Revealing the Second and the Third Causes of AgNPs Property to Restore the Bacterial Susceptibility to Antibiotics
Abstract
:1. Introduction
2. Results
2.1. Number of Isolates with Different Bacteria (In Vivo)
2.2. Anti-Adhesion Activity
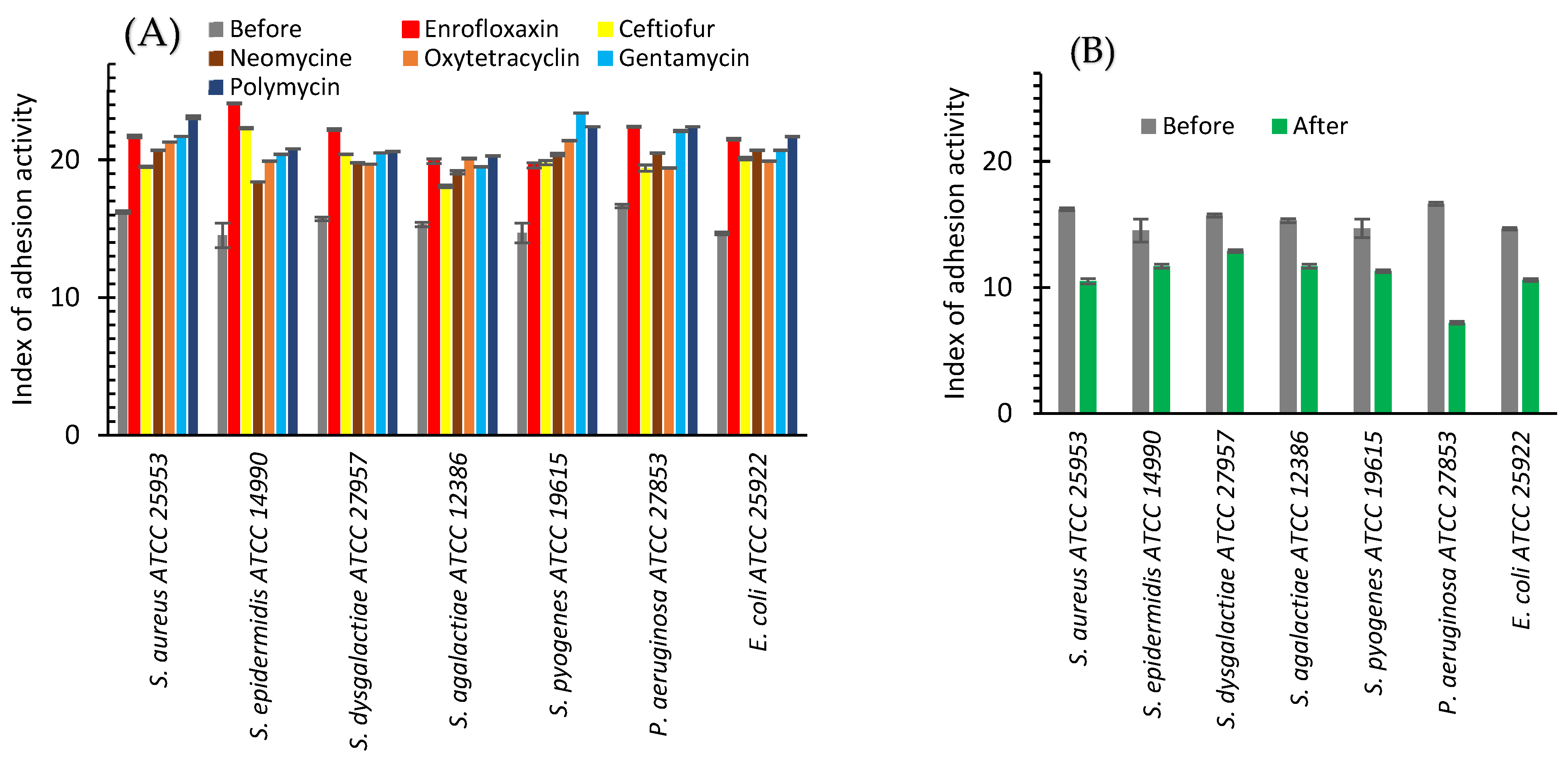
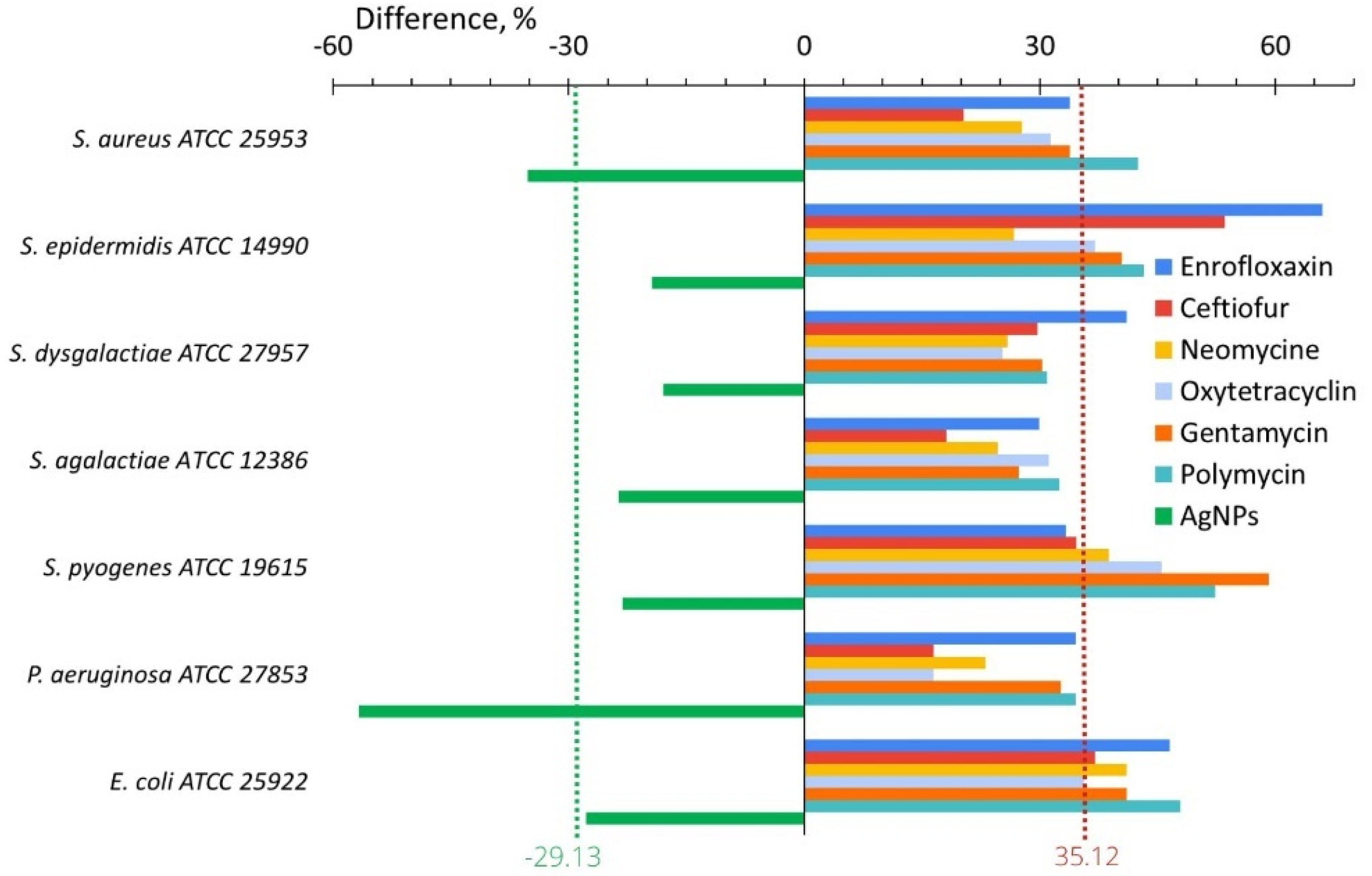
