New pecJ-n (n = 1, 2) Basis Sets for Selenium Atom Purposed for the Calculations of NMR Spin–Spin Coupling Constants Involving Selenium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Creation of pecJ-n (n = 1, 2) Basis Sets for Selenium
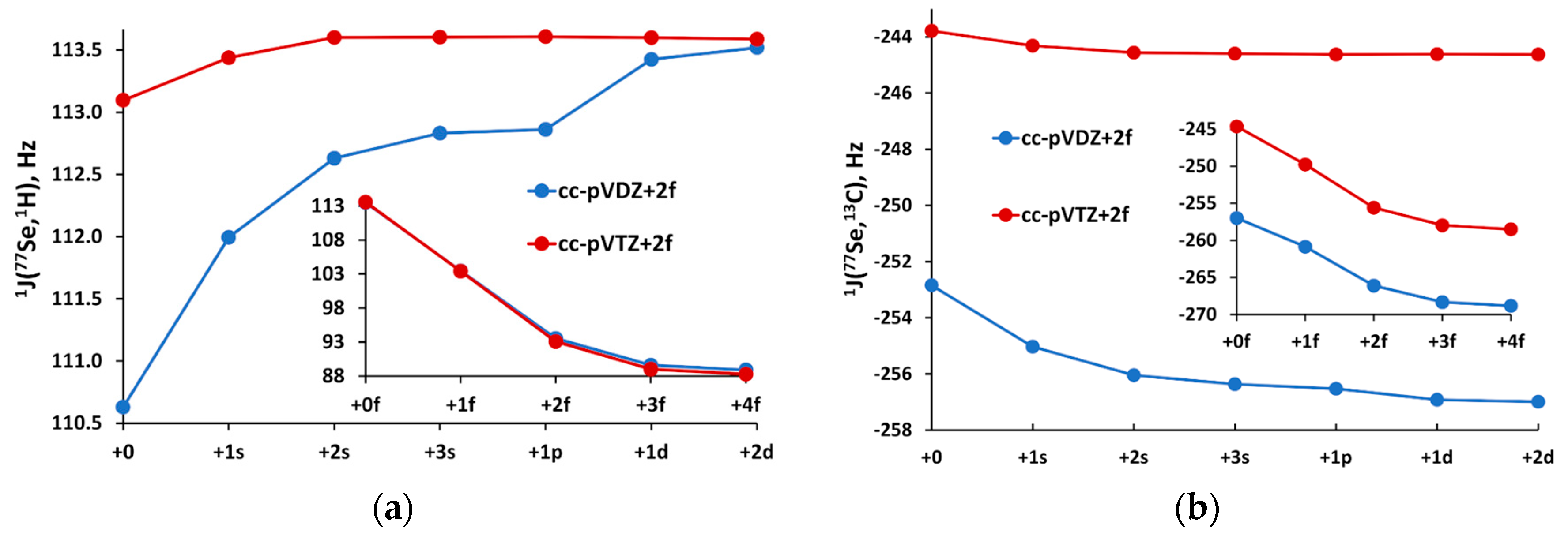
- The PEC optimization of the contraction coefficients for the s-shell with respect to the target function: is performed. The are the values obtained with chosen s-contraction pattern with the varying contraction coefficients, and are the values obtained with the fully uncontracted pecJ-n(uc) basis set on selenium. When the optimization procedure is completed, the reference values are redefined: the new ones are obtained with the modified pecJ-n basis set on selenium, in which the s-shell is contracted with the optimized coefficients, and the remaining shells are uncontracted;
- The PEC optimization of the contraction coefficients for the p-shell starts; the reference values are those that were recalculated in the previous step. When the optimized coefficients for the p-shell are obtained, the are redefined once again: the new ones are calculated with the pecJ-n basis set, in which the s-shell is contracted with the coefficients obtained in the first step and p-shell is contracted with the newly optimized coefficients, while the remaining shells are kept uncontracted;
- The PEC optimization of the contraction coefficients for the d-shell starts; the reference values are those that were recalculated in the previous step. In the end, we arrive at the final pecJ-n basis set with the contracted s-, p-, and d-shells and uncontracted f-shell, plus 1g function in the pecJ-2 basis set.
2.2. Testing New Basis Sets
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eybl, V.; Kotyzová, D.; Sýkora, J.; Topolčan, O.; Pikner, R.; Mihaljevič, M.; Brtko, J.; Glattre, E. Effects of selenium and tellurium on the activity of selenoenzymes glutathione peroxidase and Type I iodothyronine deiodinase, trace element thyroid level, and thyroid hormone status in rats. Biol. Trace Elem. Res. 2007, 117, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Gafvelin, G.; Arnér, E.S. Selenocysteine in proteins—Properties and biotechnological use. Biochim. Biophys. Acta 2005, 1726, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Standtman, T.C. Selenium biochemistry. Annu. Rev. Biochem. 1990, 59, 111–127. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; Combs, S.B. The Nutritional Biochemistry of Selenium. Annu. Rev. Nutr. 1984, 4, 257–280. [Google Scholar] [CrossRef]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef]
- Phiri, F.P.; Ander, E.L.; Lark, R.M.; Bailey, E.H.; Chilima, B.; Gondwe, J.; Joy, E.J.M.; Kalimbira, A.A.; Phuka, J.C.; Suchdev, P.S.; et al. Urine selenium concentration is a useful biomarker for assessing population level selenium status. Environ. Int. 2020, 134, 105218. [Google Scholar] [CrossRef]
- Behne, D.; Kyriakopoulos, A. Mammalian selenium-containing proteins. Annu. Rev. Nutr. 2001, 21, 453–473. [Google Scholar] [CrossRef]
- Garcia, R.I.; Lozano, R.D.; Martinez, E.A. A transimpedance circuit for use with selenium photoelectric cells. J. Phys. E Sci. Instrum. 1972, 5, 745. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; You, Q.; Huang, P.; Wang, Y.; Huang, Z.N.; Ge, Y.; Wu, L.; Dong, Z.; Dai, X.; et al. Enhanced Photodetection Properties of Tellurium-Selenium Roll-to-Roll Nanotube Heterojunctions. Nano Micro Small 2019, 15, 1900902. [Google Scholar] [CrossRef] [PubMed]
- Hadar, I.; Song, T.-B.; Ke, W.; Kanatzidis, M.G. Modern Processing and Insights on Selenium Solar Cells: The World’s First Photovoltaic Device. Adv. Energy Mater. 2019, 9, 1802766. [Google Scholar] [CrossRef]
- Malik, M.A.; Ramasamy, K.; O’Brien, P. Selenium and Tellurium Containing Precursors for Semiconducting Materials. In Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials, 1st ed.; Woollins, J.D., Laitinen, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Chapter 9; pp. 201–238. [Google Scholar] [CrossRef]
- Johnson, J.A.; Saboungi, M.-L.; Thiyagarajan, P.; Csencsits, R.; Meisel, D. Selenium Nanoparticles: A Small-Angle Neutron Scattering Study. J. Phys. Chem. B 1999, 103, 59–63. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lampis, S.; Vallini, G.; Turner, R.J. Physical–Chemical Properties of Biogenic Selenium Nanostructures Produced by Stenotrophomonas maltophilia SeITE02 and Ochrobactrum sp. MPV1. Phys. Sci. Rev. 2018, 3, 20170100. [Google Scholar] [CrossRef]
- Mayers, B.; Jiang, X.; Sunderland, D.; Cattle, B.; Xia, Y. Hollow Nanostructures of Platinum with Controllable Dimensions Can Be Synthesized by Templating Against Selenium Nanowires and Colloids. J. Am. Chem. Soc. 2003, 125, 13364–13365. [Google Scholar] [CrossRef]
- Terasaki, O.; Yamazaki, K.; Thomas, J.M.; Ohsuna, T.; Watanabe, D.; Sanders, J.V.; Barry, J.C. Isolating individual chains of selenium by incorporation into the channels of a zeolite. Nature 1987, 330, 58–60. [Google Scholar] [CrossRef]
