci. 2012, 32, 6699–6710. [Google Scholar] [CrossRef]
- Iyer, A.A.; Groves, A.K. Transcription Factor Reprogramming in the Inner Ear: Turning on Cell Fate Switches to Regenerate Sensory Hair Cells. Front. Cell. Neurosci. 2021, 15, 660748. [Google Scholar] [CrossRef]
- Ni, W.; Zeng, S.; Li, W.; Chen, Y.; Zhang, S.; Tang, M.; Sun, S.; Chai, R.; Li, H. Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea. Oncotarget 2016, 7, 66754–66768. [Google Scholar] [CrossRef]
- Borggrefe, T.; Oswald, F. The Notch signaling pathway: Transcriptional regulation at Notch target genes. Cell. Mol. Life Sci. 2009, 66, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wu, M.; Zhao, L.; Ma, J.; Li, W.; Li, H. Selective ablation of inner hair cells and subsequent in-situ hair cell regeneration in the neonatal mouse cochlea. Hear. Res. 2021, 407, 108275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Yu, P.; Hu, Y.; Zhou, H.; Guo, L.; Xu, X.; Zhu, X.; Waqas, M.; Qi, J.; et al. Characterization of Lgr5+ Progenitor Cell Transcriptomes after Neomycin Injury in the Neonatal Mouse Cochlea. Front. Mol. Neurosci. 2017, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, X.; Guo, L.; Ni, W.; Zhang, Y.; Zhao, L.; Wu, L.; Sun, S.; Zhang, S.; Tang, M.; et al. Hedgehog Signaling Promotes the Proliferation and Subsequent Hair Cell Formation of Progenitor Cells in the Neonatal Mouse Cochlea. Front. Mol. Neurosci. 2017, 10, 426. [Google Scholar] [CrossRef]
- Mizutari, K.; Fujioka, M.; Hosoya, M.; Bramhall, N.; Okano, H.J.; Okano, H.; Edge, A.S. Notch Inhibition Induces Cochlear Hair Cell Regeneration and Recovery of Hearing after Acoustic Trauma. Neuron 2013, 77, 58–69. [Google Scholar] [CrossRef]
- Heuermann, M.L.; Matos, S.; Hamilton, D.; Cox, B.C. Regenerated hair cells in the neonatal cochlea are innervated and the majority co-express markers of both inner and outer hair cells. Front. Cell. Neurosci. 2022, 16, 841864. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, L.; Wang, X.; Gao, X.; Bing, J.; Xi, C.; Wang, W.; Zhang, M.; Zhang, X.; Han, Z.; et al. Transcriptomic analysis of mouse cochleae suffering from gentamicin damage reveals the signalling pathways involved in hair cell regeneration. Sci. Rep. 2019, 9, 10494. [Google Scholar] [CrossRef]
- Li, X.-J.; Morgan, C.; Goff, L.A.; Doetzlhofer, A. Follistatin promotes LIN28B-mediated supporting cell reprogramming and hair cell regeneration in the murine cochlea. Sci. Adv. 2023, 8, eabj7651. [Google Scholar] [CrossRef]
- Udagawa, T.; Atkinson, P.J.; Milon, B.; Abitbol, J.M.; Song, Y.; Sperber, M.; Najarro, E.H.; Scheibinger, M.; Elkon, R.; Hertzano, R.; et al. Lineage-tracing and translatomic analysis of damage-inducible mitotic cochlear progenitors identifies candidate genes regulating regeneration. PLoS Biol. 2021, 19, e3001445. [Google Scholar] [CrossRef]
- White, P.M.; Doetzlhofer, A.; Lee, Y.S.; Groves, A.K.; Segil, N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 2006, 441, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Aran, J.-M.; Chappert, C.; Dulon, D.; Erre, J.-P.; Aurousseau, C. Uptake of amikacin by hair cells of the guinea pig cochlea and vestibule and ototoxicity: Comparison with gentamicin. Hear. Res. 1995, 82, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.P.; Forge, A.; Kros, C.J.; Marcotti, W.; Becker, D.; Williams, D.S.; Thorpe, J.; Fleming, J.; Brown, S.D.; Steel, K.P. A missense mutation in myosin VIIA prevents aminoglycoside accumulation in early postnatal cochlear hair cells. Ann. N. Y. Acad. Sci. 1999, 884, 110–124. [Google Scholar]
