Review on Generation and Characterization of Copper Particles and Copper Composites Prepared by Mechanical Milling on a Lab-Scale
Abstract
1. Introduction
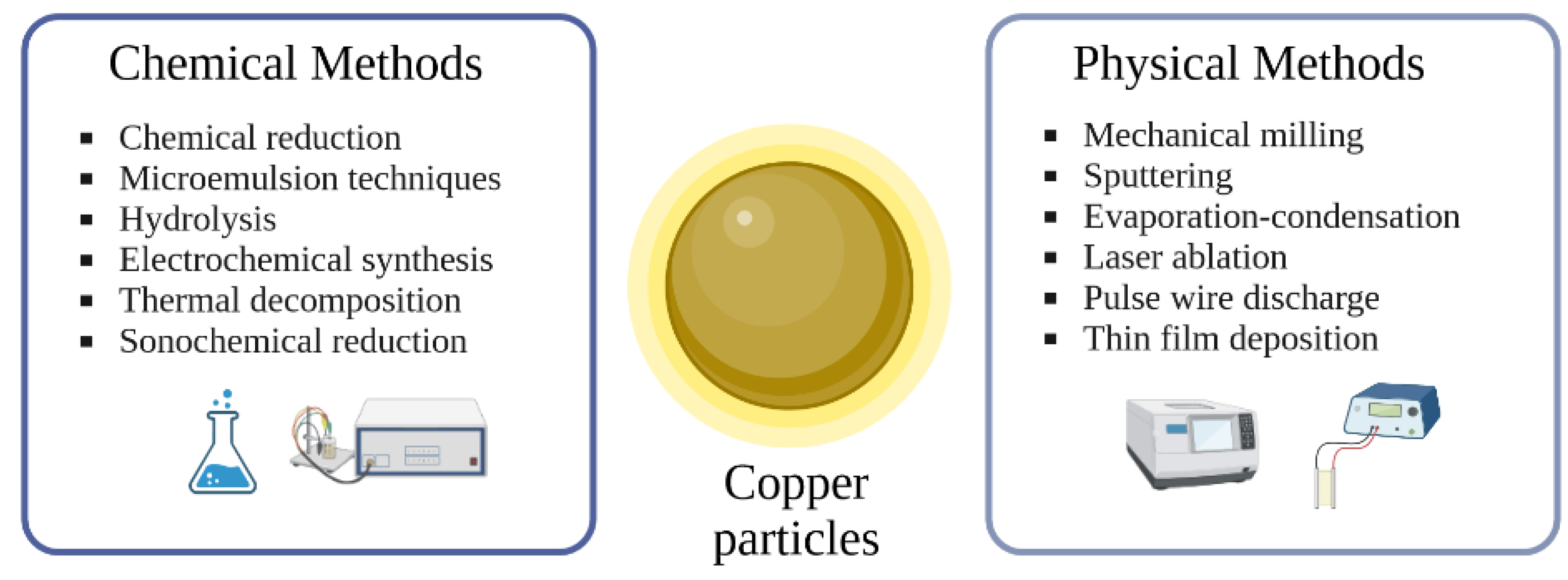
2. Generation of Copper Particles and Composites
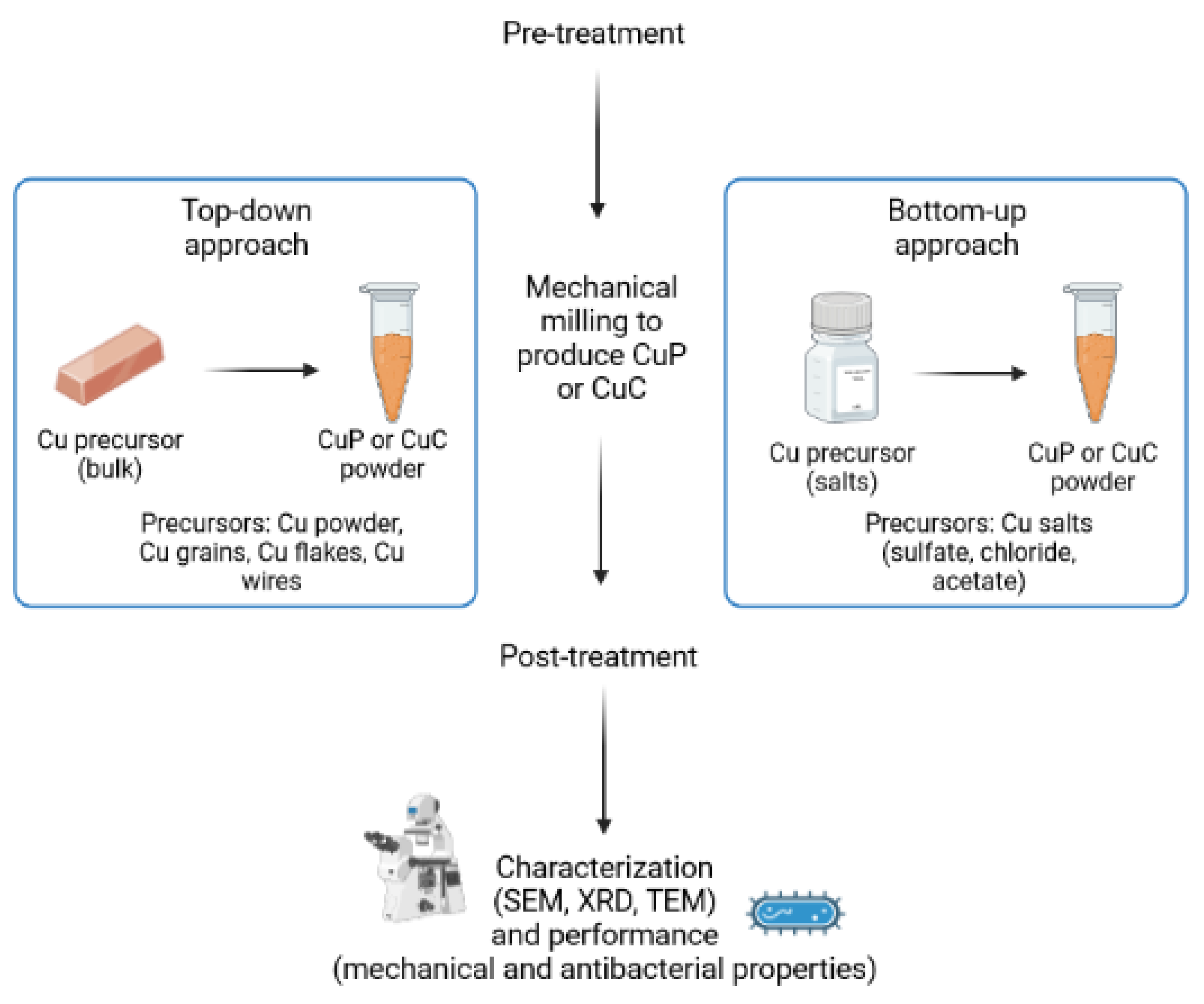
3. Pretreatment of the Starting Materials
4. Synthesis of CuP and CuC Using Mechanical Milling
4.1. Top-Down Approach Using Mechanical Milling
4.2. Bottom-Up Approach Using Mechanical Milling
5. Post-Treatment of the Obtained CuP and CuC
6. Characterization Techniques of the Synthesized CuP and CuC
6.1. X-ray Powder Diffraction (XRPD)
6.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectroscopy (EDS)
6.3. Transmission Electron Microscopy (TEM) and Selected Area Electron Diffraction (SAED)
6.4. UV-Visible Spectrophotometry (UV-Vis)
7. Evaluation of the Properties and Performance of the Obtained CuP and CuC
7.1. Mechanical Properties
7.2. Anti-Microbial Activity Assays
8. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Din, M.I.; Rehan, R. Synthesis, Characterization, and Applications of Copper Nanoparticles. Anal. Lett. 2017, 50, 50–62. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Abdul Kadir, A. Nanoparticles in Construction Materials and Other Applications, and Implications of Nanoparticle Use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef]
- Singh, M.; Jagaran, K. Nanomedicine for COVID-19: Potential of Copper Nanoparticles. Biointerface Res. Appl. Chem. 2020, 11, 10716–10728. [Google Scholar] [CrossRef]
- Zuniga, J.M.; Cortes, A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev. Med. Devices 2020, 17, 477–481. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Ganesh, M.R.S.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral properties of copper and its alloys to inactivate COVID-19 virus: A review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef]
- Achimovičová, M.; Dutková, E.; Tóthová, E.; Bujňáková, Z.; Briančin, J.; Kitazono, S. Structural and optical properties of nanostructured copper sulfide semiconductor synthesized in an industrial mill. Front. Chem. Sci. Eng. 2019, 13, 164–170. [Google Scholar] [CrossRef]
- Silva, N.; Arellano, E.; Castro, C.; Yutronic, N.; Lang, E.; Chornik, B.; Jara, P. Cyclodextrin inclusion compound crystals for growth of Cu–Au core–shell nanoparticles. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 497–504. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of copper nanoparticles: An overview of the various methods. Korean J. Chem. Eng. 2014, 31, 1105–1109. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; El-Hack, M.E.A.; Taha, A.E.; Fouda, M.M.G.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Elshaer, N. Ecofriendly Synthesis and Insecticidal Application of Copper Nanoparticles against the Storage Pest Tribolium castaneum. Nanomaterials 2020, 10, 587. [Google Scholar] [CrossRef]