- Parise, J.B.; MacDougall, J.; Herron, N.; Farlee, R.; Sleight, A.W.; Wang, Y.; Bein, T.; Moller, K.; Moroney, L.M. Characterization of selenium-loaded molecular sieves A, X, Y, AlPO-5, and mordenite. Inorg. Chem. 1988, 27, 221–228. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y. Quantum chemical calculations of 77Se and 125Te nuclear magnetic resonance spectral parameters and their structural applications. Magn. Reson. Chem. 2021, 59, 359–407. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S. 77Se NMR: Theoretical Aspects and Practical Applications. In Organoselenium Chemistry: Between Synthesis and Biochemistry, 1st ed.; Santi, C., Ed.; Bentham Science Publishers (eBook): Dubai, United Arab Emirates, 2014; Chapter 13; pp. 379–415. [Google Scholar] [CrossRef]
- Krivdin, L.B. Recent advances in computational liquid-phase 77Se NMR. Russ. Chem. Rev. 2021, 90, 265–279. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L.; Krivdin, L.B. MP2 calculation of 77Se NMR chemical shifts taking into account relativistic corrections. Magn. Reson. Chem. 2015, 53, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Rusakov, Y.Y.; Rusakova, I.L.; Krivdin, L.B. On the significant relativistic heavy atom effect on 13C NMR chemical shifts of β- and γ-carbons in seleno- and telluroketones. Mol. Phys. 2017, 115, 3117–3127. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. Long-range relativistic heavy atom effect on 1H NMR chemical shifts of selenium- and tellurium-containing compounds. Int. J. Quantum Chem. 2018, 119, e25809. [Google Scholar] [CrossRef]
- Vícha, J.; Novotný, J.; Komorovsky, S.; Straka, M.; Kaupp, M.; Marek, R. Relativistic Heavy-Neighbor-Atom Effects on NMR Shifts: Concepts and Trends Across the Periodic Table. Chem. Rev. 2020, 120, 7065–7103. [Google Scholar] [CrossRef]
- Vícha, J.; Komorovsky, S.; Repisky, M.; Marek, R.; Straka, M. Relativistic Spin−Orbit Heavy Atom on the Light Atom NMR Chemical Shifts: General Trends Across the Periodic Table Explained. J. Chem. Theory Comput. 2018, 14, 3025–3039. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y. Relativistic Effects from Heavy Main Group p-Elements on the NMR Chemical Shifts of Light Atoms: From Pioneering Studies to Recent Advances. Magnetochemistry 2023, 9, 24. [Google Scholar] [CrossRef]
- Wrackmeyer, B. Indirect Nuclear 77Se–77Se Spin–Spin Coupling Constants. Application of Density Functional Theory (DFT) Calculations. Struct. Chem. 2005, 16, 67–71. [Google Scholar] [CrossRef]
- Åstrand, P.-O.; Mikkelsen, K.V. Solvent effects on nuclear shieldings and spin–spin couplings of hydrogen selenide. J. Chem. Phys. 1998, 108, 2528–2537. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y. Correlated ab initio calculations of one-bond 31P-77Se and 31P-125Te spin–spin coupling constants in a series of P=Se and P=Te systems accounting for relativistic effects (part 2). Magn. Reson. Chem. 2020, 58, 929–940. [Google Scholar]
- Rusakov, Y.Y.; Rusakova, I.L.; Krivdin, L.B. Full four-component relativistic calculations of the one-bond 77Se–13C spin-spin coupling constants in the series of selenium heterocycles and their parent open-chain selenides. Magn. Reson. Chem. 2014, 52, 214–221. [Google Scholar] [CrossRef]
- Feindel, K.W.; Wasylishen, R.E. A relativistic DFT study of one-bond fluorine-X indirect spin–spin coupling tensors. Magn. Reson. Chem. 2004, 42, S158–S167. [Google Scholar] [CrossRef] [PubMed]
- Tanioku, A.; Hayashi, S.; Nakanishi, W. Analysis of One-Bond Se-Se Nuclear Couplings in Diselenides and 1,2-Diselenoles on the Basis of Molecular Orbital Theory: Torsional Angular Dependence, Electron Density Influence, and Origin in 1J(Se,Se). Bioinorg. Chem. Appl. 2009, 2009, 381925. [Google Scholar] [CrossRef] [PubMed]
- Jokisaari, J.; Autschbach, J. 13C–77Se and 77Se–77Se spin–spin coupling tensors in carbon diselenide: NMR experiments and ZORA DFT calculations. Phys. Chem. Chem. Phys. 2003, 5, 4551–4555. [Google Scholar] [CrossRef]
- Komorovsky, S.; Jakubowska, K.; Swider, P.; Repisky, M.; Jaszunski, M. NMR Spin–Spin Coupling Constants Derived from Relativistic Four-Component DFT Theory—Analysis and Visualization. J. Phys. Chem. A 2020, 124, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P.; Wiesenfeld, L. Relativistically parameterized extended Hückel calculations. IV. Nuclear spin-spin coupling tensors for main group elements. Mol. Phys. 1981, 43, 557–580. [Google Scholar] [CrossRef]
- Gomez, S.S.; Romero, R.H.; Aucar, G.A. Fully relativistic calculation of nuclear magnetic shieldings and indirect nuclear spin-spin couplings in group-15 and -16 hydrides. J. Chem. Phys. 2002, 117, 7942–7946. [Google Scholar] [CrossRef]
- Aucar, G.A.; Saue, T.; Visscher, L.; Jensen, H.A. On the origin and contribution of the diamagnetic term in four-component relativistic calculations of magnetic properties. J. Chem. Phys. 1999, 110, 6208–6218. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y.; Krivdin, L.B. First example of the correlated calculation of the one-bond tellurium-carbon spin-spin coupling constants: Relativistic effects, vibrational corrections, and solvent effects. J. Comput. Chem. 2016, 37, 1367–1372. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Østerstrøm, F.F.; Sauer, S.P.A.; Potapov, V.A.; Amosova, S.V. First example of a high-level correlated calculation of the indirect spin–spin coupling constants involving tellurium: Tellurophene and divinyl telluride. Phys. Chem. Chem. Phys. 2013, 15, 13101–13107. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Krivdin, L.B. Relativistic effects in the NMR spectra of compounds containing heavy chalcogens. Mendeleev Commun. 2018, 28, 1–13. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y.; Krivdin, L.B. Relativistic effects in the one-bond spin–spin coupling constants involving selenium. Magn. Reson. Chem. 2014, 52, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, J. Perspective: Relativistic effects. J. Chem. Phys. 2012, 136, 150902. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, J. Relativistic Effects on NMR Parameters. In High Resolution NMR Spectroscopy. Understanding Molecules and Their Electronic Structures, 1st ed.; Contreras, R.H., Ed.; Elsevier B.V.: London, UK, 2013; Volume 3, Chapter 4; pp. 69–117. [Google Scholar]