2.2.1. In Vitro
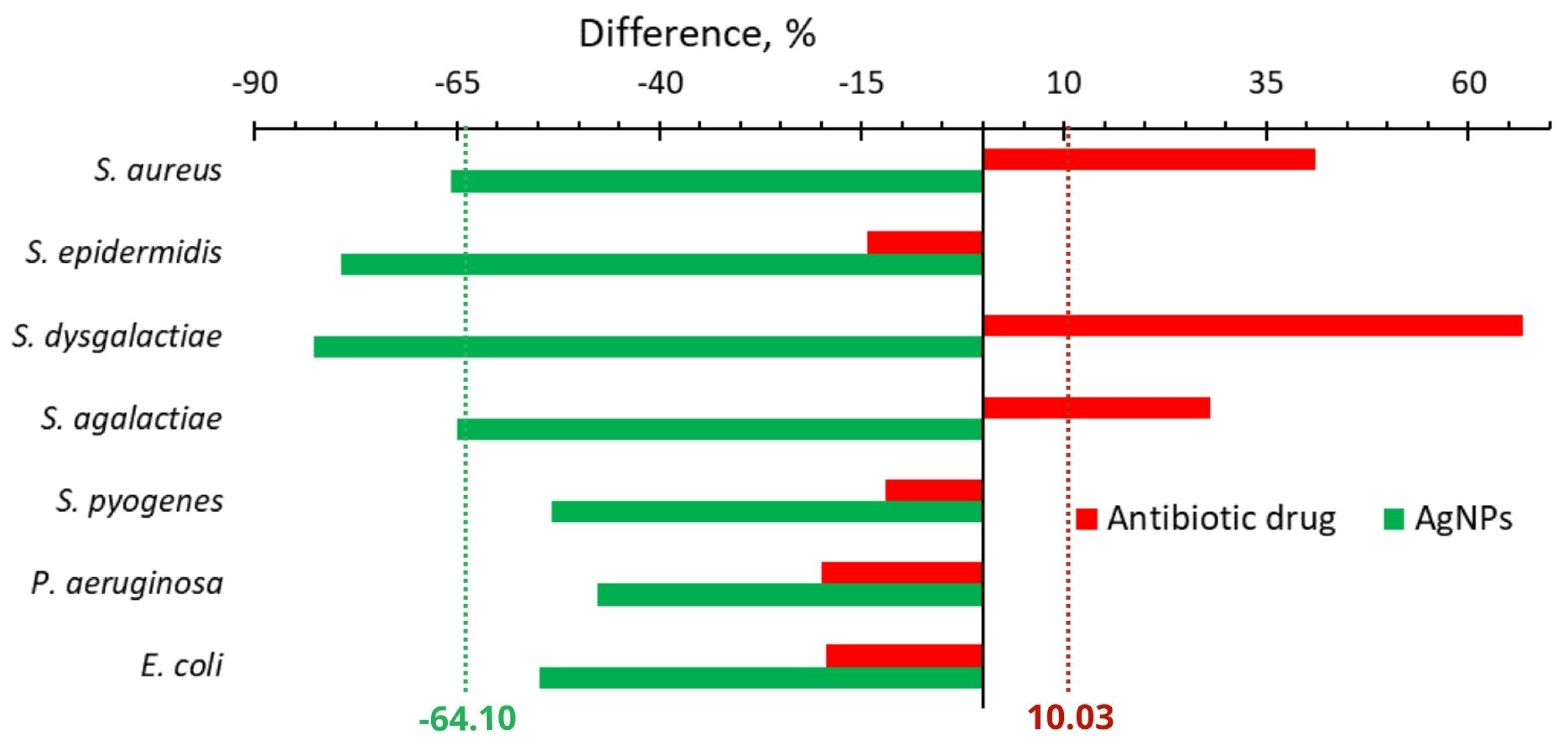
2.2.2. In Vivo
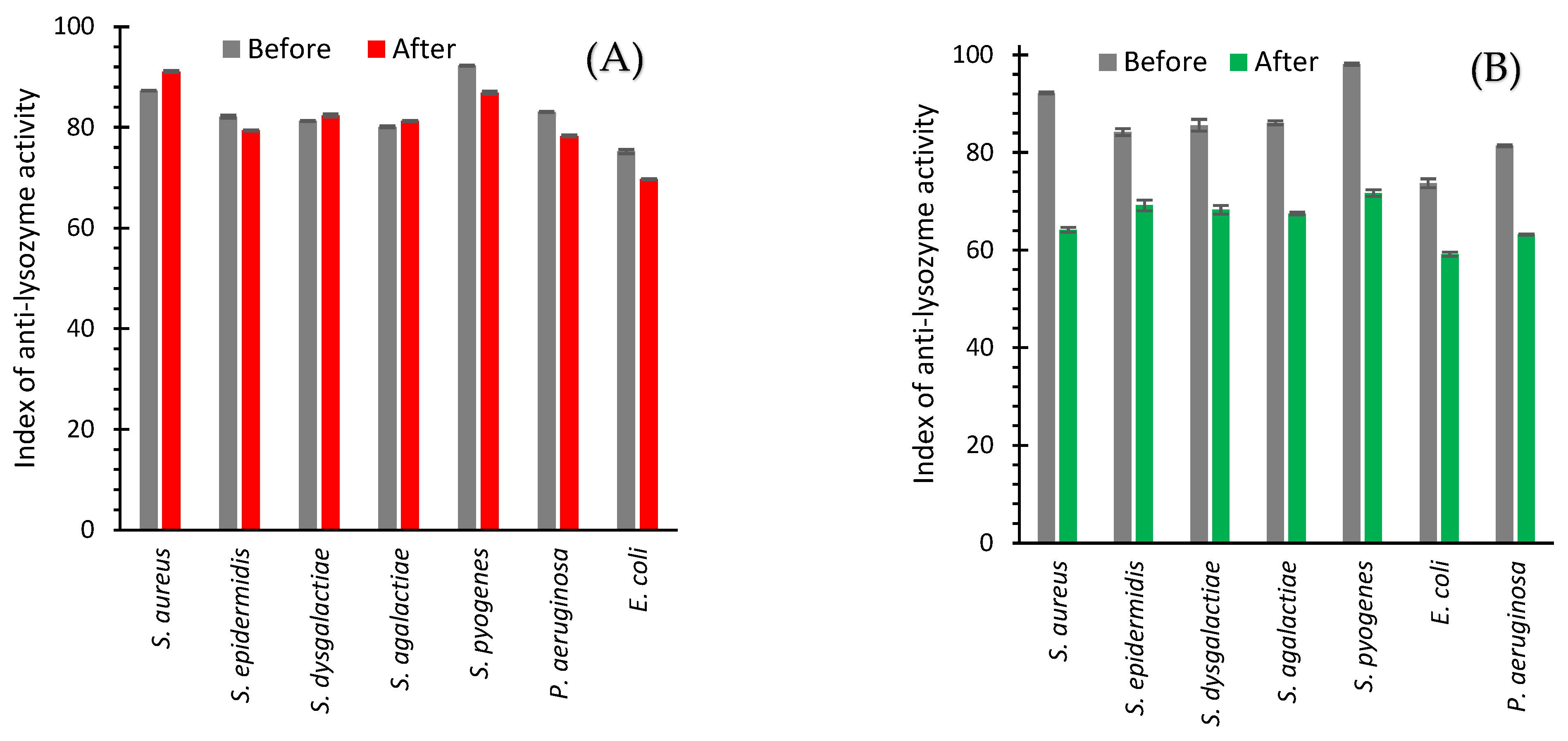
2.3. Anti-Lysozyme Activity
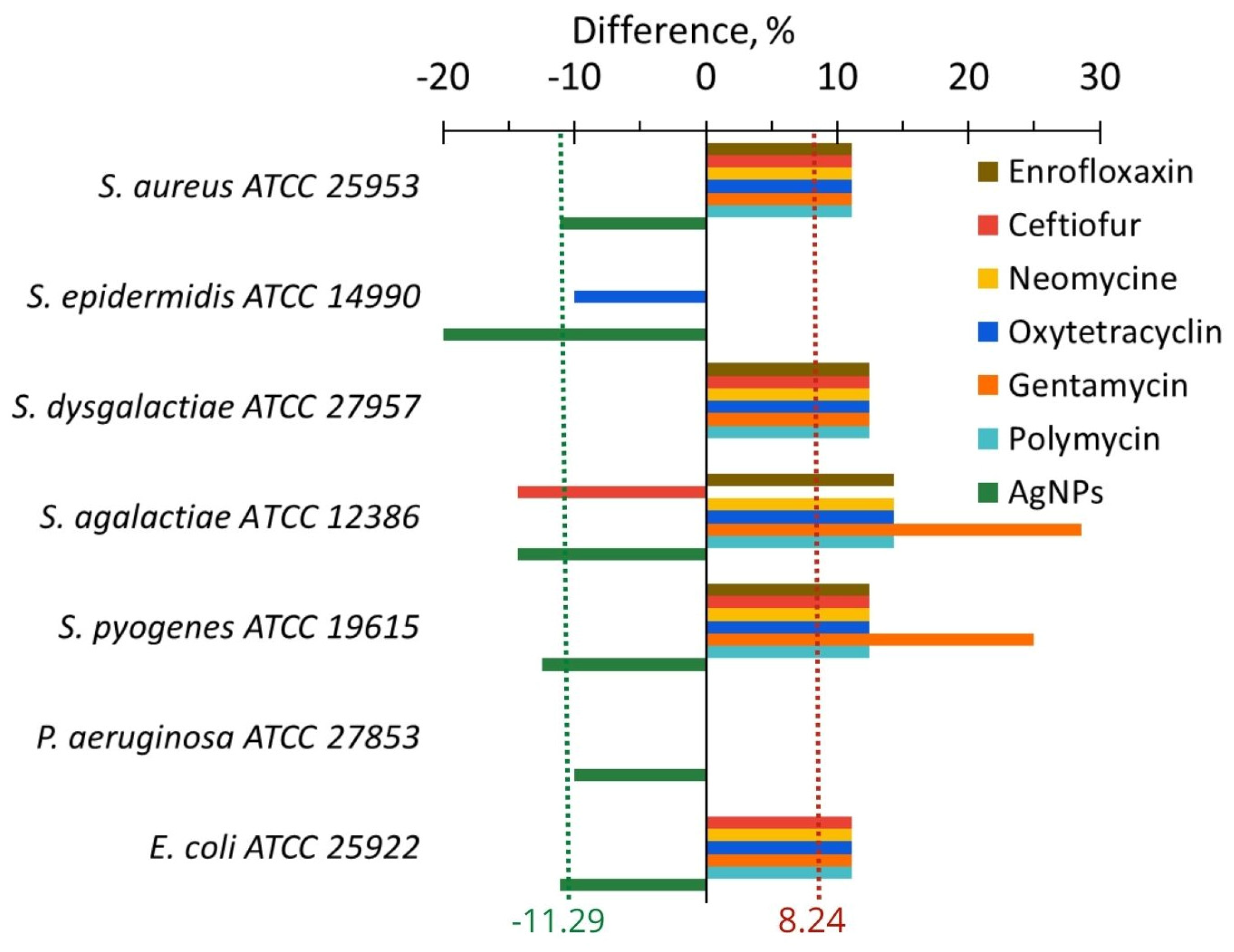
2.3.1. In Vitro
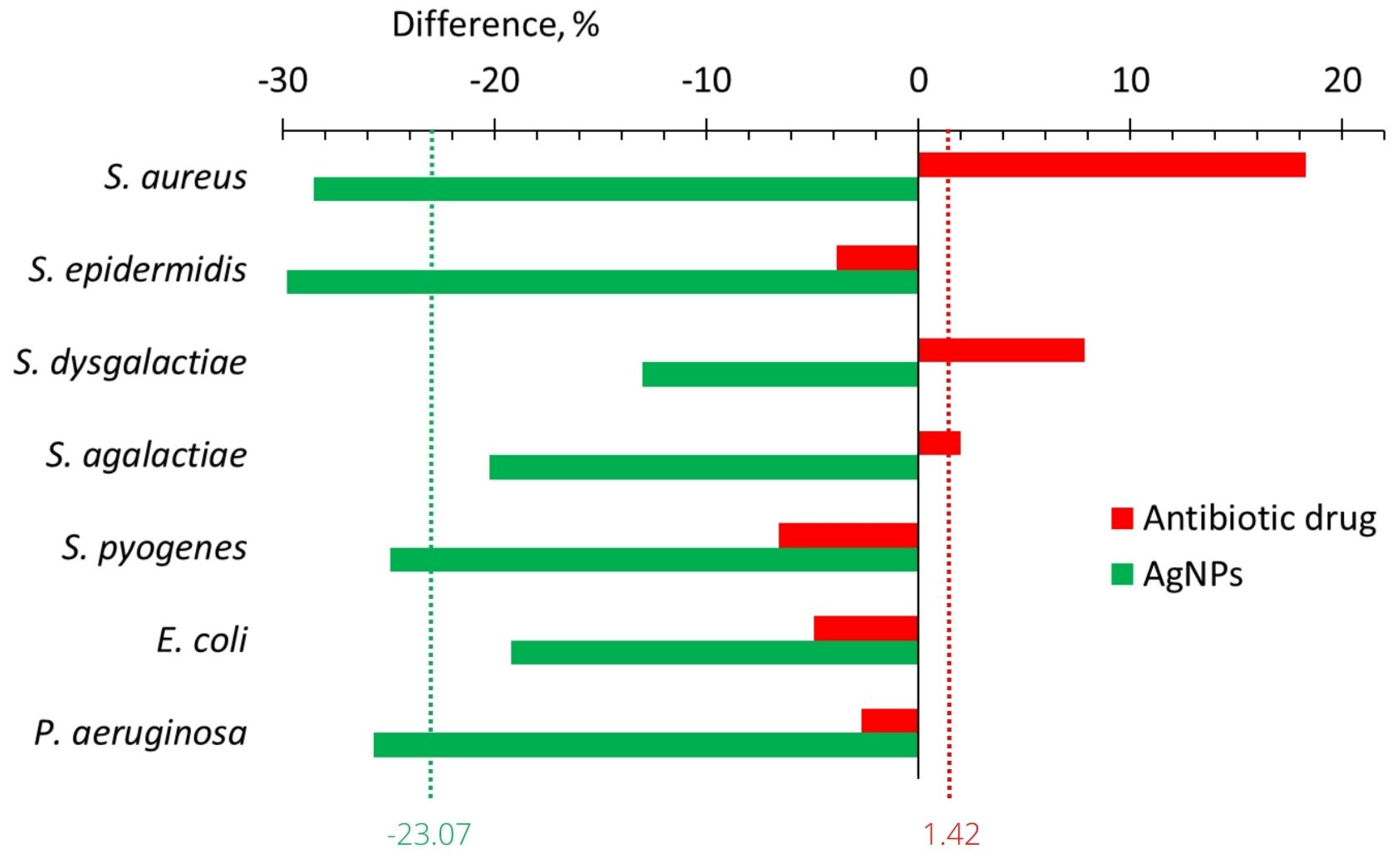
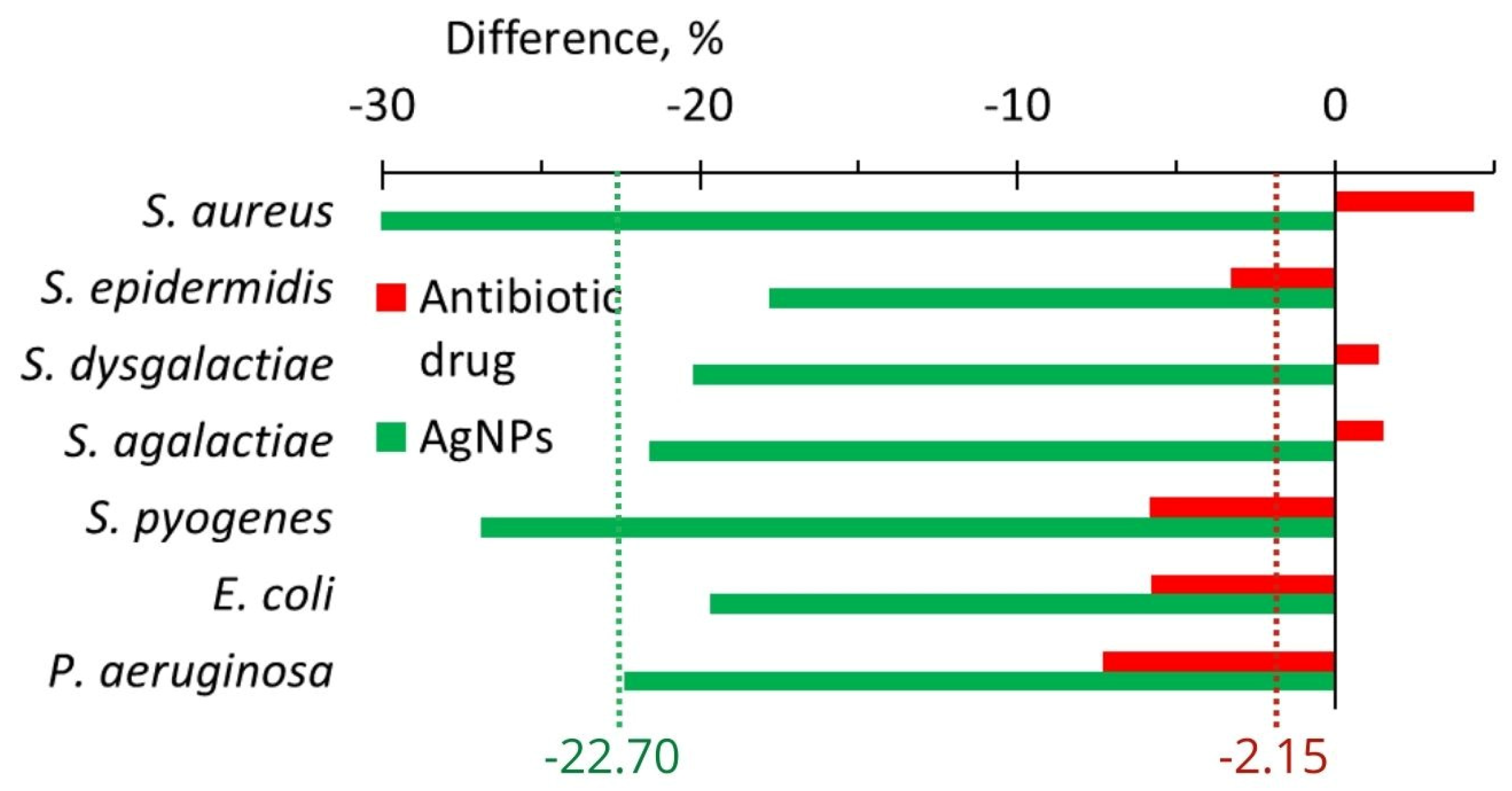
2.3.2. In Vivo
3. Discussion
3.1. Isolated Bacteria Strains
3.2. Adhesion Activity
3.3. Anti-Lysozyme Activity
3.4. Problem of Bacteria Resistance to Antibiotics
4. Materials and Methods
4.1. Experimental Design
4.1.1. In Vivo
4.1.2. In Vitro
4.2. Sampling
4.3. Treatment Formulations
4.3.1. In Vitro
4.3.2. In Vivo
4.4. Isolation and Identification of Bacterial Isolates
4.4.1. In Vivo
4.4.2. In Vitro
4.5. Adhesive Activity
4.6. Anti-Lysozyme Activity
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Lee, S.; Jeong, K.C. Mitigating Antibiotic Resistance at the Livestock-Environment Interface: A Review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the Use of Antibiotics in Food-Producing Animals and Its Associations with Antibiotic Resistance in Food-Producing Animals and Human Beings: A Systematic Review and Meta-Analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance, 1st ed.; World Health Organization: Geneva, Switzerland, 2016; Volume 1.