- Richardson, G.P.; Forge, A.; Kros, C.J.; Fleming, J.; Brown, S.D.M.; Steel, K.P. Myosin VIIA Is Required for Aminoglycoside Accumulation in Cochlear Hair Cells. J. Neurosci. 1997, 17, 9506–9519. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Rovers, J.; Vink, H.A.; Ramekers, D.; Maccarone, R.; Versnel, H. No Protective Effects of Hair Cells or Supporting Cells in Ototoxically Deafened Guinea Pigs upon Administration of BDNF. Brain Sci. 2022, 12, 2. [Google Scholar] [CrossRef]
- Izumikawa, M.; Minoda, R.; Kawamoto, K.; Abrashkin, K.A.; Swiderski, D.L.; Dolan, D.F.; Brough, D.E.; Raphael, Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 2005, 11, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Raphael, Y. Cell Division and Maintenance of Epithelial Integrity in the Deafened Auditory Epithelium. Cell Cycle 2007, 6, 612–619. [Google Scholar] [CrossRef]
- Mittal, R.; Nguyen, D.; Patel, A.P.; Debs, L.H.; Mittal, J.; Yan, D.; Eshraghi, A.A.; Van De Water, T.R.; Liu, X.Z. Recent Advancements in the Regeneration of Auditory Hair Cells and Hearing Restoration. Front. Mol. Neurosci. 2017, 10, 236. [Google Scholar] [CrossRef]
- Layman, W.S.; Zuo, J. Epigenetic regulation in the inner ear and its potential roles in development, protection, and regeneration. Front. Cell. Neurosci. 2015, 8, 446. [Google Scholar] [CrossRef]
- Tao, L.; Yu, H.V.; Llamas, J.; Trecek, T.; Wang, X.; Stojanova, Z.; Groves, A.K.; Segil, N. Enhancer decommissioning imposes an epigenetic barrier to sensory hair cell regeneration. Dev. Cell 2021, 56, 2471–2485.e5. [Google Scholar] [CrossRef]
- Iyer, A.A.; Hosamani, I.; Nguyen, J.D.; Cai, T.; Singh, S.; McGovern, M.M.; Beyer, L.; Zhang, H.; Jen, H.-I.; Yousaf, R.; et al. Cellular reprogramming with ATOH1, GFI1, and POU4F3 implicate epigenetic changes and cell-cell signaling as obstacles to hair cell regeneration in mature mammals. Elife 2022, 11, e79712. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Bencie, N.; Liu, G.; Eshraghi, N.; Nisenbaum, E.; Blanton, S.H.; Yan, D.; Mittal, J.; Dinh, C.T.; Young, J.I.; et al. Recent advancements in understanding the role of epigenetics in the auditory system. Gene 2020, 761, 144996. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, S.; Qin, S.; Guo, J.; Yuan, J.; Qiang, R.; Zhou, S.; Cao, W.; Yang, J.; Ma, F.; et al. Transcriptomic and epigenomic analyses explore the potential role of H3K4me3 in neomycin-induced cochlear Lgr5+ progenitor cell regeneration of hair cells. Hum. Cell 2022, 35, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hu, Z. Generation of Cochlear Hair Cells from Sox2 Positive Supporting Cells via DNA Demethylation. Int. J. Mol. Sci. 2020, 21, 8649. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tanigaki, K.; Tsuji, M.; Yabe, D.; Ito, J.; Honjo, T. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J. Mol. Med. 2006, 84, 37–45. [Google Scholar] [CrossRef]
- Korrapati, S.; Roux, I.; Glowatzki, E.; Doetzlhofer, A. Notch Signaling Limits Supporting Cell Plasticity in the Hair Cell-Damaged Early Postnatal Murine Cochlea. PLoS ONE 2013, 8, e73276. [Google Scholar] [CrossRef]