- Colquhoun, I.J.; McFarlane, H.C.E.; McFarlane, W.; Nash, J.A.; Keat, R.; Rycroft, D.S.; Thompson, D.G. Long-range nuclear spin–spin coupling between selenium-77 and phosphorus-31 in biphosphorus compounds. Org. Magn. Res. 1979, 12, 473–475. [Google Scholar] [CrossRef]
- Krivdin, L.B.; Rusakov, Y.Y. Structural and Stereochemical Applications of Computational NMR Using 29Si–1H and 77Se–1H Indirect Spin–Spin Coupling Constants. eMagRes 2014, 3, 87–110. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B. Stereochemical behavior of geminal and vicinal 77Se–13C spin–spin coupling constants studied at the SOPPA(CC2) level taking into account relativistic corrections. Magn. Reson. Chem. 2015, 53, 93–98. [Google Scholar] [CrossRef]
- Kövér, K.E.; Kumar, A.A.; Rusakov, Y.Y.; Krivdin, L.B.; Illyés, T.-Z.; Szilágyi, L. Experimental and computational studies of nJ(77Se,1H) selenium–proton couplings in selenoglycosides. Magn. Reson. Chem. 2011, 49, 190–194. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Penzik, M.V.; Potapov, V.A.; Amosova, S.V. Open-chain unsaturated selanyl sulfides: Stereochemical structure and stereochemical behavior of their 77Se–1H spin–spin coupling constants. Magn. Reson. Chem. 2012, 50, 653–658. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Kumar, A.A.; Szilágyi, L.; Kövér, K.E. Resonance assignments of diastereotopic CH2 protons in the anomeric side chain of selenoglycosides by means of 2J(Se,H) spin-spin coupling constants. Magn. Reson. Chem. 2012, 50, 488–495. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S. Torsional Angular Dependence of 1J(Se,Se) and Fermi Contact Control of 4J(Se,Se): Analysis of nJ(Se,Se) (n = 1–4) Based on Molecular Orbital Theory. Chem. Eur. J. 2008, 14, 5645–5655. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Orlov, N.V.; Ananikov, V.P. Stereochemical study of the sterically crowded phenylselanylalkenes by means of 77Se-1H spin–spin coupling constants. Magn. Reson. Chem. 2011, 49, 570–574. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Potapov, V.A.; Penzik, M.V.; Amosova, S.V. Conformational analysis and diastereotopic assignments in the series of selenium-containing heterocycles by means of 77Se-1H spin-spin coupling constants: A combined theoretical and experimental study. Magn. Reson. Chem. 2011, 49, 389–398. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B. Stereochemical behavior of 2J(Se,H) and 3J(Se,H) spin-spin coupling constants across sp3 carbons: A theoretical scrutiny. Magn. Reson. Chem. 2012, 50, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, J.; Ziegler, T. Relativistic computation of NMR shieldings and spin-spin coupling constants. In Encyclopedia of Nuclear Magnetic Resonance: Advances in NMR, 1st ed.; Grant, D.M., Harris., R.K., Eds.; John Wiley and Sons: Chichester, UK, 2002; Volume 9, pp. 306–323. [Google Scholar]
- Autschbach, J. Relativistic calculations of magnetic resonance parameters: Background and some recent developments. Phil. Trans. R. Soc. A 2014, 372, 20120489. [Google Scholar] [CrossRef] [PubMed]
- Facelli, J.C. Chemical shift tensors: Theory and application to molecular structural problems. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 58, 176–201. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P. Theory of NMR parameters. From Ramsey to Relativity, 1953 to 1983. In Calculation of NMR and EPR Parameters. Theory and Applications, 1st ed.; Kaupp, M., Bühl, M., Malkin, V.G., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; Chapter 2; pp. 7–19. [Google Scholar]
- Rusakov, Y.Y.; Krivdin, L.B. Modern quantum chemical methods for calculating spin–spin coupling constants: Theoretical basis and structural applications in chemistry. Russ. Chem. Rev. 2013, 82, 99–130. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y.; Krivdin, L.B. Theoretical grounds of relativistic methods for calculation of spin–spin coupling constants in nuclear magnetic resonance spectra. Russ. Chem. Rev. 2016, 85, 356–426. [Google Scholar] [CrossRef]
- Rusakova, I.L. Quantum Chemical Approaches to the Calculation of NMR Parameters: From Fundamentals to Recent Advances. Magnetochemistry 2022, 8, 50. [Google Scholar] [CrossRef]
- Vahtras, O.; Ågren, H.; Jørgensen, P.; Helgaker, T.; Jensen, H.J.A. The nuclear spin-spin coupling in N2 and CO. Chem. Phys. Lett. 1993, 209, 201–206. [Google Scholar] [CrossRef]
- Barszczewicz, A.; Helgaker, T.; Jaszuński, M.; Jørgensen, P.; Ruud, K. Multiconfigurational self-consistent field calculations of nuclear magnetic resonance indirect spin–spin coupling constants. J. Chem. Phys. 1994, 101, 6822–6828. [Google Scholar] [CrossRef]
