Overexpression of miR-4669 Enhances Tumor Aggressiveness and Generates an Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Its Clinical Value as a Predictive Biomarker
Abstract
:1. Introduction
2. Results
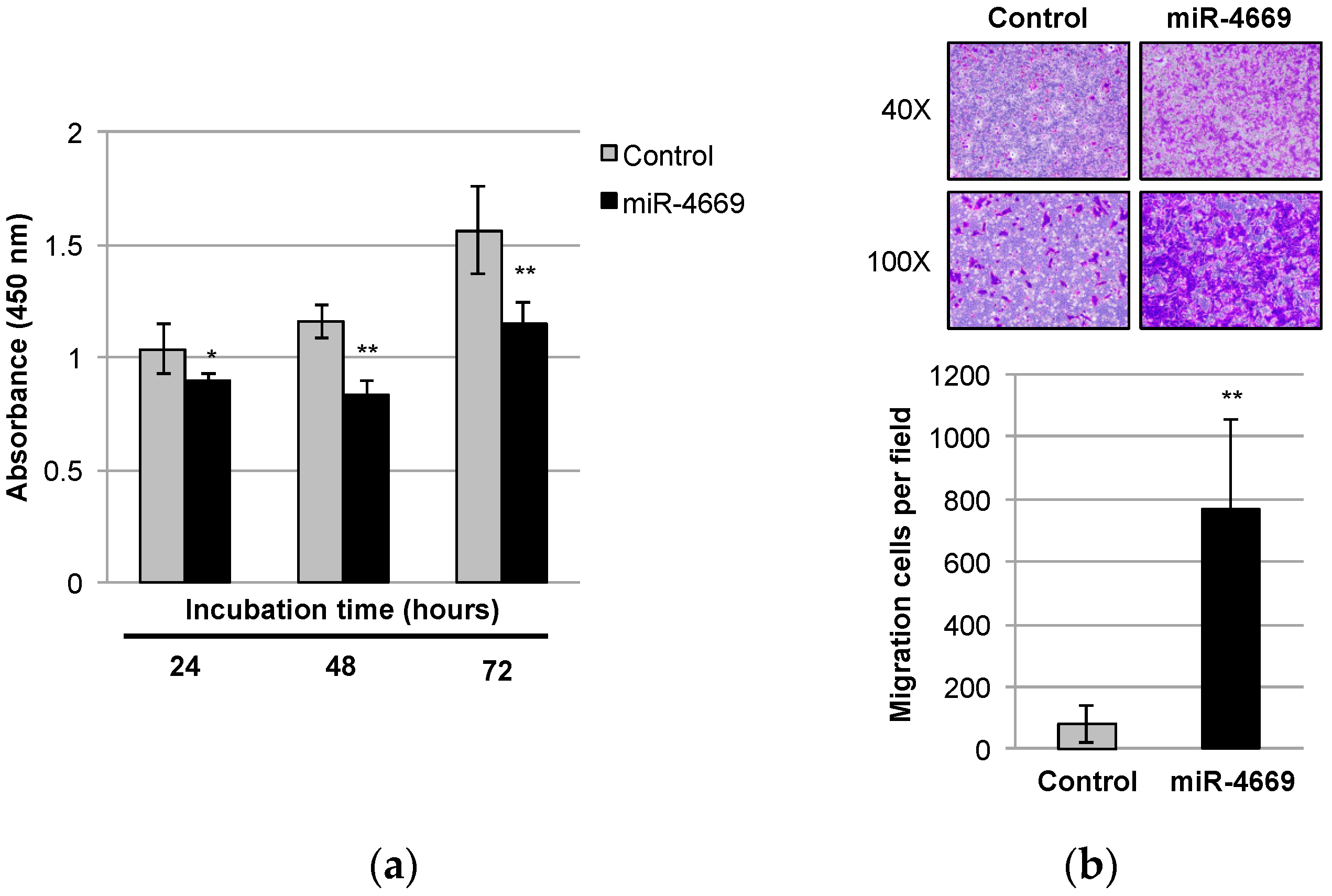
2.1. Impact of miR-4669 Overexpression on Tumor Cell Growth and Migration in HCC
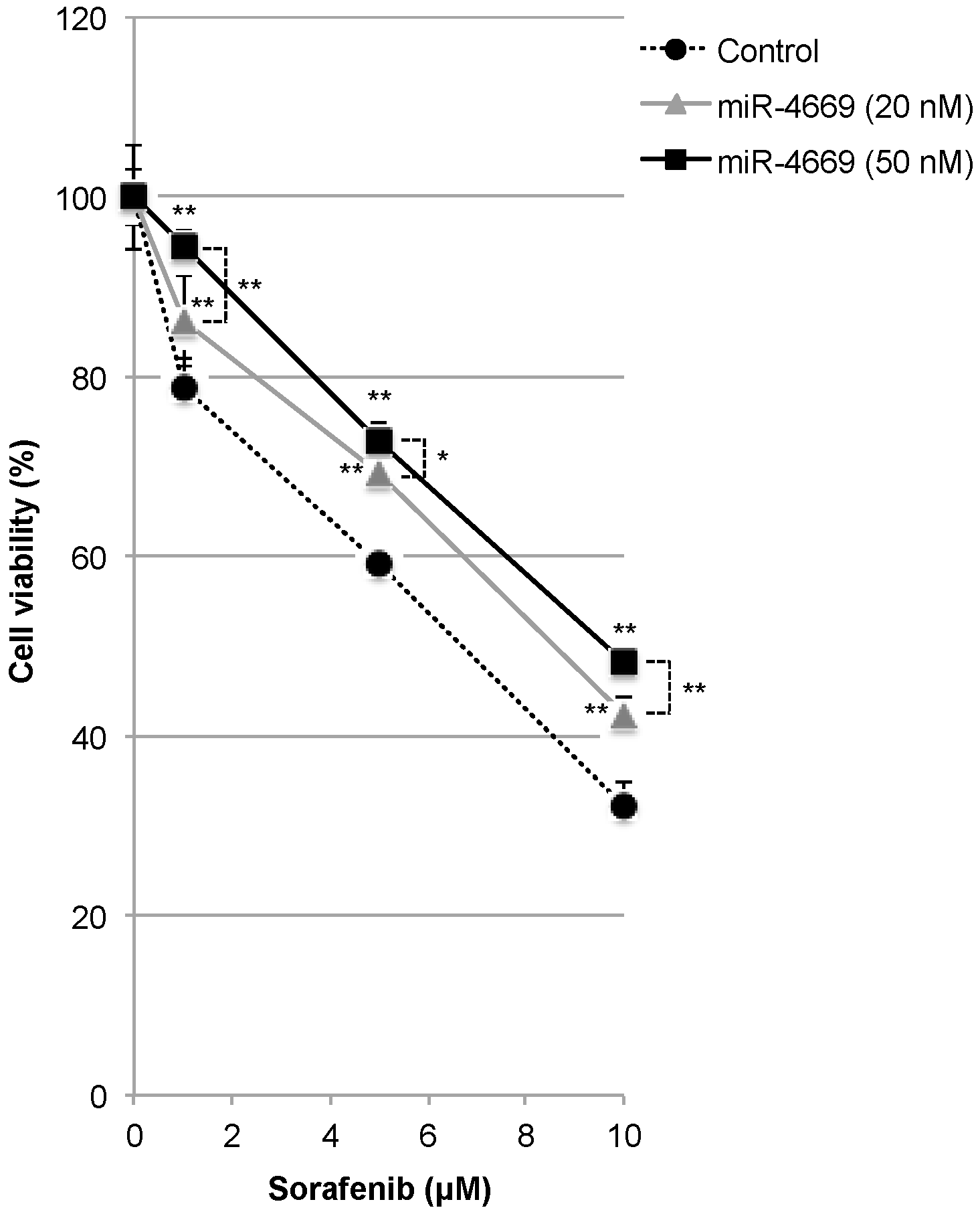
2.2. Impact of miR-4669 Overexpression on Drug Sensitivity in HCC
2.3. Impact of miR-4669 Overexpression on Genes Involved in Metastasis and Drug Resistance
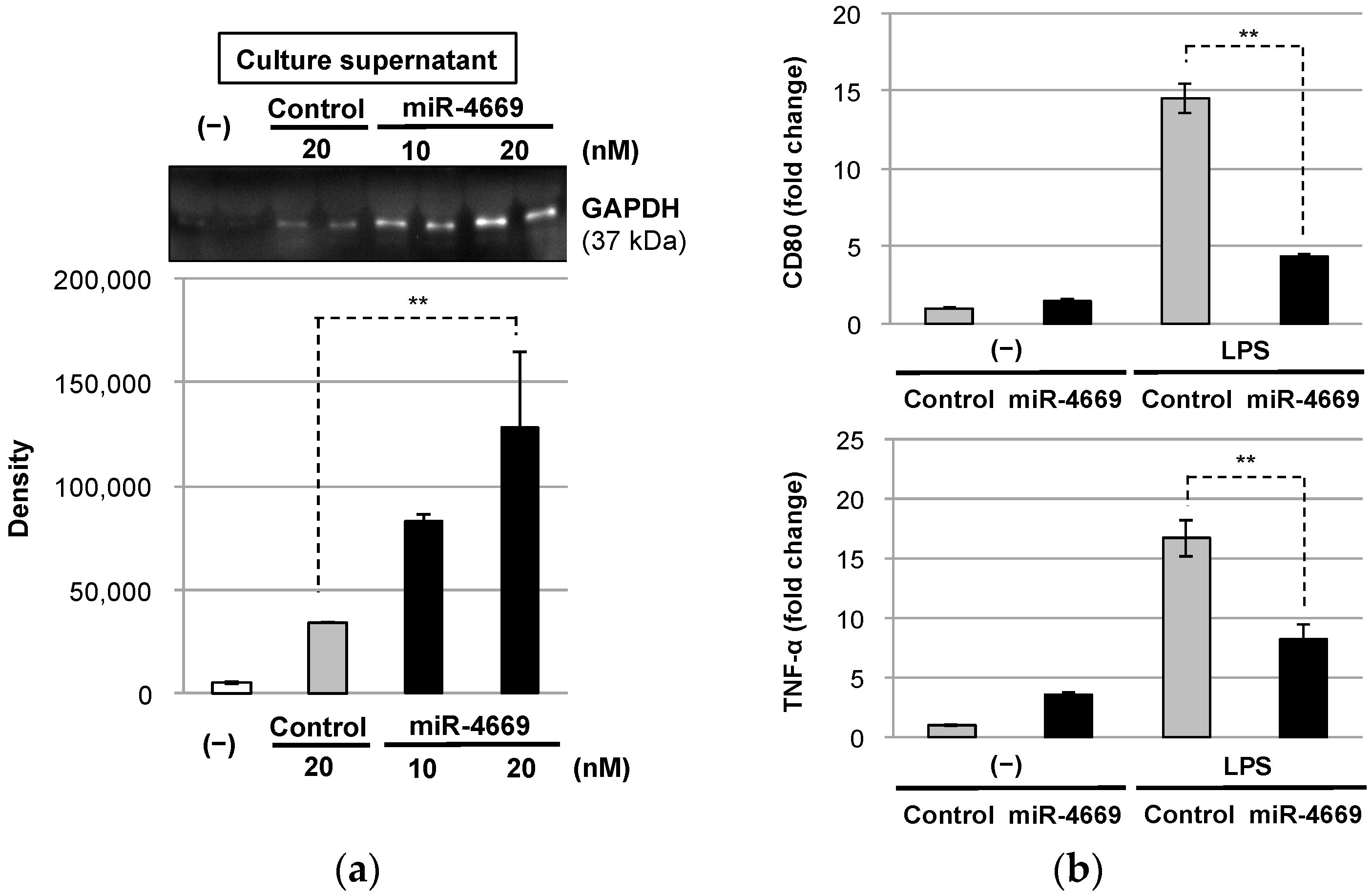
2.4. Impact of Tumor-Derived Extracellular Factors on Macrophages in the Tumor Microenvironment
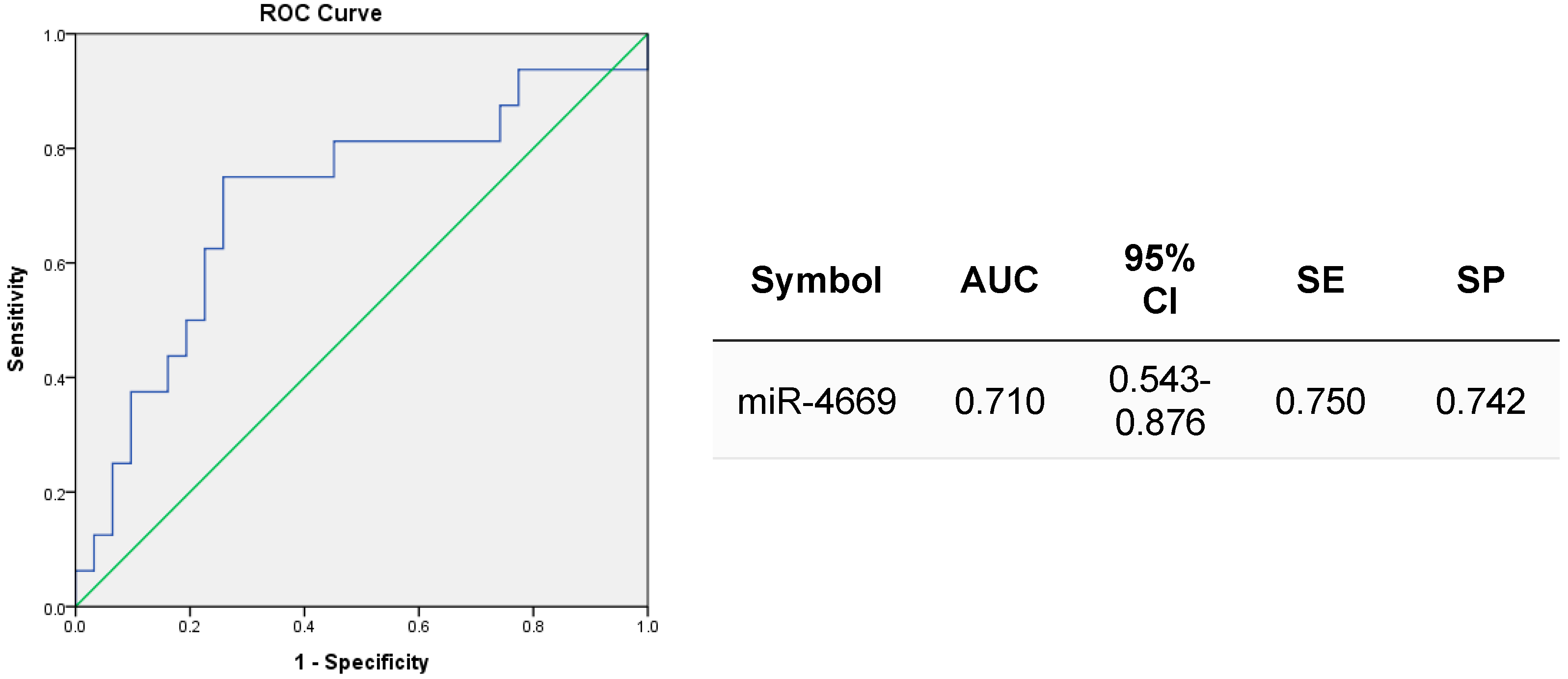
2.5. Clinical Value of Exosomal miR-4669 for Prediction of Treatment Response to HCC Downstaging Therapies and for Risk Assessment of Posttransplant HCC Recurrence
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Drug Sensitivity Assay
4.3. RNA Extraction and Quantitative Real-Time PCR
4.4. Western Blot Analysis
4.5. Tumor-Derived Exosome Isolation and Macrophage Polarization
4.6. Clinical Verification
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; EI-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Gbolahan, O.B.; Schacht, M.A.; Beckley, E.W.; LaRoche, T.P.; O’Neil, B.H.; Pyko, M. Locoregional and systemic therapy for hepatocellular carcinoma. J. Gastrointest. Oncol. 2017, 8, 215–228. [Google Scholar] [CrossRef]
- Jones, D.A.; Smith, J.; Mei, X.M.; Hawkins, M.A.; Maughan, T.; van den Heuvel, F.; Mee, T.; Kirkby, K.; Kirkby, N.; Gray, A. A systemic review of health economic evaluations of proton beam therapy for adult cancer: Appraising methodology and quality. Clin. Transl. Radiat. Oncol. 2019, 20, 19–26. [Google Scholar] [CrossRef]