- Javadhesari, S.M.; Alipour, S.; Mohammadnejad, S.; Akbarpour, M.R. Antibacterial activity of ultra-small copper oxide (II) nanoparticles synthesized by mechanochemical processing against S. aureus and E. coli. Mater. Sci. Eng. C-Mater. 2019, 105, 110011. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Zhao, W.; Cao, L.; Sun, Z.; Zhang, F. A facile synthesis of copper nanoparticles supported on an ordered mesoporous polymer as an efficient and stable catalyst for solvent-free sonogashira coupling Reactions. Green Chem. 2017, 19, 1949–1957. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Ul-Islam, M.; Asiri, A.M. Microwave Assisted Synthesis and Carboxymethyl Cellulose Stabilized Copper Nanoparticles on Bacterial Cellulose Nanofibers Support for Pollutants Degradation. J. Polym. Environ. 2019, 27, 2867–2877. [Google Scholar] [CrossRef]
- Waris, A.; Din, M.; Ali, A.; Ali, M.; Afridi, S.; Baset, A.; Khan, A.U. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg. Chem. Commun. 2021, 123, 108369. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Priyadarshini, B.G. Mechanical Milling of Copper Oxide Nanoparticles. In Proceedings of the First International Conference on Combinatorial and Optimization, ICCAP 2021, Chennai, India, 7–8 December 2021. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Hazza, A.; Al-Hajji, L.A.; Ali, N.; Al-Duweesh, A.A.; Banyan, M.; Al-Ajmi, F. Mechanical Milling: A Superior Nanotechnological Tool for Fabrication of Nanocrystalline and Nanocomposite Materials. Nanomaterials 2021, 11, 2484. [Google Scholar] [CrossRef]
- Yadav, T.P.; Yadav, R.M.; Singh, D. Mechanical Milling: A Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Balázs, B.Z.; Geier, N.; Takács, M.; Davim, J.P. A review on micro-milling: Recent advances and future trends. Int. J. Adv. Manuf. Technol. 2021, 112, 655–684. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B. Materials Development Using High-Energy Ball Milling: A Review Dedicated to the Memory of M.A. Korchagin. J. Compos. Sci. 2022, 6, 188. [Google Scholar] [CrossRef]
- Chen, Y. Chapter 3 Solid-State Formation of Carbon Nanotubes; Elsevier Ltd.: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Fu, E.; Du, J.; Wang, P.; Fan, Y.; Zhao, Y. Effect of Ball Milling Parameters on the Refinement of Tungsten Powder. Metals 2018, 8, 281. [Google Scholar] [CrossRef]
- Ramesh, S.; Vetrivel, S.; Suresh, P.; Kaviarasan, V. Characterization techniques for nano particles: A practical top down approach to synthesize copper nano particles from copper chips and determination of its effect on planes. Mater. Today Proc. 2020, 33, 2626–2630. [Google Scholar] [CrossRef]
- Menapace, C.; Leonardi, M.; Perricone, G.; Bortolotti, M.; Straffelini, G.; Gialanella, S. Pin-on-disc study of brake friction materials with ball-milled nanostructured components. Mater. Des. 2017, 115, 287–298. [Google Scholar] [CrossRef]
- Baláž, M.; Tešinský, M.; Marquardt, J.; Škrobian, M.; Daneu, N.; Rajňák, M.; Baláž, P. Synthesis of copper nanoparticles from refractory sulfides using a semi-industrial mechanochemical approach. Adv. Powder Technol. 2020, 31, 782–791. [Google Scholar] [CrossRef]
- Yadav, S.K. Synthesis and Characterization of Copper Nanoparticles, Using Combination of Two Different Sizes of Balls in Wet Ball Milling. Int. J. Emerg. Trends Sci. Technol. 2016, 3, 3795–3799. [Google Scholar] [CrossRef]