- Malkin, V.G.; Malkina, O.L.; Salahub, D.R. Calculation of spin-spin coupling constants using density functional theory. Chem. Phys. Lett. 1994, 221, 91–99. [Google Scholar] [CrossRef]
- Helgaker, T.; Watson, M.; Handy, N.C. Analytical calculation of nuclear magnetic resonance indirect spin–spin coupling constants at the generalized gradient approximation and hybrid levels of density-functional theory. J. Chem. Phys. 2000, 113, 9402–9409. [Google Scholar] [CrossRef]
- Autschbach, J.; Ziegler, T. Nuclear spin–spin coupling constants from regular approximate relativistic density functional calculations. I. Formalism and scalar relativistic results for heavy metal compounds. J. Chem. Phys. 2000, 113, 936–947. [Google Scholar] [CrossRef]
- Sychrovský, V.; Gräfenstein, J.; Cremer, D. Nuclear magnetic resonance spin–spin coupling constants from coupled perturbed density functional theory. J. Chem. Phys. 2000, 113, 3530–3547. [Google Scholar] [CrossRef]
- Barone, V.; Peralta, J.E.; Contreras, R.H.; Snyder, J.P. DFT Calculation of NMR JFF Spin−Spin Coupling Constants in Fluorinated Pyridines. J. Phys. Chem. A 2002, 106, 5607–5612. [Google Scholar] [CrossRef]
- Sekino, H.; Bartlett, R.J. Nuclear spin–spin coupling constants evaluated using many body methods. J. Chem. Phys. 1986, 85, 3945–3949. [Google Scholar] [CrossRef]
- Perera, S.A.; Sekino, H.; Bartlett, R.J. Coupled-cluster calculations of indirect nuclear coupling constants: The importance of non-Fermi contact contributions. J. Chem. Phys. 1994, 101, 2186–2191. [Google Scholar] [CrossRef]
- Perera, S.A.; Nooijen, M.; Bartlett, R.J. Electron correlation effects on the theoretical calculation of nuclear magnetic resonance spin–spin coupling constants. J. Chem. Phys. 1996, 104, 3290–3305. [Google Scholar] [CrossRef]
- Auer, A.; Gauss, J. Triple excitation effects in coupled-cluster calculations of indirect spin–spin coupling constants. J. Chem. Phys. 2001, 115, 1619–1622. [Google Scholar] [CrossRef]
- Christiansen, O.; Koch, H.; Jørgensen, P. The second-order approximate coupled cluster singles and doubles model CC2. Chem. Phys. Lett. 1995, 243, 409–418. [Google Scholar] [CrossRef]
- Bartlett, R.J. Many-Body Perturbation Theory and Coupled Cluster Theory for Electron Correlation in Molecules. Annu. Rev. Phys. Chem. 1981, 32, 359–401. [Google Scholar] [CrossRef]
- Geertsen, J.; Oddershede, J. Second-order polarization propagator calculations of indirect nuclear spin-spin coupling tensors in the water molecule. Chem. Phys. 1984, 90, 301–311. [Google Scholar] [CrossRef]
- Geertsen, J.; Oddershede, J. Higher RPA and second-order polarization propagator calculations of coupling constants in acetylene. Chem. Phys. 1986, 104, 67–72. [Google Scholar] [CrossRef]
- Kjær, H.; Sauer, S.P.A.; Kongsted, J. Benchmarking NMR indirect nuclear spin-spin coupling constants: SOPPA, SOPPA(CC2), and SOPPA(CCSD) versus CCSD. J. Chem. Phys. 2010, 133, 144106. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.P.A. Second-order polarization propagator approximation with coupled-cluster singles and doubles amplitudes—SOPPA(CCSD): The polarizability and hyperpolarizability of Li−. J. Phys. B At. Mol. Opt. Phys. 1997, 30, 3773–3780. [Google Scholar] [CrossRef]
- Enevoldsen, T.; Oddershede, J.; Sauer, S.P.A. Correlated calculations of indirect nuclear spin-spin coupling constants using second-order polarization propagator approximations: SOPPA and SOPPA(CCSD). Theor. Chem. Acc. 1998, 100, 275–284. [Google Scholar] [CrossRef]
- Ramsey, N.F. Electron Coupled Interactions between Nuclear Spins in Molecules. Phys. Rev. 1953, 91, 303–307. [Google Scholar] [CrossRef]
- Jensen, F. The basis set convergence of spin–spin coupling constants calculated by density functional methods. J. Chem. Theory Comput. 2006, 2, 1360–1369. [Google Scholar] [CrossRef]
- Helgaker, T.; Jaszuński, M.; Ruud, K.; Górska, A. Basis-set dependence of nuclear spin-spin coupling constants. Theor. Chem. Acc. 1998, 99, 175–182. [Google Scholar] [CrossRef]
- Provasi, P.F.; Aucar, G.A.; Sauer, S.P.A. The effect of lone pairs and electronegativity on the indirect nuclear spin–spin coupling constants in CH2X (X=CH2, NH, O, S): Ab initio calculations using optimized contracted basis sets. J. Chem. Phys. 2001, 115, 1324–1334. [Google Scholar] [CrossRef]
- Provasi, P.F.; Sauer, S.P.A. Optimized basis sets for the calculation of indirect nuclear spin-spin coupling constants involving the atoms B, Al, Si, P, and Cl. J. Chem. Phys. 2010, 133, 054308. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Sauer, S.P.A.; Levanova, E.P.; Levkovskaya, G.G. Structural trends of 77Se-1H spin-spin coupling constants and conformational behavior of 2-substituted selenophenes. Magn. Reson. Chem. 2010, 48, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, C.; Sauer, S.P.A. The aug-cc-pVTZ-J basis set for the p-block fourth-row elements Ga, Ge, As, Se, and Br. Magn. Reson. Chem. 2021, 59, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Provasi, P.F.; Peralta, J.E.; Snyder, J.P.; Sauer, S.P.A.; Contreras, R.H. Substituent effects on scalar 2J(19F,19F) and 3J(19F,19F) NMR couplings: A comparison of SOPPA and DFT methods. J. Phys. Chem. A 2003, 107, 4748–4754. [Google Scholar] [CrossRef]
- Sauer, S.P.A.; Raynes, W.T. Unexpected differential sensitivity of nuclear spin-spin-coupling constants to bond stretching in BH4−, NH4+, and SiH4. J. Chem. Phys. 2000, 113, 3121–3129. [Google Scholar] [CrossRef]