- Concejero, A.; Chen, C.L.; Wang, C.C.; Wang, S.H.; Lin, C.C.; Liu, Y.W.; Yang, C.H.; Yong, C.C.; Lin, T.S.; Jawan, B.; et al. Living donor liver transplantation for hepatocellular carcinoma: A single-center experience in Taiwan. Transplantation 2008, 85, 398–406. [Google Scholar] [CrossRef]
- Yu, C.Y.; Ou, H.Y.; Huang, T.L.; Chen, T.Y.; Tsang, L.L.; Chen, C.L.; Cheng, Y.F. Hepatocellular carcinoma downstaging in liver transplantation. Trasplant. Proc. 2012, 44, 412–414. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, C.L. Living donor liver transplantation for hepatocellular carcinoma achieves better outcomes. Hepatobiliary Surg. Nutr. 2016, 5, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Wang, Y.C.; Cheng, C.H.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Chan, K.M.; Lee, W.C. Outcomes associated with the intention of loco-regional therapy prior to living donor liver transplantation for hepatocellular carcinoma. World J. Gastrointest. Surg. 2020, 12, 17–27. [Google Scholar] [CrossRef]
- Tong, Y.S.; Huang, T.L.; Chen, T.Y.; Tsang, L.L.; Ou, H.Y.; Hsu, H.W.; Xiong, L.W.; Liao, C.C.; Eng, H.L.; Chen, C.L.; et al. Imaging Validation of Drug-Eluting Beads Transarterial Chemoembolization of Hepatocellular Carcinomas in Living Donor Liver Transplantation. Transplant. Proc. 2018, 50, 2622–2625. [Google Scholar] [CrossRef] [PubMed]
- Piñero, F.; Dirchwolf, M.; Pessôa, M.G. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020, 9, 1370. [Google Scholar] [CrossRef]
- Sasaki, R.; Kanda, T.; Yokosuka, O.; Kato, N.; Matsuoka, S.; Moriyama, M. Exosomes and Hepatocellular Carcinoma: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1406. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, M.; Grossi, I.; Corsi, J.; D’Agostino, V.G.; Jurikova, K.; Cusanelli, E.; Molfino, S.; Portolani, N.; Salvi, A.; De Petro, G. Expression of Cellular and Extracellular TERRA, TERC and TERT in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 6183. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Chen, I.H.; Wang, C.C.; Chen, P.J.; Tsang, H.P.; Huang, K.Z.; Hu, T.H.; Li, L.C.; Goto, S.; Cheng, Y.F.; et al. Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am. J. Transplant. 2019, 19, 3250–3262. [Google Scholar] [CrossRef]
- Wang, Y.N.; Chen, Z.H.; Chen, W.C. Novel circulating microRNAs expression profiles in colon cancer: A pilot study. Eur. J. Med.Res. 2017, 22, 51. [Google Scholar] [CrossRef]
- Bao, M.H.; Wong, C.C. Hypoxia, Metabolic Reprograming, and Drug Resistance in Liver Cancer. Cells 2021, 10, 1715. [Google Scholar] [CrossRef]
- Hao, C.; Zhu, P.X.; Yang, X.; Han, Z.P.; Jiang, J.H.; Zong, C.; Zhang, X.G.; Liu, W.T.; Zhao, Q.D.; Fan, T.T.; et al. Overexpression of SIRT1 promotes metastasis through epithelial-mesenchymal transition in hepatocellular carcinoma. BMC Cancer 2014, 14, 978. [Google Scholar] [CrossRef]
- Farcas, M.; Gavrea, A.A.; Gulei, D.; Ionescu, C.; Irimie, A.; Catana, C.S.; Berindan-Neagoe, I. SIRT1 in the Development and Treatment of Hepatocellular Carcinoma. Front. Nutr. 2019, 6, 148. [Google Scholar] [CrossRef]
- Garten, A.; Grohmann, T.; Kluckova, K.; Lavery, G.G.; Kiess, W.; Penke, M. Sorafenib-induced Apoptosis in Hepatocellular Carcinoma Is Reversed by SIRT1. Int. J. Mol. Sci. 2019, 20, 4048. [Google Scholar] [CrossRef]
- Ünal, E.; İdilman, İ.S.; Akata, D.; Özmen, M.N.; Karçaaltıncaba, M. Microvascular invasion in hepatocellular carcinoma. Diagn. Interv. Radiol. 2016, 22, 125–132. [Google Scholar] [CrossRef]