- Erni, S.T.; Gill, J.C.; Palaferri, C.; Fernandes, G.; Buri, M.; Lazarides, K.; Grandgirard, D.; Edge, A.S.B.; Leib, S.L.; Roccio, M. Hair Cell Generation in Cochlear Culture Models Mediated by Novel γ-Secretase Inhibitors. Front. Cell Dev. Biol. 2021, 9, 710159. [Google Scholar] [CrossRef]
- Samarajeewa, A.; Lenz, D.R.; Xie, L.; Chiang, H.; Kirchner, R.; Mulvaney, J.F.; Edge, A.S.B.; Dabdoub, A. Transcriptional response to Wnt activation regulates the regenerative capacity of the mammalian cochlea. Development 2018, 145, dev166579. [Google Scholar] [CrossRef]
- Wu, J.; Li, W.; Guo, L.; Zhao, L.; Sun, S.; Li, H. The crosstalk between the Notch, Wnt, and SHH signaling pathways in regulating the proliferation and regeneration of sensory progenitor cells in the mouse cochlea. Cell Tissue Res. 2021, 386, 281–296. [Google Scholar] [CrossRef]
- Wu, J.; Dong, X.; Li, W.; Zhao, L.; Zhou, L.; Sun, S.; Li, H. Dibenzazepine promotes cochlear supporting cell proliferation and hair cell regeneration in neonatal mice. Cell Prolif. 2020, 53, e12872. [Google Scholar] [CrossRef]
- Ellis, K.; Driver, E.C.; Okano, T.; Lemons, A.; Kelley, M.W. GSK3 regulates hair cell fate in the developing mammalian cochlea. Dev. Biol. 2019, 453, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Li, W.; Huang, M.; Quan, Y.-Z.; Scheffer, D.; Tian, C.; Tao, Y.; Liu, X.; Hochedlinger, K.; Indzhykulian, A.A.; et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat. Commun. 2019, 10, 5530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, L.; Zhu, M.-S.; Wan, G. High-throughput screening on cochlear organoids identifies VEGFR-MEK-TGFB1 signaling promoting hair cell reprogramming. Stem Cell Rep. 2021, 16, 2257–2273. [Google Scholar] [CrossRef] [PubMed]
- Cassinotti, L.R.; Ji, L.; Borges, B.C.; Cass, N.D.; Desai, A.S.; Kohrman, D.C.; Liberman, M.C.; Corfas, G. Cochlear Neurotrophin-3 overexpression at mid-life prevents age-related inner hair cell synaptopathy and slows age-related hearing loss. Aging Cell 2022, 21, e13708. [Google Scholar] [CrossRef]
- Gillespie, L.N.; Clark, G.M.; Marzella, P.L. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport 2004, 15, 1121–1125. [Google Scholar] [CrossRef]
- Staecker, H.; Van De Water, T.R.; Lefebvre, P.P.; Liu, W.; Moghadassi, M.; Galinovic-Schwartz, V.; Malgrange, B.; Moonen, G. NGF, BDNF and NT-3 play unique roles in the in vitro development and patterning of innervation of the mammalian inner ear. Dev. Brain Res. 1996, 92, 49–60. [Google Scholar] [CrossRef]
- Vink, H.A.; Versnel, H.; Kroon, S.; Klis, S.F.; Ramekers, D. BDNF-mediated preservation of spiral ganglion cell peripheral processes and axons in comparison to that of their cell bodies. Hear. Res. 2021, 400, 108114. [Google Scholar] [CrossRef]
- Vink, H.A.; Ramekers, D.; Thomeer, H.G.X.M.; Versnel, H. Combined brain-derived neurotrophic factor and neurotrophin-3 treatment is preferred over either one separately in the preservation of the auditory nerve in deafened guinea pigs. Front. Mol. Neurosci. 2022, 15, 935111. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Gómez-Casati, M.E.; Gigliello, A.R.; Liberman, M.C.; Corfas, G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife 2014, 3, e03564. [Google Scholar] [CrossRef]
- Nevoux, J.; Alexandru, M.; Bellocq, T.; Tanaka, L.; Hayashi, Y.; Watabe, T.; Lahlou, H.; Tani, K.; Edge, A.S.B. An antibody to RGMa promotes regeneration of cochlear synapses after noise exposure. Sci. Rep. 2021, 11, 2937. [Google Scholar] [CrossRef]