- Sauer, S.P.A.; Raynes, W.T.; Nicholls, R.A. Nuclear spin-spin coupling in silane and its isotopomers: Ab initio calculation and experimental investigation. J. Chem. Phys. 2001, 115, 5994–6006. [Google Scholar] [CrossRef]
- Hedegård, E.D.; Kongsted, J.; Sauer, S.P.A. Optimized basis sets for calculation of electron paramagnetic resonance hyperfine coupling constants: Aug-cc-pVTZ-J for the 3d atoms Sc-Zn. J. Chem. Theory Comput. 2011, 7, 4077–4087. [Google Scholar] [CrossRef]
- Kjær, H.; Sauer, S.P.A. Pople Style Basis Sets for the Calculation of NMR Spin–Spin Coupling Constants: The 6-31G-J and 6-311G-J Basis Sets. J. Chem. Theory Comput. 2011, 7, 4070–4076. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. Hierarchical Basis Sets for the Calculation of Nuclear Magnetic Resonance Spin–Spin Coupling Constants Involving Either Selenium or Tellurium Nuclei. J. Phys. Chem. A 2019, 123, 6564–6571. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. Efficient J-oriented tin basis sets for the correlated calculations of indirect nuclear spin–spin coupling constants. Magn. Reson. Chem. 2021, 59, 713–722. [Google Scholar] [CrossRef]
- Jensen, F. The optimum contraction of basis sets for calculating spin–spin coupling constants. Theor. Chem. Acc. 2010, 126, 371–382. [Google Scholar] [CrossRef]
- Aggelund, P.A.; Sauer, S.P.A.; Jensen, F. Development of polarization consistent basis sets for spin-spin coupling constant calculations for the atoms Li, Be, Na, and Mg. J. Chem. Phys. 2018, 149, 044117. [Google Scholar] [CrossRef] [PubMed]
- Benedikt, U.; Auer, A.A.; Jensen, F. Optimization of augmentation functions for correlated calculations of spin-spin coupling constants and related properties. J. Chem. Phys. 2008, 129, 064111. [Google Scholar] [CrossRef] [PubMed]
- Rusakov, Y.Y.; Rusakova, I.L. An efficient method for generating property-energy consistent basis sets. New pecJ-n (n = 1, 2) basis sets for high-quality calculations of indirect nuclear spin–spin coupling constants involving 1H, 13C, 15N, and 19F nuclei. Phys. Chem. Chem. Phys. 2021, 23, 14925–14939. [Google Scholar] [CrossRef] [PubMed]
- Rusakov, Y.Y.; Rusakova, I.L. New pecJ-n (n = 1, 2) Basis Sets for High-Quality Calculations of Indirect Nuclear Spin–Spin Coupling Constants Involving 31P and 29Si: The Advanced PEC Method. Molecules 2022, 27, 6145. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. New pecS-n (n = 1, 2) basis sets for quantum chemical calculations of the NMR chemical shifts of H, C, N and O nuclei. J. Chem. Phys. 2022, 156, 244112. [Google Scholar] [CrossRef]
- Metropolis, N.; Ulam, S. The Monte Carlo Method. J. Am. Stat. Assoc. 1949, 44, 335–341. [Google Scholar] [CrossRef]
- Harrison, R.L. Introduction to Monte Carlo Simulation. AIP Conf. Proc. 2010, 1204, 17–21. [Google Scholar] [CrossRef]
- Del Moral, P.; Doucet, A.; Jasra, A. Sequential Monte Carlo samplers. J. R. Statist. Soc. B 2006, 68, 411–436. [Google Scholar] [CrossRef]
- Reeves, C.M.; Fletcher, R. Use of Gaussian functions in the calculation of wavefunctions for small molecules. III. The orbital basis and its effect on valence. J. Chem. Phys. 1965, 42, 4073–4081. [Google Scholar] [CrossRef]
- Wilson, A.K.; Woon, D.E.; Peterson, K.A.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. IX. The atoms gallium through krypton. J. Chem. Phys. 1999, 110, 7667–7676. [Google Scholar] [CrossRef]
- Dyall, K.G. Relativistic and nonrelativistic finite nucleus optimized triple-zeta basis sets for the 4p, 5p and 6p elements. Theor. Chem. Acc. 2002, 108, 335–340. [Google Scholar] [CrossRef]
- Dyall, K.G. Relativistic quadruple-zeta and revised triple-zeta and double-zeta basis sets for the 4p, 5p and 6p elements. Theor. Chem. Acc. 2006, 115, 441–447. [Google Scholar] [CrossRef]
- Dyall, K.G. Relativistic and nonrelativistic finite nucleus optimized double zeta basis sets for the 4p, 5p and 6p elements. Theor. Chem. Acc. 1998, 99, 366–371. [Google Scholar] [CrossRef]
- Verbeke, J.; Cools, R. The Newton-Raphson method. Int. J. Math. Educ. Sci. Technol. 1995, 26, 177–193. [Google Scholar] [CrossRef]
- Gil, V.M.S.; von Philipsborn, W. Effect of Electron Lone-Pairs on Nuclear Spin-Spin Coupling Constants. Magn. Reson. Chem. 1989, 27, 409–430. [Google Scholar] [CrossRef]
- Wu, A.; Gräfenstein, J.; Cremer, D. Analysis of the Transmission Mechanism of NMR Spin-Spin Coupling Constants Using Fermi Contact Spin Density Distribution, Partial Spin Polarization, and Orbital Currents: XHn Molecules. J. Phys. Chem. A 2003, 107, 7043–7056. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Papernaya, L.K.; Shatrova, A.A. Stereochemical behavior of 77Se-1 H spin-spin coupling constants in pyrazolyl-1,3-diselenanes and 1,2-diselenolane. Magn. Reson. Chem. 2012, 50, 169–173. [Google Scholar] [CrossRef]
- Aidas, K.; Angeli, C.; Bak, K.L.; Bakken, V.; Bast, R.; Boman, L.; Christiansen, O.; Cimiraglia, R.; Coriani, S.; Dahle, P.; et al. The Dalton quantum chemistry program system. WIREs Comput. Mol. Sci. 2014, 4, 269–284. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Cheng, M.E.; Harding, D.A.; Matthews, P.G.; Asthana, A.; Auer, A.A.; Bartlett, R.J.; Benedikt, U.; Berger, C.; et al. CFOUR, A Quantum Chemical Program Package. Available online: http://www.cfour.de (accessed on 27 March 2023).