- Shuai, C.; He, C.; Peng, S.; Qi, F.; Wang, G.; Min, A.; Yang, W.; Wang, W. Mechanical Alloying of Immiscible Metallic Systems: Process, Microstructure, and Mechanism. Adv. Eng. Mater. 2021, 23, 2001098. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Taha, M.A.; Youness, R.A.; Zawrah, M. Review on nanocomposites fabricated by mechanical alloying. Int. J. Miner. Met. Mater. 2019, 26, 1047–1058. [Google Scholar] [CrossRef]
- Bonomi, S.; Armenise, V.; Accorsi, G.; Colella, S.; Rizzo, A.; Fracassi, F.; Malavasi, L.; Listorti, A. The Effect of Extended Ball-Milling upon Three-Dimensional and Two-Dimensional Perovskite Crystals Properties. Appl. Sci. 2020, 10, 4775. [Google Scholar] [CrossRef]
- Baláž, M. Environmental Mechanochemistry; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Mussapyrova, L.; Nadirov, R.; Baláž, P.; Rajňák, M.; Bureš, R.; Baláž, M. Selective room-temperature leaching of copper from mechanically activated copper smelter slag. J. Mater. Res. Technol. 2021, 12, 2011–2025. [Google Scholar] [CrossRef]
- Barai, K.; Tiwary, C.; Chattopadhyay, P.; Chattopadhyay, K. Synthesis of free standing nanocrystalline Cu by ball milling at cryogenic temperature. Mater. Sci. Eng. A 2012, 558, 52–58. [Google Scholar] [CrossRef]
- Joy, J.; Krishnamoorthy, A.; Tanna, A.; Kamathe, V.; Nagar, R.; Srinivasan, S. Recent Developments on the Synthesis of Nanocomposite Materials via Ball Milling Approach for Energy Storage Applications. Appl. Sci. 2022, 12, 9312. [Google Scholar] [CrossRef]
- Bor, A.; Jargalsaikhan, B.; Uranchimeg, K.; Lee, J.; Choi, H. Particle morphology control of metal powder with various experimental conditions using ball milling. Powder Technol. 2021, 394, 181–190. [Google Scholar] [CrossRef]
- Jo, Y.; Park, H.J.; Jeong, S. Synthesis of disc-shaped copper flakes by mechanical milling: Green laser-sintered, highly conductive printed Cu features. J. Alloys Compd. 2021, 886, 161093. [Google Scholar] [CrossRef]
- Musza, K.; Szabados, M.; Ádám, A.A.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Ball Milling of Copper Powder Under Dry and Surfactant-Assisted Conditions—On the Way Towards Cu/Cu2O Nanocatalyst. J. Nanosci. Nanotechnol. 2019, 19, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Güler, O.; Varol, T.; Alver, Ü.; Kaya, G.; Yıldız, F. Microstructure and wear characterization of Al2O3 reinforced silver coated copper matrix composites by electroless plating and hot pressing methods. Mater. Today Commun. 2021, 27, 102205. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.; Zhou, C.; Arif, M.; Sun, M.; Chen, Y.; Liu, Y. Water-assisted-mechanical activation of copper pyrometallurgical tailings for molybdenum leaching and selective removal of environmentally-hazardous elements. Sep. Purif. Technol. 2023, 310, 123088. [Google Scholar] [CrossRef]
- Rahmanifard, R.; Javidan, S.M.; Asadabad, M.A. Effects of Process Control Agents on Characteristics of Cu-Ta Nanocomposite during Milling and Subsequent Sintering. J. Mater. Eng. Perform. 2019, 28, 4102–4110. [Google Scholar] [CrossRef]
- Jia, C.; Zhu, J.; Zhang, L. An Anti-Corrosion Superhydrophobic Copper Surface Fabricated by Milling and Chemical Deposition. Coatings 2022, 12, 442. [Google Scholar] [CrossRef]