- Yanhan, W.; Lianfang, L.; Hao, L.; Yunfeng, D.; Nannan, S.; Fanfan, L.; Chengzhan, Z.; Meilong, W.; Chuandong, S. Effect of Microvascular Invasion on the Prognosis in Hepatocellular Carcinoma and Analysis of Related Risk Factors: A Two-Center Study. Front. Surg. 2021, 8, 733343. [Google Scholar] [CrossRef]
- Yuan, S.X.; Yang, F.; Yang, Y.; Tao, Q.F.; Zhang, J.; Huang, G.; Yang, Y.; Wang, R.Y.; Yang, S.; Huo, X.S.; et al. noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology 2012, 56, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.C.; Liu, P.S. Metabolic communication in tumors: A new layer of immunoregulation for immune evasion. J. Immunother. Cancer 2016, 4, 4. [Google Scholar] [CrossRef]
- Nakano, T.; Goto, S.; Takaoka, Y.; Tseng, H.P.; Fujimura, T.; Kawamotyo, S.; Ono, K.; Chen, C.L. A novel moonlight function of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for immunomodulation. Biofactors 2018, 44, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, S.; Sun, M.Z. Novel insight into the role of GAPDH playing in tumor. Clin. Transl. Oncol. 2013, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhan, F.; Hong, C.Q.; Giuliano, A.E.; Cui, X.J.; Zhou, G.J.; Zhang, G.J.; Cui, Y.K. Critical protein GAPDH and tts regulatory mechanisms in cancer cells. Cancer Biol. Med. 2015, 12, 10–22. [Google Scholar] [PubMed]
- Evdokimova, V.; Tognon, C.; Ng, T.; Sorensen, P.H. Reduced proliferation and enhanced migration: Two sides of the same coin? Molecular mechanisms of metastatic progression by YB-1. Cell Cycle 2009, 8, 2901–2906. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Li, J.; Zheng, L.; Feng, M.; Wang, X.; Han, K.; Pi, H.; Li, M.; Huang, X.; et al. SIRT1 facilitates hepatocellular carcinoma metastasis by promoting PGC-1α-mediated mitochondrial biogenesis. Oncotarget 2016, 7, 29255–29274. [Google Scholar] [CrossRef]