- Raffenetti, R.C. General contraction of Gaussian atomic orbitals: Core, valence, polarization, and diffuse basis sets; molecular integral evaluation. J. Chem. Phys. 1973, 58, 4452–4458. [Google Scholar] [CrossRef]
- Slater, J.C.; Phillips, J.C. Quantum Theory of Molecules and Solids: The Self-Consistent Field for Molecules and Solids, 1st ed.; McGraw-Hill: New York, NY, USA, 1974; Volume 4, pp. 1–583. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 59, 1200–1211. [Google Scholar] [CrossRef]
- Dirac, P.A.M. Note on exchange phenomena in the Thomas atom. Proc. Camb. Philos. Soc. 1930, 26, 376–385. [Google Scholar] [CrossRef]
- Slater, J.C. A simplification of the Hartree-Fock method. Phys. Rev. 1951, 81, 385–390. [Google Scholar] [CrossRef]
- Lutnæs, O.B.; Helgaker, T.; Jaszuński, M. Spin-spin coupling constants and triplet instabilities in Kohn-Sham theory. Mol. Phys. 2010, 108, 2579–2590. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. THEOCHEM 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Ruden, T.A.; Lutnæs, O.B.; Helgaker, T. Vibrational corrections to indirect nuclear spin–spin coupling constants calculated by density-functional theory. J. Chem. Phys. 2003, 118, 9572–9581. [Google Scholar] [CrossRef]
- Ruud, K.; Åstrand, P.-O.; Taylor, P.R. Zero-point vibrational effects on proton shieldings: Functional-group contributions from ab initio calculations. J. Am. Chem. Soc. 2001, 123, 4826–4833. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. Relativistic heavy atom effect on 13C NMR chemical shifts initiated by adjacent multiple chalcogens. Magn. Reson. Chem. 2018, 56, 716–726. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L.; Krivdin, L.B. Relativistic heavy atom effect on the 31P NMR parameters of phosphine chalcogenides. Part 1. Chemical shifts. Magn. Reson. Chem. 2018, 56, 1061–1073. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y. On the heavy atom on light atom relativistic effect in the NMR shielding constants of phosphine tellurides. Magn. Reson. Chem. 2019, 57, 1071–1083. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. What Most Affects the Accuracy of 125Te NMR Chemical Shift Calculations. J. Phys. Chem. A 2020, 124, 6714–6725. [Google Scholar] [CrossRef] [PubMed]
- Rusakova, I.L.; Rusakov, Y.Y. On the Utmost Importance of the Basis Set Choice for the Calculations of the Relativistic Corrections to NMR Shielding Constants. Int. J. Mol. Sci. 2023, 24, 6231. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.F.; Aucar, G.A. The UKB prescription and the heavy atom effects on the nuclear magnetic shielding of vicinal heavy atoms. Phys. Chem. Chem. Phys. 2009, 11, 5615–5627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, W.; Kutzelnigg, W. Comparison of restricted, unrestricted, inverse, and dual kinetic balances for four-component relativistic calculations. Theor. Chem. Acc. 2011, 129, 423–436. [Google Scholar] [CrossRef]
- Anderson, J.A.; Odom, J.D. Carbon-13 Chemical Shifts and 77Se-13C Spin-Spin Coupling Constants in Symmetrical Dialkyl Diselenides, Unsymmetrical Alkyl Methyl and Alkyl Phenyl Diselenides, and Related Alkyl Selenols. Organometallics 1988, 7, 267–271. [Google Scholar] [CrossRef]
- Poleschner, H.; Radeglia, R.; Kuprat, M.; Richter, A.M.; Fanghänel, E. Organylselenoacetylene und selenocyanate; 77Se- und 13C-NMR-chemische verschiebungen und 77Se-13C-spin-kopplungskonstanten. J. Organomet. Chem. 1987, 327, 7–15. [Google Scholar] [CrossRef]
- Schröder, H.F.; Müller, J. Dialkylphosphinsaureazide II. Z. Für Anorg. Allg. Chem. 1979, 451, 158–174. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Liu, H. A Nuclear Magnetic Resonance Study of the Binding of Trimethylphosphine Selenide to Cadmium Oleate. J. Phys. Chem. A 2014, 118, 7314–7319. [Google Scholar] [CrossRef]
- Glidewell, C.; Rankin, D.W.H.; Sheldrick, G.M. N.M.R. Studies of Mixed Group IV/Group VI Hydrides. Trans. Faraday Soc. 1969, 65, 1409–1412. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Jensen, F. Polarization consistent basis sets: Principles. J. Chem. Phys. 2001, 115, 9113–9125. [Google Scholar] [CrossRef]