- Rosen, Y.; Marrach, R.; Gutkin, V.; Magdassi, S. Thin Copper Flakes for Conductive Inks Prepared by Decomposition of Copper Formate and Ultrafine Wet Milling. Adv. Mater. Technol. 2019, 4, 1800426. [Google Scholar] [CrossRef]
- Singhal, S.K.; Lal, M.; Sharma, I.; Mathur, R.B. Fabrication of copper matrix composites reinforced with carbon nanotubes using a combination of molecular-level-mixing and high energy ball milling. J. Compos. Mater. 2013, 47, 613–621. [Google Scholar] [CrossRef]
- Pattison, T.G.; Hess, A.E.; Arellano, N.; Lanzillo, N.; Nguyen, S.; Bui, H.; Rettner, C.; Truong, H.; Friz, A.; Topuria, T.; et al. Surface Initiated Polymer Thin Films for the Area Selective Deposition and Etching of Metal Oxides. ACS Nano 2020, 14, 4276–4288. [Google Scholar] [CrossRef] [PubMed]
- Drozda, M.; Miszczyk, A. Selection of Organic Coating Systems for Corrosion Protection of Industrial Equipment. Coatings 2022, 12, 523. [Google Scholar] [CrossRef]
- Salvo, C.; Mangalaraja, R.; Udayabashkar, R.; Lopez, M.; Aguilar, C. Enhanced mechanical and electrical properties of novel graphene reinforced copper matrix composites. J. Alloys Compd. 2019, 777, 309–316. [Google Scholar] [CrossRef]
- Güler, O.; Varol, T.; Alver, Ü.; Çanakçı, A. The effect of flake-like morphology on the coating properties of silver coated copper particles fabricated by electroless plating. J. Alloys Compd. 2019, 782, 679–688. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Chen, C.-H.; Zhang, J.; Ren, F. Fabrication of nanosized metallic copper by electrochemical milling process. J. Mater. Sci. 2008, 43, 1492–1496. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Aldhameer, A. Synthesizing and characterizations of one-dimensional Cu-based antibiofilm surface protective coating. J. Nanopart. Res. 2020, 22, 120. [Google Scholar] [CrossRef]
- Park, H.J.; Jo, Y.; Lee, S.Y.; Choi, Y.; Jeong, S. One-pot synthesis of surface oxidation-suppressed multidimensional copper particles for photonically sintered, directly printed conductive features. J. Alloys Compd. 2021, 867, 159152. [Google Scholar] [CrossRef]
- Marinca, T.; Chicinaş, I.; Isnard, O. Synthesis, structural and magnetic characterization of nanocrystalline CuFe2O4 as obtained by a combined method reactive milling, heat treatment and ball milling. Ceram. Int. 2012, 38, 1951–1957. [Google Scholar] [CrossRef]
- Sayyad, R.; Ghambari, M.; Ebadzadeh, T.; Pakseresht, A.H.; Ghasali, E. Preparation of Ag/reduced graphene oxide reinforced copper matrix composites through spark plasma sintering: An investigation of microstructure and mechanical properties. Ceram. Int. 2020, 46, 13569–13579. [Google Scholar] [CrossRef]
- Amal, M.I.; Wibowo, J.T.; Nuraini, L.; Senopati, G.; Hasbi, M.Y.; Priyotomo, G. Antibacterial activity of copper oxide nanoparticles prepared by mechanical milling. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 012039. [Google Scholar] [CrossRef]
- Ansell, T.Y.; Hanneman, T.; Gonzalez-Perez, A.; Park, C.; Nieto, A. Effect of high energy ball milling on spherical metallic powder particulates for additive manufacturing. Part. Sci. Technol. 2021, 39, 981–989. [Google Scholar] [CrossRef]
