Topical Alginate Protection against Pepsin-Mediated Esophageal Damage: E-Cadherin Proteolysis and Matrix Metalloproteinase Induction
Abstract
1. Introduction
2. Results
2.1. Cell Viability
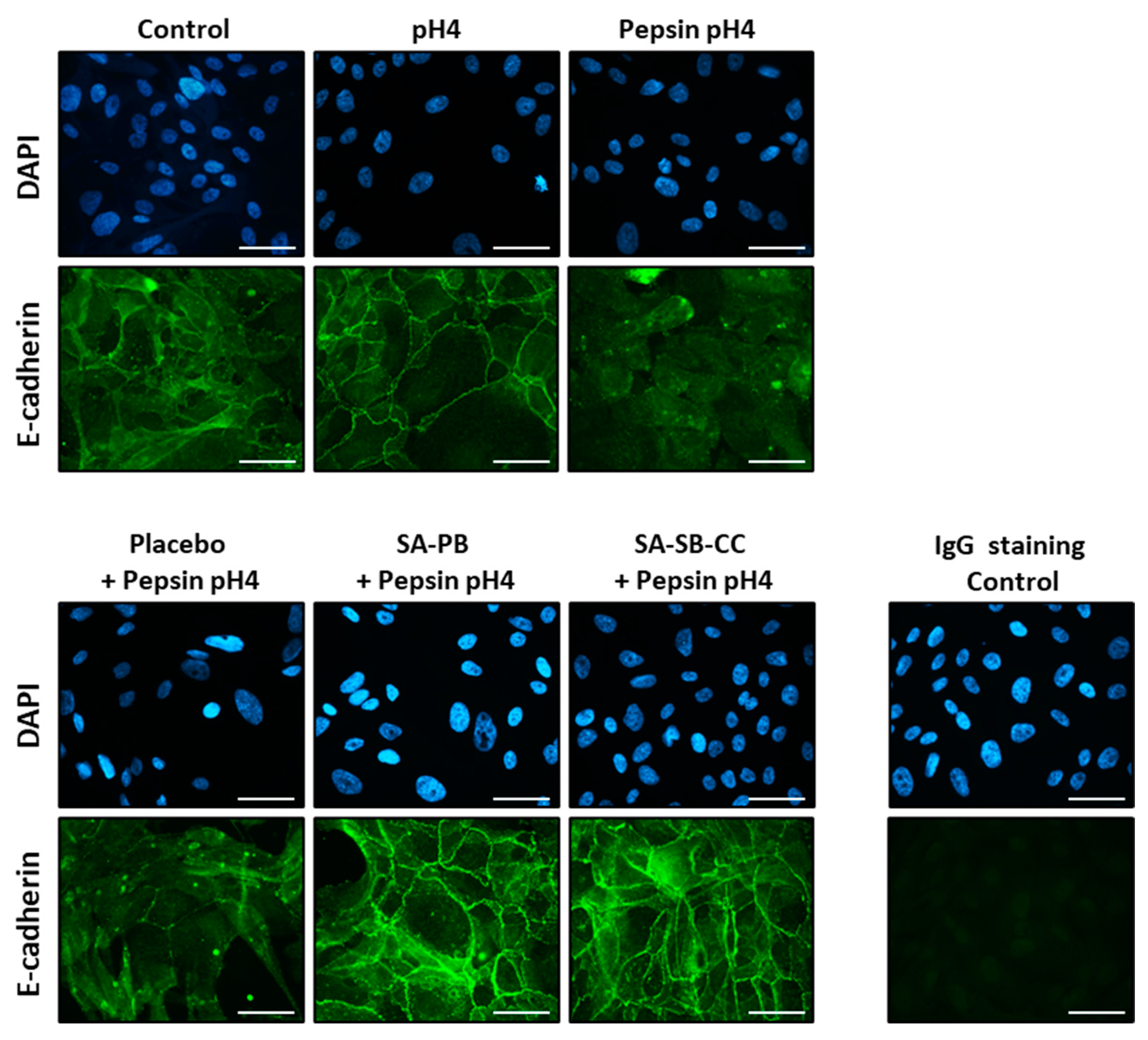
2.2. E-Cadherin and ADAM-10 Cleavage
2.3. MMP Expression
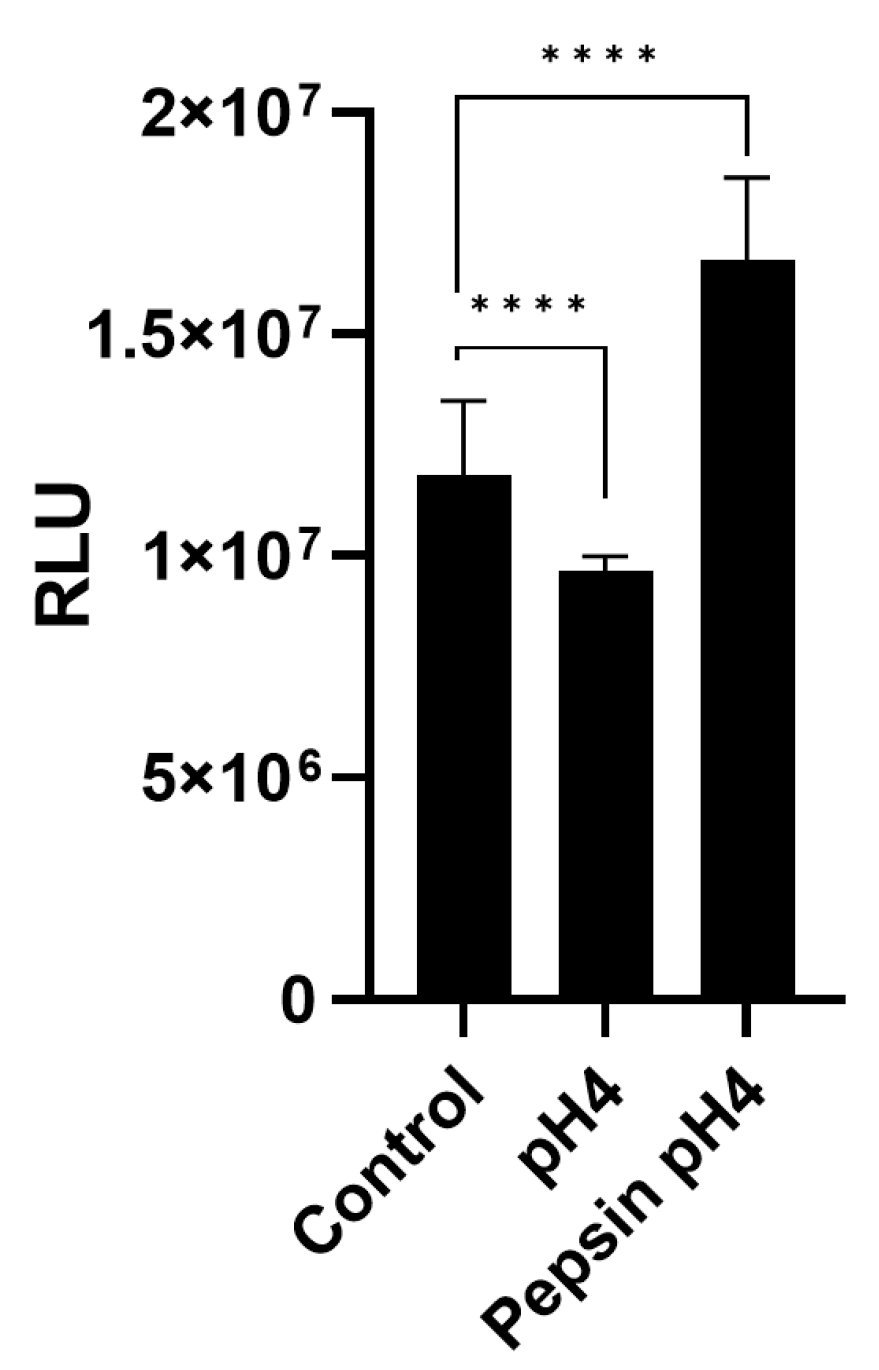
2.4. Proliferation Assay
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. ATP Assay
4.3. Western Blot
4.4. Immunofluoresence
4.5. Real Time qPCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the Epidemiology of Gastro-Oesophageal Reflux Disease: A Systematic Review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef][Green Version]
- Richter, J.E.; Rubenstein, J.H. Presentation and Epidemiology of Gastroesophageal Reflux disease. Gastroenterology 2018, 154, 267–276. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Ratnakumaran, R.; Yuan, Y.; Solaymani-Dodaran, M.; Bazzoli, F.; Ford, A.C. Global Prevalence of, and Risk Factors for, Gastro-Oesophageal Reflux Symptoms: A Meta-Analysis. Gut 2018, 67, 430–440. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.U.; Conway, B.R.; Ghori, M.U. Global Prevalence and Risk Factors of Gastro-Oesophageal Reflux Disease (Gord): Systematic Review with Meta-Analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11. [Google Scholar] [CrossRef][Green Version]
- Wahlqvist, P.; Reilly, M.C.; Barkun, A. Systematic Review: The Impact of Gastro-Oesophageal Reflux Disease on Work Productivity. Aliment. Pharmacol. Ther. 2006, 24, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Hayeck, T.J.; Kong, C.Y.; Spechler, S.J.; Gazelle, G.S.; Hur, C. The Prevalence of Barrett’s Esophagus in the Us: Estimates from a Simulation Model Confirmed by Seer Data. Dis. Esophagus 2010, 23, 451–457. [Google Scholar] [CrossRef][Green Version]