- Jensen, F. Polarization consistent basis sets. II. Estimating the Kohn-Sham basis set limit. J. Chem. Phys. 2002, 116, 7372–7379. [Google Scholar] [CrossRef]
- Jensen, F.; Helgaker, T. Polarization consistent basis sets. V. The elements Si-Cl. J. Chem. Phys. 2004, 121, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F. Polarization consistent basis sets. VII. The elements K, Ca, Ga, Ge, As, Se, Br, and Kr. J. Chem. Phys. 2012, 136, 114107. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Gomes, A.S.P.; Saue, T.; Visscher, L.; Jensen, H.J.A.; Bast, R.; Aucar, A.; Bakken, V.; Dyall, K.G.; Dubillard, S.; Ekström, U.; et al. DIRAC, a Relativistic Ab Initio Electronic Structure Program, Release DIRAC19. 2019. Available online: http://www.diracprogram.org (accessed on 29 March 2023).
- Irkutsk Supercomputer Center of SB RAS. Irkutsk: ISDCT SB RAS. Available online: https://hpc.icc.ru (accessed on 29 March 2023).
| Starting Configuration | Total Modification | Resulting Configuration |
|---|---|---|
| cc-pVDZ+2f: (14s11p6d2f) | +2s, +0p, +1d, +1f | pecJ-1: (16s11p7d3f) |
| cc-pVTZ+2f: (20s13p9d2f) | +2s, +0p, –1d, +2f, +1g | pecJ-2: (22s13p8d4f1g) |
| Step | Basis Set | Contraction Pattern | Detailed Contraction Pattern | Final Contraction Error Δ (in Relation to Current Reference Values), in Hz |
|---|---|---|---|---|
| Step 1 (s-shell) | pecJ-1 | 10s | (8,8,1,1,1,1,1,1,1,1) | 0.133 |
| 16s11s | (7,7,1,1,1,1,1,1,1,1,1) | 0.024 | ||
| 12s | (6,6,1,1,1,1,1,1,1,1,1,1) | 0.011 | ||
| pecJ-2 | 14s | (10,10,1,1,1,1,1,1,1,1,1,1,1,1) | 0.133 | |
| 22s15s | (9,9,1,1,1,1,1,1,1,1,1,1,1,1,1) | 0.091 | ||
| 16s | (8,8,1,1,1,1,1,1,1,1,1,1,1,1,1,1) | 0.077 | ||
| Step 2 (p-shell) | pecJ-1 | 6p | (7,7,1,1,1,1) | 1.080 |
| 11p7p | (6,6,1,1,1,1,1) | 0.095 | ||
| 8p | (5,5,1,1,1,1,1,1) | 0.076 | ||
| pecJ-2 | 7p | (8,8,1,1,1,1,1) | 0.998 | |
| 13p8p | (7,7,1,1,1,1,1,1) | 0.047 | ||
| 9p | (6,6,1,1,1,1,1,1,1) | 0.032 | ||
| Step 3 (d-shell) | pecJ-1 | 4d | (4,1,1,1) | 0.768 |
| 7d5d | (3,1,1,1,1) | 0.123 | ||
| 6d | (2,1,1,1,1,1) | 0.098 | ||
| pecJ-2 | 4d | (5,1,1,1) | 0.676 | |
| 8d5d | (4,1,1,1,1) | 0.127 | ||
| 6d | (3,1,1,1,1,1) | 0.124 |
| Final Configuration | Nbas/Nbas(uc) | ε1/2, in % 1 |
|---|---|---|
| pecJ-1: [16s11p7d3f|11s7p5d3f] | 78/105 | ε1 = 0.35; ε2 = 0.08 |
| pecJ-2: [22s13p8d4f1g|15s8p5d4f1g] | 101/138 | ε1 = 0.18; ε2 = 0.04 |
| Basis Set | N | N4 (DFT, RPA) | N5 (SOPPA, SOPPA (CC2)) | N6 (SOPPA(CCSD), CCSD) | N8 (CCSDT) |
|---|---|---|---|---|---|
| pecJ-1 | 78(Se) + 2 × 11(H) = 100 | 1.00 × 108, R = 0.16 | 1.00 × 1010, R = 0.10 | 1.00 × 1012, R = 0.07 | 1.00 × 1016, R = 0.03 |
| pecJ-2 | 101(Se) + 2 × 20(H) = 141 | 3.95 × 108, R = 0.65 | 5.57 × 1010, R = 0.58 | 7.86 × 1012, R = 0.52 | 1.56 × 1017, R = 0.42 |
| aug-cc-pVTZ-J | 117(Se) + 2 × 20(H) = 157 | 6.08 × 108 R = 1.00 | 9.54 × 1010 R = 1.00 | 1.50 × 1013 R = 1.00 | 3.69 × 1017, R = 1.00 |
| Molecule | SSCC | Basis Set | JFC | JSD | JPSO | JDSO | Total JCCSD |
|---|---|---|---|---|---|---|---|
| Me2Se2 (1) | 1J(77Se,13C) | pecJ-1 | −77.21 | 11.26 | 6.61 | 0.09 | −59.25 |
| pecJ-2 | −81.26 | 12.32 | 6.74 | 0.09 | −62.11 | ||
| 2J(77Se,13C) | pecJ-1 | 4.13 | 1.60 | 2.00 | −0.02 | 7.71 | |
| pecJ-2 | 3.34 | 1.57 | 1.77 | −0.02 | 6.66 | ||
| MeSe-C≡N (2) | 1J(77Se,13Csp3) | pecJ-1 | −57.23 | 12.17 | 9.32 | 0.05 | −35.69 |
| pecJ-2 | −60.95 | 12.53 | 9.25 | 0.05 | −39.12 | ||
| 1J(77Se,13Csp) | pecJ-1 | −173.73 | 1.96 | −6.90 | 0.10 | −178.57 | |
| pecJ-2 | −182.11 | 2.21 | −6.17 | 0.10 | −185.97 | ||
| MeSeH (3) | 1J(77Se,13C) | pecJ-1 | −60.72 | 12.25 | 11.77 | 0.04 | −36.66 |
| pecJ-2 | −64.61 | 12.68 | 12.00 | 0.04 | −39.89 | ||
| Me2P(Se)Cl (4) | 1J(77Se,31P) | pecJ-1 | −688.03 | −1.55 | −152.96 | 0.12 | −842.42 |
| pecJ-2 | −700.90 | −0.74 | −151.21 | 0.12 | −852.73 | ||
| C4H4Se (5) | 2J(77Se,1H) | pecJ-1 | 47.50 | −0.15 | −4.61 | −0.21 | 42.53 |
| pecJ-2 | 48.97 | −0.16 | −4.92 | −0.21 | 43.68 | ||
| 3J(77Se,1H) | pecJ-1 | 8.59 | 0.26 | 0.56 | −0.36 | 9.05 | |
| pecJ-2 | 8.74 | 0.45 | 0.38 | −0.36 | 9.21 | ||
| 1J(77Se,13C) | pecJ-1 | −59.91 | 2.31 | −25.79 | 0.09 | −83.3 | |
| pecJ-2 | −65.34 | 2.16 | −26.53 | 0.09 | −89.62 | ||