- Fernandez, K.A.; Watabe, T.; Tong, M.; Meng, X.; Tani, K.; Kujawa, S.G.; Edge, A.S. Trk agonist drugs rescue noise-induced hidden hearing loss. J. Clin. Investig. 2021, 6, e142572. [Google Scholar] [CrossRef] [PubMed]
- Szobota, S.; Mathur, P.D.; Siegel, S.; Black, K.; Saragovi, H.U.; Foster, A.C. BDNF, NT-3 and Trk receptor agonist monoclonal antibodies promote neuron survival, neurite extension, and synapse restoration in rat cochlea ex vivo models relevant for hidden hearing loss. PLoS ONE 2019, 14, e0224022. [Google Scholar] [CrossRef] [PubMed]
- Seist, R.; Tong, M.; Landegger, L.D.; Vasilijic, S.; Hyakusoku, H.; Katsumi, S.; McKenna, C.E.; Edge, A.S.B.; Stankovic, K.M. Regeneration of Cochlear Synapses by Systemic Administration of a Bisphosphonate. Front. Mol. Neurosci. 2020, 13, 87. [Google Scholar] [CrossRef]
- Yamahara, K.; Asaka, N.; Kita, T.; Kishimoto, I.; Matsunaga, M.; Yamamoto, N.; Omori, K.; Nakagawa, T. Insulin-like growth factor 1 promotes cochlear synapse regeneration after excitotoxic trauma in vitro. Hear. Res. 2019, 374, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ogino-Nishimura, E.; Hiraumi, H.; Sakamoto, T.; Yamamoto, N.; Ito, J. Audiometric Outcomes of Topical IGF1 Treatment for Sudden Deafness Refractory to Systemic Steroids. Otol. Neurotol. 2012, 33, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kumakawa, K.; Usami, S.-I.; Hato, N.; Tabuchi, K.; Takahashi, M.; Fujiwara, K.; Sasaki, A.; Komune, S.; Sakamoto, T.; et al. A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Naeve, G.S.; Ramakrishnan, M.; Kramer, R.; Hevroni, D.; Citri, Y.; Theill, L.E. Neuritin: A gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, X.; Wang, H.; Chen, R.; Yang, Y.; Hu, J.; Zhang, Y.; Gui, F.; Huang, J.; Yang, L.; et al. Conditional overexpression of neuritin in supporting cells (SCs) mitigates hair cell (HC) damage and induces HC regeneration in the adult mouse cochlea after drug-induced ototoxicity. Hear. Res. 2022, 420, 108515. [Google Scholar] [CrossRef] [PubMed]
- Senn, P.; Mina, A.; Volkenstein, S.; Kranebitter, V.; Oshima, K.; Heller, S. Progenitor Cells from the Adult Human Inner Ear. Anat. Rec. 2020, 303, 461–470. [Google Scholar] [CrossRef]
- Matsunaga, M.; Nakagawa, T. Future Pharmacotherapy for Sensorineural Hearing Loss by Protection and Regeneration of Auditory Hair Cells. Pharmaceutics 2023, 15, 777. [Google Scholar] [CrossRef]
- Foster, A.C.; Jacques, B.E.; Piu, F. Hearing loss: The final frontier of pharmacology. Pharmacol. Res. Perspect. 2022, 10, e00970. [Google Scholar] [CrossRef] [PubMed]
- McLean, W.J.; Hinton, A.S.; Herby, J.T.; Salt, A.N.; Hartsock, J.J.; Wilson, S.; Lucchino, D.L.; Lenarz, T.; Warnecke, A.; Prenzler, N.; et al. Improved Speech Intelligibility in Subjects with Stable Sensorineural Hearing Loss Following Intratympanic Dosing of FX-322 in a Phase 1b Study. Otol. Neurotol. 2021, 42, e849–e857. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Nyberg, S.; Abbott, N.J.; Shi, X.; Steyger, P.S.; Dabdoub, A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019, 11, eaao0935. [Google Scholar] [CrossRef] [PubMed]