- Eusebi, L.H.; Cirota, G.G.; Zagari, R.M.; Ford, A.C. Global Prevalence of Barrett’s Oesophagus and Oesophageal Cancer in Individuals with Gastro-Oesophageal Reflux: A Systematic Review and Meta-Analysis. Gut 2021, 70, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.G.; Xie, S.H.; Lagergren, J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018, 154, 390–405. [Google Scholar] [CrossRef]
- Njei, B.; McCarty, T.R.; Birk, J.W. Trends in Esophageal Cancer Survival in United States Adults from 1973 to 2009: A Seer Database Analysis. J. Gastroenterol. Hepatol. 2016, 31, 1141–1146. [Google Scholar] [CrossRef][Green Version]
- Bustillos, H.; Leer, K.; Kitten, A.; Reveles, K.R. A Cross-Sectional Study of National Outpatient Gastric Acid Suppressant Prescribing in the United States between 2009 and 2015. PLoS ONE 2018, 13, e0208461. [Google Scholar] [CrossRef][Green Version]
- Torres-Bondia, F.; de Batlle, J.; Galván, L.; Buti, M.; Barbé, F.; Piñol-Ripoll, G. Evolution of the Consumption Trend of Proton Pump Inhibitors in the Lleida Health Region between 2002 and 2015. BMC Public Health 2022, 22, 818. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, M.; Le Tri, T.; Bardou, M.; Biour, M.; Kirchgesner, J.; Rouby, F.; Dumarcet, N.; Zureik, M.; Dray-Spira, R. Use of Proton Pump Inhibitors in Adults in France: A Nationwide Drug Utilization Study. Eur. J. Clin. Pharmacol. 2020, 76, 449–457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muheim, L.; Signorell, A.; Markun, S.; Chmiel, C.; Neuner-Jehle, S.; Blozik, E.; Ursprung, P.; Rosemann, T.; Senn, O. Potentially Inappropriate Proton-Pump Inhibitor Prescription in the General Population: A Claims-Based Retrospective Time Trend Analysis. Ther. Adv. Gastroenterol. 2021, 14, 1756284821998928. [Google Scholar] [CrossRef]