- Portmann, S.; Fahrner, R.; Lechleiter, A.; Keogh, A.; Overney, S.; Laemmle, A.; Mikami, K.; Nontani, M.; Tschan, M.P.; Candinas, D.; et al. Antitumor effect of SIRT1 inhibition in human HCC tumor models in vitro and in vivo. Mol. Cancer Ther. 2013, 12, 499–508. [Google Scholar] [CrossRef]
- Ceballos, M.P.; Angel, A.; Delprato, C.B.; Linore, V.I.; Ferretti, A.C.; Lucci, A.; Comanzo, C.G.; Alvarez, M.L.; Quiroga, A.D.; Mottino, A.D.; et al. Sirtuin 1 and 2 nhibitors enhance the inhibitory effect of sorafenib in hepatocellular carcinoma cells. Eur. J. Pharmacol. 2021, 892, 173736. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Mano, Y.; Muto, J.; Matono, R.; Motomura, T.; Toshima, T.; Takeishi, K.; Uchiyama, H.; Yoshizumi, T.; Taketomi, A.; et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg. Today 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Meng, Y.; Xu, X.; Zuo, D. The role of glycolysis and lactate in the induction of tumor-associated macrophages immunosuppressive phenotype. Int. Immunopharmacol. 2022, 110, 108994. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, S.; Fan, R.; Tang, Q.; Zhang, H.; Wang, F.; Xie, S.; Gao, K.; Zhang, J.; Xie, Z.; et al. Exosomes Derived hsa-miR-4669 as a Novel Biomarker for Early Predicting the Response of Subcutaneous Immunotherapy in Pediatric Allergic Rhinitis. J. Inflamm. Res. 2022, 15, 5063–5074. [Google Scholar] [CrossRef]
- Lou, H.; Huang, Y.; Chen, H.; Wang, Y.; Zhang, L.; Wang, C. M2 macrophages correlated with symptom severity and promote type 2 inflammation in allergic rhinitis. Allergy 2019, 74, 2255–2257. [Google Scholar] [CrossRef]