- Han, T.; Li, J.; Zhao, N.; He, C. Microstructure and properties of copper coated graphene nanoplates reinforced Al matrix composites developed by low temperature ball milling. Carbon 2020, 159, 311–323. [Google Scholar] [CrossRef]
- Andrade-Gamboa, J.; Gennari, F.; Larochette, P.A.; Neyertz, C.; Ahlers, M.; Pelegrina, J. Stability of Cu–Zn phases under low energy ball milling. Mater. Sci. Eng. A 2007, 447, 324–331. [Google Scholar] [CrossRef]
- Helle, S.; Pedron, M.; Assouli, B.; Davis, B.; Guay, D.; Roué, L. Structure and high-temperature oxidation behaviour of Cu–Ni–Fe alloys prepared by high-energy ball milling for application as inert anodes in aluminium electrolysis. Corros. Sci. 2010, 52, 3348–3355. [Google Scholar] [CrossRef]
- Dong, L.; Li, L.; Li, X.; Zhang, W.; Fu, Y.; Elmarakbi, A.; Zhang, Y. Enhancing mechanisms of arc-erosion resistance for copper tungsten electrical contact using reduced graphene oxides in situ modified by copper nanoparticles. Int. J. Refract. Met. Hard Mater. 2022, 108, 105934. [Google Scholar] [CrossRef]
- Lu, T.; Chen, C.; Li, P.; Zhang, C.; Han, W.; Zhou, Y.; Suryanarayana, C.; Guo, Z. Enhanced mechanical and electrical properties of in situ synthesized nano-tungsten dispersion-strengthened copper alloy. Mater. Sci. Eng. A 2021, 799, 140161. [Google Scholar] [CrossRef]
- Bettge, M.; Chatterjee, J.; Haik, Y. Physically synthesized Ni-Cu nanoparticles for magnetic hyperthermia. Biomagn. Res. Technol. 2004, 2, 1. [Google Scholar] [CrossRef]
- Saberi, Y.; Oveisi, H. Development of novel cellular copper–aluminum composite materials: The advantage of powder metallurgy and mechanical milling approach for lighter heat exchanger. Mater. Chem. Phys. 2022, 279, 125742. [Google Scholar] [CrossRef]
- Abu-Okail, M.; Shewakh, W.; Brisha, A.M.; Abdelraouf, Y.A.; Abu-Oqail, A. Effect of GNPs content at various compaction pressures and sintering temperatures on the mechanical and electrical properties of hybrid Cu/Al2O3/xGNPs nanocomposites synthesized by high energy ball milling. Ceram. Int. 2020, 46, 18037–18045. [Google Scholar] [CrossRef]
- Abd-Elwahed, M.; Wagih, A.; Najjar, I. Correlation between micro/nano-structure, mechanical and tribological properties of copper-zirconia nanocomposites. Ceram. Int. 2020, 46, 56–65. [Google Scholar] [CrossRef]
- Huang, F.; Wang, H.; Yang, B.; Liao, T.; Wang, Z. Uniformly dispersed Y2O3 nanoparticles in nanocrystalline copper matrix via multi-step ball milling and reduction process. Mater. Lett. 2019, 242, 119–122. [Google Scholar] [CrossRef]
- Tong, W.; Fang, D.; Bao, C.; Tan, S.; Liu, Y.; Li, F.; You, X.; Tao, J.; Bao, R.; Li, C.; et al. Enhancing mechanical properties of copper matrix composite by adding SiO2 quantum dots reinforcement. Vacuum 2022, 195, 110682. [Google Scholar] [CrossRef]
- Alam, S.N.; Singh, H. Development of copper-based metal matrix composites: An analysis by SEM, EDS and XRD. Microsc. Anal. 2014, 28, S8–S13. Available online: https://www.researchgate.net/publication/271707140_metal_matrix_COMPOSITES_S8_Development_of_copper-based_metal_matrix_composites_An_analysis_by_SEM_EDS_and_XRD (accessed on 5 April 2023).