- Mordmuang, A.; Shankar, S.; Chethanond, U.; Voravuthikunchai, S. Effects of Rhodomyrtus Tomentosa Leaf Extract on Staphylococcal Adhesion and Invasion in Bovine Udder Epidermal Tissue Model. Nutrients 2015, 7, 8503–8517. [Google Scholar] [CrossRef] [PubMed]
- Garibo Ruiz, D.; Nefedova, E.; Shkil, N.N.; Shkil, N.A.; Vazquez-Gomez, R.L.; Pestryakov, A.; Bogdanchikova, N. Silver Nanoparticles Targeting the Drug Resistance Problem of Streptococcus Dysgalactiae: Susceptibility to Antibiotics and Efflux Effect. Int. J. Mol. Sci. 2022, 23, 6024. [Google Scholar] [CrossRef]
- Nefedova, E.; Shkil, N.; Luna Vazquez-Gomez, R.; Garibo, D.; Pestryakov, A.; Bogdanchikova, N. AgNPs Targeting the Drug Resistance Problem of Staphylococcus aureus: Susceptibility to Antibiotics and Efflux Effect. Pharmaceutics 2022, 14, 763. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Chagnot, C.; Zorgani, M.A.; Astruc, T.; Desvaux, M. Proteinaceous Determinants of Surface Colonization in Bacteria: Bacterial Adhesion and Biofilm Formation from a Protein Secretion Perspective. Front. Microbiol. 2013, 4, 303. [Google Scholar] [CrossRef]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A Review on Anti-Adhesion Therapies of Bacterial Diseases. Infection 2019, 47, 13–23. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From Bacterial Killing to Immune Modulation: Recent Insights into the Functions of Lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Gomes, F.; Saavedra, M.J.; Henriques, M. Bovine Mastitis Disease/Pathogenicity: Evidence of the Potential Role of Microbial Biofilms. Pathog. Dis. 2016, 74, ftw006. [Google Scholar] [CrossRef]
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The Role of Streptococcus Spp. in Bovine Mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef]
- Gao, X.; Guo, M.; Zhang, Z.; Shen, P.; Yang, Z.; Zhang, N. Baicalin Promotes the Bacteriostatic Activity of Lysozyme on S. Aureus in Mammary Glands and Neutrophilic Granulocytes in Mice. Oncotarget 2017, 8, 19894–19901. [Google Scholar] [CrossRef]
- Yu, L.; Shang, F.; Chen, X.; Ni, J.; Yu, L.; Zhang, M.; Sun, D.; Xue, T. The Anti-Biofilm Effect of Silver-Nanoparticle-Decorated Quercetin Nanoparticles on a Multi-Drug Resistant Escherichia coli Strain Isolated from a Dairy Cow with Mastitis. PeerJ 2018, 6, e5711. [Google Scholar] [CrossRef]
- Alawneh, J.I.; Vezina, B.; Ramay, H.R.; Al-Harbi, H.; James, A.S.; Soust, M.; Moore, R.J.; Olchowy, T.W.J. Survey and Sequence Characterization of Bovine Mastitis-Associated Escherichia coli in Dairy Herds. Front. Vet. Sci. 2020, 7, 582297. [Google Scholar] [CrossRef]
- Ren, Q.; Liao, G.; Wu, Z.; Lv, J.; Chen, W. Prevalence and Characterization of Staphylococcus aureus Isolates from Subclinical Bovine Mastitis in Southern Xinjiang, China. J. Dairy Sci. 2020, 103, 3368–3380. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in Therapeutic and Managemental Approaches of Bovine Mastitis: A Comprehensive Review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Reding-Roman, C.; Hewlett, M.; Duxbury, S.; Gori, F.; Gudelj, I.; Beardmore, R. The Unconstrained Evolution of Fast and Efficient Antibiotic-Resistant Bacterial Genomes. Nat. Ecol. Evol. 2017, 1, 0050. [Google Scholar] [CrossRef]
- Raie, D.S.; Mhatre, E.; Thiele, M.; Labena, A.; El-Ghannam, G.; Farahat, L.A.; Youssef, T.; Fritzsche, W.; Kovács, Á.T. Application of Quercetin and Its Bio-Inspired Nanoparticles as Anti-Adhesive Agents against Bacillus Subtilis Attachment to Surface. Mater. Sci. Eng. C 2017, 70, 753–762. [Google Scholar] [CrossRef]
- Molina Bertrán, S.d.C.; Monzote, L.; Cappoen, D.; Escalona Arranz, J.C.; Gordillo Pérez, M.J.; Rodríguez-Ferreiro, A.O.; Chill Nuñez, I.; Novo, C.P.; Méndez, D.; Cos, P.; et al. Inhibition of Bacterial Adhesion and Biofilm Formation by Seed-Derived Ethanol Extracts from Persea americana Mill. Molecules 2022, 27, 5009. [Google Scholar] [CrossRef]
- Huebinger, R.M.; Stones, D.H.; de Souza Santos, M.; Carlson, D.L.; Song, J.; Vaz, D.P.; Keen, E.; Wolf, S.E.; Orth, K.; Krachler, A.M. Targeting Bacterial Adherence Inhibits Multidrug-Resistant Pseudomonas Aeruginosa Infection Following Burn Injury. Sci. Rep. 2016, 6, 39341. [Google Scholar] [CrossRef]