- Ogulur, I.; Pat, Y.; Ardicli, O.; Barletta, E.; Cevhertas, L.; Fernandez-Santamaria, R.; Huang, M.; Imam, M.B.; Koch, J.; Ma, S.; et al. Advances and highlights in biomarkers of allergic diseases. Allergy 2021, 76, 3659–3686. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ou, H.Y.; Yu, C.Y.; Huang, T.L.; Tsang, L.L.; Cheng, Y.F. Drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma: Dose size really matter? Diagn. Interv. Radiol. 2020, 26, 230–235. [Google Scholar] [CrossRef]
- Yang, T.; Qin, W.; Sun, X.; Wang, Y.; Wu, J.; Li, Z.; Ji, F.; Zhang, L.; Liu, W. Efficacy and safety of drug-eluting bead-transcatheter arterial chemoembolization using 100–300 μm versus 300–500 μm CalliSpheres microspheres in patients with advanced-stage hepatocellular carcinoma. J. Cancer Res. Ther. 2020, 16, 1582–1587. [Google Scholar]
- Chang, W.C.; Hsu, H.H.; Chiu, S.H.; Huang, W.Y.; Lo, C.H.; Lin, H.H.; Huang, P.C.; Shih, Y.L.; Wan, Y.L. Transactheter Arterial Chemoembolization with Drug-Eluting Beads for the Treatment of Hepatocellular Carcinoma: Recommended Selection for Small-Caliber (<100 μm) Beads. J. Hepatocell. Carcinoma 2021, 8, 937–949. [Google Scholar] [PubMed]
- Zhang, J.; Feng, G.A.; Li, Y.; Wang, W. Drug-eluting bead transarterial chemoembolization with medium-sized versus small-sized CalliSpheres microspheres in unresectable primary liver cancer. Asia Pac. J. Clin. Oncol. 2022, 18, 388–393. [Google Scholar] [CrossRef] [PubMed]

| HCC No Recurrence (>5 Years) | HCC Recurrence (≤2 Years) | |
|---|---|---|
| Gender (female/male) | 0/16 | 4/27 |
| Age (years) | 51.4 (31.2–66.1) | 53.6 (26.3–65.1) |
| AST (U/L) | 40.8 (16–83) | 64.4 (26–143) |
| ALT (U/L) | 39.9 (10–79) | 48.2 (13–162) |
| AFP (ng/mL) | 267.9 (3–4385) | 76.9 (3–410.8) |
| Tumor number | 1.69 (0–6) | 3.17 (1–11) |
| Tumor total size (cm) | 3.26 (0–8) | 8.16 (2.7–20.3) ** |
| Patient | Pathology | HCC Recurrence | Exosomal miR-4669 (Fold Change) |