- Calka, A.; Wexler, D.; Monaghan, B.; Mosbah, A.; Balaz, P. Rapid reduction of copper sulfide (Cu2S) with elemental Fe and Mg using electrical discharge assisted mechanical milling (EDAMM). J. Alloys Compd. 2009, 486, 492–496. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Q.; Yang, H.; Shi, D.; Qian, J. Photocatalytic antibacterial properties of copper doped TiO2 prepared by high-energy ball milling. Ceram. Int. 2020, 46, 16716–16724. [Google Scholar] [CrossRef]
- Kalajahi, S.T.; Rasekh, B.; Yazdian, F.; Neshati, J.; Taghavi, L. Green mitigation of microbial corrosion by copper nanoparticles doped carbon quantum dots nanohybrid. Environ. Sci. Pollut. Res. 2020, 27, 40537–40551. [Google Scholar] [CrossRef]
- Bahador, A.; Umeda, J.; Hamzah, E.; Yusof, F.; Li, X.; Kondoh, K. Synergistic strengthening mechanisms of copper matrix composites with TiO2 nanoparticles. Mater. Sci. Eng. A 2020, 772, 138797. [Google Scholar] [CrossRef]
- Ďurišinová, K.; Ďurišin, J.; Orolínová, M.; Ďurišin, M.; Szabó, J. Effect of mechanical milling on nanocrystalline grain stability and properties of Cu–Al2O3 composite prepared by thermo-chemical technique and hot extrusion. J. Alloys Compd. 2015, 618, 204–209. [Google Scholar] [CrossRef]
- Majzoobi, G.; Jafari, S.; Rahmani, K. A study on damage evolution in Cu-TiO2 composite fabricated using powder metallurgy followed by hot extrusion. Mater. Chem. Phys. 2022, 290, 126140. [Google Scholar] [CrossRef]
- Chesnokov, A.E.; Smirnov, A.V.; Batraev, I.S.; Vidyuk, T.M. Detonation Spraying of Copper Pretreated with High-Energy Impacts. J. Appl. Mech. Technol. Phys. 2020, 61, 1042–1047. [Google Scholar] [CrossRef]
- Sadoun, A.M.; Najjar, I.M.R.; Abd-Elwahed, M.S.; Meselhy, A. Experimental study on properties of Al-Al2O3 nanocomposite hybridized by graphene nanosheets. J. Mater. Res. Technol. 2020, 9, 14708–14717. [Google Scholar] [CrossRef]
- Oanh, N.T.H.; Viet, N.H.; Kim, J.-S.; Junior, A.M.J. Characterization of In-Situ Cu-TiH2-C and Cu-Ti-C Nanocomposites Produced by Mechanical Milling and Spark Plasma Sintering. Metals 2017, 7, 117. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Dudina, D.V.; Korchagin, M.A.; Gavrilov, A.I.; Ukhina, A.V.; Bulanova, U.E.; Legan, M.A.; Novoselov, A.N.; Esikov, M.A.; Anisimov, A.G. Manufacturing of TiC-Cu composites by mechanical milling and spark plasma sintering using different carbon sources. Surf. Interfaces 2021, 27, 101445. [Google Scholar] [CrossRef]
- Kumari, R.M.; Sharma, N.; Gupta, N.; Chandra, R.; Nimesh, S. Synthesis and Evolution of Polymeric Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Wang, K. Synthesis and Optical Properties of CuS Nanocrystals by Mechanical Alloying Process. Curr. Nanosci. 2010, 6, 163–168. [Google Scholar] [CrossRef]

| Pretreatment | Precursors | Type of Milling | Milling Conditions | Post-Treatment of the Obtained CuP or CuC | Morphology, Average Size | References |
|---|---|---|---|---|---|---|
| Toluene used as PCA to prevent oxidation and agglomeration | Cu powder (99% purity), initial size of 200 nm | Wet ball mill | 40 h, 250 rpm. Ball size of 5 and 3 mm. Ball weight of 540 g. BPR 8:1 | - | Agglomerates, 21 nm | [34] |
| The milling media cooled with liquid nitrogen | Cu powder (99% purity) | Cryogenic ball mill | Ar atmosphere, 3 h, 150 K. BPR 100:1 | Washing with methanol, ultra-sonication for 15 min | Spherical, 30 nm | [33] |
| The waste Cu chips cleaned through ultrasonication | Cu chips (30 g) obtained from machining areas | Planetary mill | Ar atmosphere, 75 h, 400 rpm | - | Agglomerates, 50 nm | [23] |
| - | Cu powder (99% purity, initial size of 2–5 µm) | HEBM | 4 to 16 h. Al2O3 balls of 1 mm. BPR 1:1 | - | Spherical with some agglomerates, 100 nm | [54] |
| The precursors polished with SiC abrasive and further washed with acetone | Elemental Cu spheres (99% purity), 25 mm in diameter | Vibratory disc mill and planetary mill | Disc mill: 30 s, 1500 rpm. Planetary mill: 50 h, 300 rpm | - | Spherical, 0.25–1 µm | [38] |
| The precursors mixed in sealed quartz tubes under Ar atmosphere in a resistance furnace | Cu powder (99% purity) | CBM | Ar atmosphere, 177 rpm, 0.5 MPa. Rotating cylindrical steel cell: 200 mm/diameter; 29 mm/height. Seven balls of 25 mm, each of 67 g. BPR of 47. | - | Spherical, crystallite size of 5 nm | [57] |
| Stearic acid (0.5 wt.%) added as PCA | Cu powder (99.5% purity). Ni and Fe powders of similar purity added to the mixture to prepare a Cu-Ni-Fe composite | HEBM | Ar atmosphere, 40 h. Hardened steel vial of 55 mL, two balls of 14 and 11 mm, BPR of 2:1. | - | Spherical, 10–30 nm | [58] |