- Othman, F.; Card, T.R.; Crooks, C.J. Proton Pump Inhibitor Prescribing Patterns in the UK: A Primary Care Database Study. Pharmacoepidemiol. Drug. Saf. 2016, 25, 1079–1087. [Google Scholar] [CrossRef]
- Savarino, V.; Dulbecco, P.; de Bortoli, N.; Ottonello, A.; Savarino, E. The Appropriate Use of Proton Pump Inhibitors (Ppis): Need for a Reappraisal. Eur. J. Intern. Med. 2017, 37, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.H.; Henderson, E.E.; Maradey-Romerao, C.; George, N.; Fass, R.; Lacy, B.E. The Proton Pump Inhibitor Non-Responder: A Clinical Conundrum. Clin. Transl. Gastroenterol. 2015, 6, e106. [Google Scholar] [CrossRef]
- Delshad, S.D.; Almario, C.V.; Chey, W.D.; Spiegel, B.M.R. Prevalence of Gastroesophageal Reflux Disease and Proton Pump Inhibitor-Refractory Symptoms. Gastroenterology 2020, 158, 1250–1261.e2. [Google Scholar] [CrossRef]
- Mainie, I.; Tutuian, R.; Shay, S.; Vela, M.; Zhang, X.; Sifrim, D.; Castell, D.O. Acid and Non-Acid Reflux in Patients with Persistent Symptoms Despite Acid Suppressive Therapy: A Multicentre Study Using Combined Ambulatory Impedance-Ph Monitoring. Gut 2006, 55, 1398–1402. [Google Scholar] [CrossRef][Green Version]
- Hu, Q.; Sun, T.T.; Hong, J.; Fang, J.Y.; Xiong, H.; Meltzer, S.J. Proton Pump Inhibitors Do Not Reduce the Risk of Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169691. [Google Scholar] [CrossRef][Green Version]