- Campana, R.; Casettari, L.; Ciandrini, E.; Illum, L.; Baffone, W. Chitosans Inhibit the Growth and the Adhesion of Klebsiella Pneumoniae and Escherichia coli Clinical Isolates on Urinary Catheters. Int. J. Antimicrob. Agents 2017, 50, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, S.; Toivanen, M.; Liu, C.; Tikkanen-Kaukanen, C. Novel Anti-Infective Potential of Salvianolic Acid B against Human Serious Pathogen Neisseria Meningitidis. BMC Res. Notes 2016, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Yik Hean, N.; Md Othman, S.N.A.; Basar, N.; Jemon, K. Antibiofilm and Antiadhesion Activities of Phaleria Macrocarpa against Oral Streptococcus mutans. J. Teknol. 2015, 77, 31–35. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, C.W. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Vidya, K.; Mallya, P.; Rao, P. Inhibition of Bacterial Adhesion by Subinhibitory Concentrations of Antibiotics. Indian J. Med. Microbiol. 2005, 23, 102–105. [Google Scholar] [CrossRef]
- Breines, D.M.; Burnham, J.C. The Effects of Quinolones on the Adherence of Type-1 Fimbriated Escherichia coli to Mannosylated Agarose Beads. J. Antimicrob. Chemother. 1995, 36, 911–925. [Google Scholar] [CrossRef]
- Piatti, G.; Mannini, A.; Balistreri, M.; Schito, A.M. Virulence Factors in Urinary Escherichia coli Strains: Phylogenetic Background and Quinolone and Fluoroquinolone Resistance. J. Clin. Microbiol. 2008, 46, 480–487. [Google Scholar] [CrossRef]
- Rasigade, J.P.; Moulay, A.; Lhoste, Y.; Tristan, A.; Bes, M.; Vandenesch, F.; Etienne, J.; Lina, G.; Laurent, F.; Dumitrescu, O. Impact of Sub-Inhibitory Antibiotics on Fibronectin-Mediated Host Cell Adhesion and Invasion by Staphylococcus aureus. BMC Microbiol. 2011, 11, 263. [Google Scholar] [CrossRef]
- Shabunin, S.; Pashentsev, A.; Klimov, N.; Morgunova, V.; Gritsyuk, V. Using Biferon-B for the Prevention of Mastitis in Cows. BIO Web Conf. 2020, 17, 00099. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Bovine Mastitis Prevention and Control in the Post-Antibiotic Era. Trop. Anim. Health Prod. 2021, 53, 236. [Google Scholar] [CrossRef]
- Vilar, M.J.; Rajala-Schultz, P.J. Dry-off and Dairy Cow Udder Health and Welfare: Effects of Different Milk Cessation Methods. Vet. J. 2020, 262, 105503. [Google Scholar] [CrossRef]
- Nyhetsbrev. DeLaval California Mastitis Test CMT; AgroLab: Novosibirsk, Russia, 2014; Available online: https://agrolab-nsk.ru/products/mastit-test-reaktiv (accessed on 10 March 2023).
- Garcia Garcia, M.R.; Casares, N.; Martinez Perez, L.A.; Juarez Curiel, E.; Alberto de Jesus Hernandez, A.; Bogdanchikova, N.; Garibo, D.; Rodriguez-Hernandez, A.G.; Pestryakov, A.; Castro Gamboa, S.; et al. Toxicological effects of silver nanoparticles and their effects in the induction of immunogenic cell death in cancer. J. Immunotoxicol. 2023, 20, 2175078. [Google Scholar] [CrossRef]
- Boone, D.R.; Castenholz, R.W.; Garrity, G.M. Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Systematic Bacteriology; Springer: New York, NY, USA, 2001. [Google Scholar]
- Persson, G.; Bojesen, A.M. Bacterial Determinants of Importance in the Virulence of Gallibacterium Anatis in Poultry. Vet. Res. 2015, 46, 57. [Google Scholar] [CrossRef]
- Brilis, V.I.; Brilene, T.A.; Levkov, L.A.; Lentsner, K.P.; Lentsner, A.A. Effect of antibiotics on the adhesive properties of microorganisms and an available model for its study. Antibiot. Med. Biotekhnol. 1986, 31, 353–357. [Google Scholar]
- Bukharin, O.V.; Nemtseva, N.V.; Shabanov, S.V.; Plotnikov, A.O. Antilysozyme Activity as a Factor of Algae Survival in Aquatic Biocenoses. Russ. J. Ecol. 2001, 32, 94–97. [Google Scholar] [CrossRef]




| Parameter | Experiment | Observed Effect | ||
|---|---|---|---|---|
| After Treatment with AgNPs | After Treatment with Antibiotics | Total Benefit from AgNPs Use Compared with Antibiotic Use | ||
| Treatment time, days | In vivo | 2.9 | 7.1 | 4.2 days (2.5 times faster) |
| Change of number of isolates, % | In vivo | −64.1 | +10.0 | 74.1 |
| Change of adhesive capacity, % | In vitro | −29.1 | +35.2 | 64.2 |
| In vivo | −23.1 | +1.45 | 24.5 | |
| Change of anti-lysozyme activity, % | In vitro In vivo | −11.3 −22.7 | +8.2 −2.2 | 19.5 20.5 |
| Compound | Mechanism | Bacteria | Adhesive Activity Measurement Method | Effect on Adhesion | Experiments | Ref. |