| The precursor powders washed with NaOH and submerged in acetone. Ethanol added as PCA | Cu powder (99% purity, 50 nm). Graphene nanoplatelets and Al2O3 added as reinforcement. | Planetary mill | 2 h, 100 rpm. BPR 8:1 | The obtained composite consolidated at 850 °C, under pressure of 850 MPa | Agglomerates, over 600 nm | [63] |
| - | Cu powder, initial size ranging between 4 and 7 µm | Ball milling | Drum mill, 5 h, 300 rpm. BPR 3:1 | The CuNPs densified and mixed with W particles to generate a CuW composite | Spherical, 100 nm | [59] |
| - | Cu powder and stainless-steel powder of high purity (99%) | HEBM | 2–60 min. 40 g of starting powder. Steel balls of 1.0 g each., BPR: 1:10, 1:5, 2:1, 1:1. | The milled powders cold-mounted and further polished | Agglomerates or flakes, depending on the milling times and BPR | [55] |
| Stearic acid (0.5 wt.%) added as PCA | Cu powder (99% purity, <75 µm average diameter). Graphene was also added to reinforce the Cu matrix | Planetary mill | Ar atmosphere, 4 h, 150 rpm, ZrO2 as grinding media, BPR 10:1 | The consolidation of the powders by hot-pressing techniques | Agglomerates, flattened particles or flakes, depending on the milling time | [46] |
| The precursors physically mixed in a desired composition (71% Ni, 29% Cu w/w) | Cu powder (99% purity). Ni powder of similar purity was added to obtain a Cu-Ni composite | HEBM | 2 h | The mixture placed in an Al2O3 crucible and heated at 1465 °C for 3 h | Spherical, 200 nm | [61] |
| - | CuO and α-Fe2O3 of 99% purity to obtain a CuO-Fe2O3 composite | HEBM | 20 h. Agate balls, BPR of 11:1. Two milling processes under the same conditions were performed | - | Irregular shapes with some agglomerates, 30–50 nm | [52] |
| Stearic acid (2 wt.%) added as PCA | CuMPs (99%, <45 µm) and Al microparticles (99% purity, <45 µm) to develop a Cu-Al composite | Planetary mill | 10 h, 300 rpm. BPR 10:1 | The milled Cu-Al composite was further mixed with PEG, compacted (749 MPa), and sintered at 950 °C | From spherical to flake upon addition of Al to the Cu powder, microporosity | [62] |
| Stearic acid (3 wt.%) added as PCA | Cu powder (99% purity, 40 µm) and ZrO2 (99% purity) were used as raw materials to develop a Cu-ZrO2 composite | HEBM | 250 rpm. Vertical milling machine, BPR 10:1 | The composites mixed with paraffin to reduce the friction during compaction. Cold compaction at 700 MPa and further sintered at 950 °C for 2 h | Spherical large composites and flake-shaped particles | [64] |
| Methanol added as PCA | Cu powder (99% purity, oxygen-free) | Planetary mill | Ar atmosphere. A chamber of 250 mL | The Cu powder reinforced with Al2O3 by electroless Ag plater | Flake-like Cu clusters or Cu grains | [38] |
| Ethanol used as PCA | CuO (99% purity, <20 µm) mixed with Y2O3 particles (50 nm, 99% purity) to obtain Cu-Y2O3 composites | Planetary mill | 1. Ethanol medium, 2 h, 200 rpm, BPR 15:1. 2. Ar atmosphere, 8 h, 250 rpm, BPR 15:1 | The first obtained powder was reduced at 120 °C, the second milled powder was compacted and annealed by spark plasma sintering | Agglomerates, spherical upon second milling under the same conditions as the first | [65] |
| Methanol (0.25 wt. %) used as PCA | Cu particles (99%, average size of 61 µm) | Planetary mill | Ar atmosphere, methanol medium, room temperature, 300 min, 300 rpm. BPR 5:1, | A plating process was applied to obtain a uniform Ag layer on the CuP | Spherical after 60 min of milling; flake-like after 300 min | [47] |
| Stearic acid (1 wt.%) added as PCA | Cu powder (D50 = 10 µm) and WO3 (D50 = 50 nm) to obtain a Cu-W composite | HEBM | Ar atmosphere, 16 h, 650 rpm. BPR 10:1 | The CuC was submitted to hydrogen atmosphere at 800 °C, then consolidated by hot pressing and sintered at 1000 °C for 2 h under 50 MPa | Mostly spheres or small agglomerates, 50–100 nm | [60] |
| The powder precursors annealed at 850 °C for 30 min to remove impurities | Cu powder (99% purity, average size of 40 µm) and Ti powder (98.5%, 15 µm) were used as precursors to obtain a Cu-Ti composite | HEBM | Ar atmosphere, stainless-steel balls of 8 mm, acceleration of 400 m/s2. BPR 18:1. | The CuC were sintered using spark plasma to obtain a Cu-Ti-C alloy. Graphite, nanodiamonds, and carbon black tested as carbon sources | Agglomerates, with black dots in the structure that represent the C source | [67] |