|---|---|---|---|
| DC-1 | CR | No | 6.5 |
| DC-2 | CR | No | 3.3 |
| DC-3 | PR | Late | 31.1 |
| DC-4 | PR | Early | 6.3 |
| DC-5 | PR | Early | 12.3 |
| DC-6 | PR | Early | 59.0 |
| DC-7 | PR | Early | 41.4 |
| DC-8 | PR | No | 7.1 |
| DC-9 | PR | No | 3.1 |
| DC-10 | PR | Early | 25.8 |
| DC-11 | PR | No | 9.3 |
| DC-12 | PR | No | 5.0 |
| DC-13 | PR | No | 2.4 |
| DC-14 | PR | No | 5.9 |
| DC-15 | PR | Early | 37.3 |
| DC-16 | PR | No | 4.5 |
| DC-17 | PR | No | 4.4 |
| DC-18 | PR | No | 7.2 |
| DC-19 | PR | Late | 27.3 |
| DC-20 | PR | No | 2.2 |
| DC-21 | PR | No | 4.5 |
| DC-22 | PR | No | 4.7 |
| DC-23 | PR | Early | 40.0 |
| Patient | Pathology | HCC Recurrence | Exosomal miR-4669 (Fold Change) |
|---|---|---|---|
| Tan-1 | CR | Early | 25.8 |
| Tan-2 | PR | Early | 13.1 |
| Tan-3 | PR | No | 5.7 |
| Tan-4 | PR | No | 2.4 |
| Tan-5 | PR | Early | 21.0 |
| Tan-6 | PR | No | 2.0 |
| Tan-7 | PR | No | 4.3 |
| Tan-8 | PR | No | 1.4 |
| Tan-9 | PR | No | 5.0 |
| Tan-10 | PR | No | 1.6 |
| Tan-11 | PR | No | 2.0 |
| Tan-12 | PR | No | 1.9 |
| Tan-13 | PR | No | 9.0 |
| Tan-14 | PR | No | 1.8 |
| Tan-15 | PR | No | 7.4 |
| Tan-16 | CCC | Early | 24.9 |
| Tan-17 | CCC | No | 9.8 |
| Patient | Pathology | HCC Recurrence | Exosomal miR-4669 (Fold Change) |
|---|---|---|---|
| Hep-1 | CR | No | 4.6 |
| Hep-2 | PR | No | 7.2 |
| Hep-3 | PR | No | 9.0 |
| Forward Primer | Reverse Primer | |
|---|---|---|
| SIRT1 | 5′-TAGACACGCTGGAACAGGTTGC-3′ | 5′-CTCCTCGTACAGCTTCACAGTC-3′ |
| lncRNA MVIH | 5′-AATTTTGCACATCTGAACAGCC-3′ | 5′-TTCAAAATCCCACTACGCCCA-3′ |
| β-actin | 5′-CACCATTGGCAATGAGCGGTTC-3′ | 5′-AGGTCTTTGCGGATGTCCACGT-3′ |
| Forward Primer | Reverse Primer | |
|---|---|---|
| CD80 | 5′-GCAGGATACACCACTCCTCAA-3′ | 5′-AAAGACGAATCAGCAGCACAA-3′ |