|---|---|---|---|---|---|---|
| AgNPs stabilized by PVP and protein hydrolysate | - | S. aureus, S. epidermidis, S. dysgalactiae, S. agalactiae, S. pyogenes, P. aeruginosa, E. coli | Standard method of V.I. Brilis (cow erythrocytes with a concentration of 108 cells/mL) | Inhibition adhesive capacity for 7 bacteria in average: in vitro by 29.1%, in vivo by 23.1% | In vitro In vivo | Present work |
| AgNPs synthesized with Rhodomyrtus tomentosa Leaf extract and pure extract | 2 Staphylococcus isolates 2 Staphylococcus aureus ATCC 29213 and S. epidermidis ATCC 35984 | Microbial Adhesion to Hydrocarbon (MATH) Test Modified excision-based sampling method | 106 CFU/mL decreased to 105–103 CFU/mL | In vitro In vivo (Bovine udder epidermal tissue) | [4] | |
| Coated glass slides by quercetin (a plant pigment/flavonoid) TiO2 and WO3 nanoparticles | Antiadhesive activity against B. subtilis biofilm | Bacillus subtilis | Confocal Laser Scanning Microscopy (CLSM) | Anti-adhesive efficacies were 96.71% and 98.97% for the surface coated by TiO2 and 79.35 and 87.10% by WO3 | In vitro | [19] |
| Seed-derived ethanol extracts (polyphenols and neolignan) from Persea americana Mill (avocado) | Modulation of the quorum sensing system by downregulation of the virulence factors such as mexT and lasA genes | Pseudomonas aeruginosa, 64 mg/mL Staphylococcus aureus, 64 mg/mL Escherichia coli, 512 mg/mL Staphylococcus pneumoniae, 128 mg/mL | Standard viable plate count method Inhibition of the bacterial adhesion to A549 lung epithelial cells after 60 min of incubation | 108 CFU/mL decreased to 103–106 CFU/mL 106 CFU/mL decreased to 101 CFU/mL 1016 CFU/mL decreased to 103 CFU/mL 103 CFU/mL decreased to 10−1–101 CFU/mL | In vitro | [20] |
| Multivalent adhesion molecule coupled to polystyrene microbeads | Blocking pilus assembly | Pseudomonas aeruginosa | Adhesion was not measured directly; other parameters were measured | Inhibition or mostly reduced 22 and 30% | In vivo rat model | [21] |
| Chitosans | Inhibition of the growth and adhesion of human uropathogens on urinary catheters | Klebsiella pneumoniae E. coli | Colony forming units were enumerated in the plates | Inhibition pH = 5, 107 CFU/mL -> 102–103 CFU/mL pH = 6, 107 CFU/mL -> 105 CFU/mL, 106 CFU/mL -> 104 CFU/mL pH = 5, 107 CFU/mL -> 102–104 CFU/mL pH = 6, 107 CFU/mL -> 105 | In vitro | [22] |
| Salvianolic acid B | Anti-pili of N. meningitidis | Neisseria meningitidis | A microtiter plate assay | Inhibition of Meningococcal pili binding to bovine thyroglobulin (80–93%) | In vitro | [23] |
| Phaleria macrocarpa plant extract | S. mutans adhering to the glass surface in the presence of 5 P. macrocarpa extracts (6.5 mg/mL) | Staphylococcus aureus | Spectrophotometer at 600 nm | Adhesion decreases from 100% down to 10–26% | In vitro | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanchikova, N.; Maklakova, M.; Villarreal-Gómez, L.J.; Nefedova, E.; Shkil, N.N.; Plotnikov, E.; Pestryakov, A. Revealing the Second and the Third Causes of AgNPs Property to Restore the Bacterial Susceptibility to Antibiotics. Int. J. Mol. Sci. 2023, 24, 7854. https://doi.org/10.3390/ijms24097854
Bogdanchikova N, Maklakova M, Villarreal-Gómez LJ, Nefedova E, Shkil NN, Plotnikov E, Pestryakov A. Revealing the Second and the Third Causes of AgNPs Property to Restore the Bacterial Susceptibility to Antibiotics. International Journal of Molecular Sciences. 2023; 24(9):7854. https://doi.org/10.3390/ijms24097854
Chicago/Turabian StyleBogdanchikova, Nina, Maria Maklakova, Luis Jesús Villarreal-Gómez, Ekaterina Nefedova, Nikolay N. Shkil, Evgenii Plotnikov, and Alexey Pestryakov. 2023. "Revealing the Second and the Third Causes of AgNPs Property to Restore the Bacterial Susceptibility to Antibiotics" International Journal of Molecular Sciences 24, no. 9: 7854. https://doi.org/10.3390/ijms24097854
APA StyleBogdanchikova, N., Maklakova, M., Villarreal-Gómez, L. J., Nefedova, E., Shkil, N. N., Plotnikov, E., & Pestryakov, A. (2023). Revealing the Second and the Third Causes of AgNPs Property to Restore the Bacterial Susceptibility to Antibiotics. International Journal of Molecular Sciences, 24(9), 7854. https://doi.org/10.3390/ijms24097854