| 2J(77Se,13C) | pecJ-1 | −1.83 | 2.92 | −1.20 | −0.02 | −0.13 | |
| pecJ-2 | −2.31 | 3.7 | −1.51 | −0.02 | −0.14 | ||
| SePMe3 (6) | 1J(77Se,31P) | pecJ-1 | −619.15 | 4.24 | −108.92 | 0.1 | −723.73 |
| pecJ-2 | −621.69 | 6.15 | −104.12 | 0.1 | −719.56 | ||
| SiH3SeH (7) | 1J(77Se,1H) | pecJ-1 | 47.12 | −0.48 | 23.12 | 0.12 | 69.88 |
| pecJ-2 | 48.41 | −0.04 | 22.5 | 0.11 | 70.98 | ||
| 2J(77Se,1H) | pecJ-1 | 15.81 | −0.25 | 0.13 | −0.18 | 15.51 | |
| pecJ-2 | 15.91 | −0.25 | 0.13 | −0.18 | 15.61 |
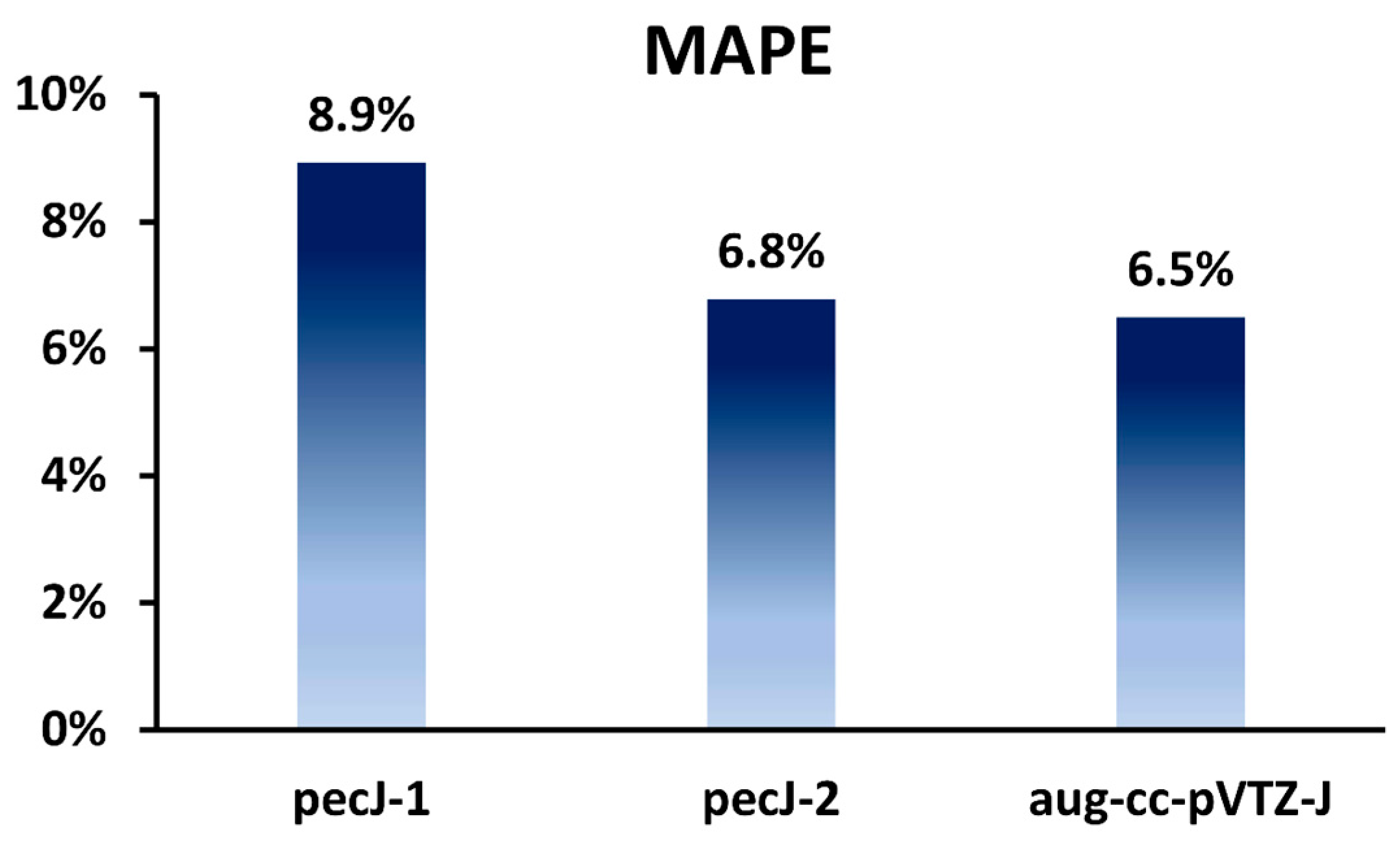
| Molecule | SSCC | Basis Set | JCCSD | Δrel 3 | Δsolv 3 | Δvib 3 | Jtot | Jexp |
|---|---|---|---|---|---|---|---|---|
| Me2Se2 (1) | 1J(77Se,13C) | pecJ-1 | −59.25 | −7.02 | 1.51 | −0.9 | −65.66 | (−)73.87 ± 0.26 |
| pecJ-2 | −62.11 | −5.48 | 1.56 | −0.9 | −66.93 | |||
| aug-cc-pVTZ-J | −63.56 | −4.51 | 1.54 | −0.9 | −67.43 | |||
| 2J(77Se,13C) | pecJ-1 | 7.71 | −0.29 | 0.24 | −0.11 | 7.55 | 7.47 ± 0.26 | |
| pecJ-2 | 6.66 | 0.36 | 0.29 | −0.11 | 7.20 | |||
| aug-cc-pVTZ-J | 6.55 | −0.64 | 0.28 | −0.11 | 6.08 | |||
| MeSe-C≡N (2) | 1J(77Se,13Csp3) | pecJ-1 | −35.69 | −5.76 | 0.24 | −1.00 | −42.21 | (−)52.3 |
| pecJ-2 | −39.12 | −6.46 | 0.24 | −1.00 | −46.34 | |||
| aug-cc-pVTZ-J | −40.74 | −5.49 | 0.24 | −1.00 | −46.99 | |||
| 1J(77Se,13Csp) | pecJ-1 | −178.57 | −37.39 | −5.84 | −3.22 | −225.02 | (−)237.8 | |
| pecJ-2 | −185.97 | −37.45 | −5.05 | −3.22 | −231.69 | |||
| aug-cc-pVTZ-J | −188.71 | −34.50 | −5.28 | −3.22 | −231.71 | |||
| MeSeH (3) | 1J(77Se,13C) | pecJ-1 | −36.66 | −5.69 | 2.30 | −1.90 | −41.95 | (−)48.26 ± 0.26 |
| pecJ-2 | −39.89 | −6.31 | 2.39 | −1.90 | −45.71 | |||
| aug-cc-pVTZ-J | −41.26 | −5.19 | 2.32 | −1.90 | −46.03 | |||
| Me2P(Se)Cl (4) | 1J(77Se,31P) | pecJ-1 | −842.42 | −49.43 | 11.82 | −4.08 | −884.11 | (−)838.4 |
| pecJ-2 | −852.73 | −27.01 | 14.27 | −4.08 | −869.55 | |||
| aug-cc-pVTZ-J | −861.31 | −28.44 | 14.12 | −4.08 | −879.71 | |||
| C4H4Se (5) | 2J(77Se,1H) | pecJ-1 | 42.53 | 3.73 | 0.10 | −0.35 | 46.01 | 47.6 |
| pecJ-2 | 43.68 | 3.97 | 0.21 | −0.35 | 47.51 | |||
| aug-cc-pVTZ-J | 44.46 | 3.90 | 0.19 | −0.35 | 48.20 | |||
| 3J(77Se,1H) | pecJ-1 | 9.05 | −0.32 | 0.23 | −0.32 | 8.64 | 9.4 | |
| pecJ-2 | 9.21 | −0.32 | 0.33 | −0.32 | 8.90 | |||
| aug-cc-pVTZ-J | 9.53 | −0.37 | 0.32 | −0.32 | 9.16 | |||
| 1J(77Se,13C) | pecJ-1 | −83.30 | −16.51 | 0.27 | −2.58 | −102.12 | (−)113.5 | |
| pecJ-2 | −89.62 | −17.61 | 0.62 | −2.58 | −109.19 | |||
| aug-cc-pVTZ-J | −89.18 | −17.95 | 0.60 | −2.58 | −109.11 | |||
| 2J(77Se,13C) | pecJ-1 | −0.13 | −1.50 | −0.37 | 0.19 | −1.81 | (−)2.7 | |
| pecJ-2 | −0.14 | −1.59 | −0.37 | 0.19 | −1.91 | |||
| aug-cc-pVTZ-J | −1.21 | −1.65 | −0.38 | 0.19 | −3.05 | |||
| SePMe3 (6) | 1J(77Se,31P) | pecJ-1 | −723.73 | −56.56 | 25.26 | −7.45 | −762.48 | (−)720.0 |
| pecJ-2 | −719.56 | −35.36 | 27.87 | −7.45 | −734.50 | |||
| aug-cc-pVTZ-J | −725.09 | −44.01 | 27.49 | −7.45 | −749.06 | |||
| SiH3SeH (7) | 1J(77Se,1H) | pecJ-1 | 69.88 | −29.49 | 5.95 | 4.59 | 50.93 | 51.0 ± 0.1 |
| pecJ-2 | 70.98 | −26.21 | 6.96 | 4.59 | 56.32 | |||
| aug-cc-pVTZ-J | 70.83 | −26.72 | 6.52 | 4.59 | 55.22 | |||
| 2J(77Se,1H) | pecJ-1 | 15.51 | 0.10 | −0.09 | −0.16 | 15.36 | 15.4 ± 0.2 | |
| pecJ-2 | 15.61 | −0.24 | 0.01 | −0.16 | 15.22 | |||
| aug-cc-pVTZ-J | 15.59 | −0.28 | −0.04 | −0.16 | 15.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusakov, Y.Y.; Rusakova, I.L. New pecJ-n (n = 1, 2) Basis Sets for Selenium Atom Purposed for the Calculations of NMR Spin–Spin Coupling Constants Involving Selenium. Int. J. Mol. Sci. 2023, 24, 7841. https://doi.org/10.3390/ijms24097841
Rusakov YY, Rusakova IL. New pecJ-n (n = 1, 2) Basis Sets for Selenium Atom Purposed for the Calculations of NMR Spin–Spin Coupling Constants Involving Selenium. International Journal of Molecular Sciences. 2023; 24(9):7841. https://doi.org/10.3390/ijms24097841
Chicago/Turabian StyleRusakov, Yuriy Yu., and Irina L. Rusakova. 2023. "New pecJ-n (n = 1, 2) Basis Sets for Selenium Atom Purposed for the Calculations of NMR Spin–Spin Coupling Constants Involving Selenium" International Journal of Molecular Sciences 24, no. 9: 7841. https://doi.org/10.3390/ijms24097841