| TNF-α | 5′-ATGAGCACAGAAAGCATGATC-3′ | 5′-TACAGGCTTGTCACTCGAATT-3′ |
| β-actin | 5′-GGCTGTATTCCCCTCCATCG-3′ | 5′-CCAGTTGGTAACAATGCCATGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, T.; Chen, C.-L.; Chen, I.-H.; Tseng, H.-P.; Chiang, K.-C.; Lai, C.-Y.; Hsu, L.-W.; Goto, S.; Lin, C.-C.; Cheng, Y.-F. Overexpression of miR-4669 Enhances Tumor Aggressiveness and Generates an Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Its Clinical Value as a Predictive Biomarker. Int. J. Mol. Sci. 2023, 24, 7908. https://doi.org/10.3390/ijms24097908
Nakano T, Chen C-L, Chen I-H, Tseng H-P, Chiang K-C, Lai C-Y, Hsu L-W, Goto S, Lin C-C, Cheng Y-F. Overexpression of miR-4669 Enhances Tumor Aggressiveness and Generates an Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Its Clinical Value as a Predictive Biomarker. International Journal of Molecular Sciences. 2023; 24(9):7908. https://doi.org/10.3390/ijms24097908
Chicago/Turabian StyleNakano, Toshiaki, Chao-Long Chen, I-Hsuan Chen, Hui-Peng Tseng, Kuei-Chen Chiang, Chia-Yun Lai, Li-Wen Hsu, Shigeru Goto, Chih-Che Lin, and Yu-Fan Cheng. 2023. "Overexpression of miR-4669 Enhances Tumor Aggressiveness and Generates an Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Its Clinical Value as a Predictive Biomarker" International Journal of Molecular Sciences 24, no. 9: 7908. https://doi.org/10.3390/ijms24097908
APA StyleNakano, T., Chen, C.-L., Chen, I.-H., Tseng, H.-P., Chiang, K.-C., Lai, C.-Y., Hsu, L.-W., Goto, S., Lin, C.-C., & Cheng, Y.-F. (2023). Overexpression of miR-4669 Enhances Tumor Aggressiveness and Generates an Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Its Clinical Value as a Predictive Biomarker. International Journal of Molecular Sciences, 24(9), 7908. https://doi.org/10.3390/ijms24097908