- Lentz, J.J.; Pan, B.; Ponnath, A.; Tran, C.M.; Nist-Lund, C.; Galvin, A.; Goldberg, H.; Robillard, K.N.; Jodelka, F.M.; Farris, H.E.; et al. Direct Delivery of Antisense Oligonucleotides to the Middle and Inner Ear Improves Hearing and Balance in Usher Mice. Mol. Ther. 2020, 28, 2662–2676. [Google Scholar] [CrossRef]
- Havenith, S.; Versnel, H.; Agterberg, M.J.; de Groot, J.C.; Sedee, R.-J.; Grolman, W.; Klis, S.F. Spiral ganglion cell survival after round window membrane application of brain-derived neurotrophic factor using gelfoam as carrier. Hear. Res. 2011, 272, 168–177. [Google Scholar] [CrossRef]
- Vink, H.A.; van Dorp, W.C.; Thomeer, H.G.X.M.; Versnel, H.; Ramekers, D. BDNF Outperforms TrkB Agonist 7,8,3′-THF in Preserving the Auditory Nerve in Deafened Guinea Pigs. Brain Sci. 2020, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Kelso, C.M.; Watanabe, H.; Wazen, J.M.; Bucher, T.; Qian, Z.J.; Olson, E.S.; Kysar, J.W.; Lalwani, A.K. Microperforations Significantly Enhance Diffusion Across Round Window Membrane. Otol. Neurotol. 2015, 36, 694–700. [Google Scholar] [CrossRef]
- Le, T.; Straatman, L.; Yanai, A.; Rahmanian, R.; Garnis, C.; Häfeli, U.; Poblete, T.; Westerberg, B.; Gregory-Evans, K. Magnetic stem cell targeting to the inner ear. J. Magn. Magn. Mater. 2017, 443, 385–396. [Google Scholar] [CrossRef]
| Gene/ Protein | Species | Stage | Location in Cochlea per Stage | References |
|---|---|---|---|---|
| EpCAM | Mouse | Mature adult | RM, Cochlear HCs, SCs | [116] |
| Human | Fetal | Chochlear duct | [115,116] | |
| Lfng | Mouse | Postnatal | DC3 | [101] |
| Mature adult | IphC, IPC, OPC, DC | [113] | ||
| Lgr4 | Mouse | Embryonic | Cochlear duct and SGN | [23] |
| Postnatal | DCs and IPCs | [23] | ||
| Adolescent | DCs | [23] | ||
| Lgr5 | Mouse | Embryonic | DC3, IPCs, IphCs, and the lateral GER | [22,82] |
| Postnatal | IPC, GER, DC3, IPHC | [20,22,77,82,122] | ||
| Adolescent | IPCs, DC3, IBC | [82,110] | ||
| Mature adult | DC3, IPCs | [22,110] | ||
| Deafened (p30) | Survival in DC3 only | [110] | ||
| Human * | Fetal | Prosensory doman, LER, SCs | [115] | |
| Lgr6 | Mouse | Embryonic | IPCs | [20] |
| Postnatal | IPCs, IBCs (disappears at p30) | [20,22] | ||
| Sox2 | Mouse | Embryonic | HCs, adjacent supporting cells and GER | [20,120] |
| Postnatal | SCs | [22,82] | ||
| Mature adult | BCs, IphCs, IPC, OPC, DCs, HeCs | [21,121] | ||
| Deafened (p120) | BCs, IphCs, IPC, OPC, DCs, HeCs | [21,121] | ||
| Human | Fetal | Organ of Corti | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith-Cortinez, N.; Tan, A.K.; Stokroos, R.J.; Versnel, H.; Straatman, L.V. Regeneration of Hair Cells from Endogenous Otic Progenitors in the Adult Mammalian Cochlea: Understanding Its Origins and Future Directions. Int. J. Mol. Sci. 2023, 24, 7840. https://doi.org/10.3390/ijms24097840
Smith-Cortinez N, Tan AK, Stokroos RJ, Versnel H, Straatman LV. Regeneration of Hair Cells from Endogenous Otic Progenitors in the Adult Mammalian Cochlea: Understanding Its Origins and Future Directions. International Journal of Molecular Sciences. 2023; 24(9):7840. https://doi.org/10.3390/ijms24097840
Chicago/Turabian StyleSmith-Cortinez, Natalia, A. Katherine Tan, Robert J. Stokroos, Huib Versnel, and Louise V. Straatman. 2023. "Regeneration of Hair Cells from Endogenous Otic Progenitors in the Adult Mammalian Cochlea: Understanding Its Origins and Future Directions" International Journal of Molecular Sciences 24, no. 9: 7840. https://doi.org/10.3390/ijms24097840