| The ball milling process modeled through discrete element model (DEM) simulations | Cu powder (99% purity, average size of 45 µm) | Planetary mill | 48 h. CEBM (10, 50, 100 rpm) HEBM (300, 500, 700 rpm). Balls of 1 and 10 mm in diameter. BPR 10:1 | - | The morphology changed from spherical to flake-like CuP as the milling time increased. The average size decreased upon increasing the rotation speeds (27 to 9 nm). | [35] |
| Pretreatment | Precursors | Type of Milling | Milling Conditions | Post-Treatment of CuP or CuC | Morphology, Average Size | References |
|---|---|---|---|---|---|---|
| - | CuSO4 NaBH4/NaOH/reducing medium, EDTA was added as PCA | Planetary mill | 3 h, 250 rpm. BPR 10:1 | The CuNPs were mixed with CNTs, then dried at 60 °C for 2 h under vacuum | Spherical, 20–50 nm as CuNPs. Agglomerated as Cu-CNTs composites | [43] |
| The precursor was decomposed under inert atmosphere | Cu(HCOO)2 | Ultrafine wet mill | 240 min | The CuNPs milled in dipropylene glycol monomethyl ether (DMP) and dispersed in oleic acid | Spherical, 200–500 nm. Flakes, 2–5 µm | [42] |
| Dichlorobenzene added as PCA | Cu(COO)2 | Vibratory disc mill | 60 min. 45 g of 0.5 mm and 1 mm Zr balls. | The Cu flakes were sieved and further separated with toluene and centrifugation | Flakes and agglomerates, 100 nm | [36] |
| Quantum dots (QDs) were added to the copper solution, sonicated, atomized, and sintered | Cu(COO)2 | Planetary mill | First ball milling (low): 6 h, 150 rpm. Stainless-steel jar. Second ball milling (high): 1 h, 300 rpm. BPR 10:1 | The obtained QDs-Cu composite reduced at 300 °C for 5 h under Ar atmosphere | Spherical, 4 nm | [70] |
| Stearic acid added as PCA | Cu(NO3)2, glucose/reducing agent. Graphene added to form a reinforced Cu-GNPs matrix | Cryogenic planetary mill | 4 h, 500 rpm, 193 K Steel milling balls of 6 and 10 mm, BPR 10:1 | The mixed powders were cold-compacted, sintered in a tubular furnace, and further heated and extruded at 823 K | Cu-GNPs bars, 5 mm diameter | [13] |
| - | CuSO4 Formaldehyde/reducing agent. Al2O3 and GNPs added to obtain a Cu-Al2O3-GNPs composite | HEBM | 20 h, 300 rpm. Al2O3 balls of 10 mm, BPR 50:1 | The composite was compacted at 1200 MPa and further sintered at 1000 °C for 2 h | Spherical CuNPs, 50 nm. CuC, 700 nm | [53] |
| The precursors CuS and Cu2S were co-milled in a stochiometric ratio | CuS and Cu2S, Fe added as a reducing agent | Planetary mill | 15–480 min, powder charge of 5 g, 250 mL tungsten carbide grinding chamber, 50 balls of 10 mm diameter, 500 rpm. Ar atmosphere | - | Grains, below 5 µm | [25] |
| NaOH or NaCl used as diluent phases | CuSO4 and CuCl2 | HEBM | Air atmosphere, 1 h, 300 rpm. Stainless-steel balls of 10 and 7 mm. BPR 10:1 | The obtained Cu powders were dispersed in an ultrasonic bath, washed with distilled water, centrifuged, and dried at 60 °C | CuONP synthesized with CuSO4: 14 nm; with CuCl2: 7 nm. | [12] |
| The precursors were mechanically pre-mixed in a conventional ball mill | Cu2S. Fe and Mg as reducing agents | Electric discharge assisted ball milling | 5 min, Ar atmosphere. During milling, pulsed discharges travelled through the milling atmosphere and the precursor powders | - | Agglomerates, 5–100 µm | [68] |
| The precursors were mixed, and ethanol was added as PCA | CuSO4 (99%) and TiO2 (98%) were used to obtain CuNPs doped with TiO2 | HEBM | 10 h, 450 rpm, Zr balls of 3, 5, and 10 mm, BPR 10:1 | The CuC was cooled, then dried, ultrasonicated, and ground | Average size of 65 nm. The average size of the Cu-Ti composite was proportional to BPR. | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval, S.S.; Silva, N. Review on Generation and Characterization of Copper Particles and Copper Composites Prepared by Mechanical Milling on a Lab-Scale. Int. J. Mol. Sci. 2023, 24, 7933. https://doi.org/10.3390/ijms24097933
Sandoval SS, Silva N. Review on Generation and Characterization of Copper Particles and Copper Composites Prepared by Mechanical Milling on a Lab-Scale. International Journal of Molecular Sciences. 2023; 24(9):7933. https://doi.org/10.3390/ijms24097933
Chicago/Turabian StyleSandoval, Sebastián Salazar, and Nataly Silva. 2023. "Review on Generation and Characterization of Copper Particles and Copper Composites Prepared by Mechanical Milling on a Lab-Scale" International Journal of Molecular Sciences 24, no. 9: 7933. https://doi.org/10.3390/ijms24097933
APA StyleSandoval, S. S., & Silva, N. (2023). Review on Generation and Characterization of Copper Particles and Copper Composites Prepared by Mechanical Milling on a Lab-Scale. International Journal of Molecular Sciences, 24(9), 7933. https://doi.org/10.3390/ijms24097933