- Hvid-Jensen, F.; Pedersen, L.; Funch-Jensen, P.; Drewes, A.M. Proton Pump Inhibitor Use May Not Prevent High-Grade Dysplasia and Oesophageal Adenocarcinoma in Barrett’s Oesophagus: A Nationwide Study of 9883 Patients. Aliment. Pharmacol. Ther. 2014, 39, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Engstrand, L.; Lagergren, J. Maintenance Proton Pump Inhibition Therapy and Risk of Oesophageal Cancer. Cancer Epidemiol. 2018, 53, 172–177. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, H.K.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Kim, N.Y.; Cho, S.J.; Nam, E.S.; Min, K.W.; et al. Possible Association between the Use of Proton Pump Inhibitors and H(2) Receptor Antagonists, and Esophageal Cancer: A Nested Case-Control Study Using a Korean National Health Screening Cohort. Pharmaceuticals 2022, 15, 517. [Google Scholar] [CrossRef] [PubMed]
- Vela, M.F.; Camacho-Lobato, L.; Srinivasan, R.; Tutuian, R.; Katz, P.O.; Castell, D.O. Simultaneous Intraesophageal Impedance and Ph Measurement of Acid and Nonacid Gastroesophageal Reflux: Effect of Omeprazole. Gastroenterology 2001, 120, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Ten Kate, R.W.; Tuynman, H.A.; Festen, H.P.; Pals, G.; Meuwissen, S.G. Effect of High Dose Omeprazole on Gastric Pepsin Secretion and Serum Pepsinogen Levels in Man. Eur. J. Clin. Pharmacol. 1988, 35, 173–176. [Google Scholar] [CrossRef]
- Alsalahi, O.; Dobrian, A.D. Proton Pump Inhibitors: The Culprit for Barrett’s Esophagus? Front. Oncol. 2014, 4, 373. [Google Scholar] [CrossRef]
- Hurley, B.P.; Jugo, R.H.; Snow, R.F.; Samuels, T.L.; Yonker, L.M.; Mou, H.; Johnston, N.; Rosen, R. Pepsin Triggers Neutrophil Migration across Acid Damaged Lung Epithelium. Sci. Rep. 2019, 9, 13778. [Google Scholar] [CrossRef][Green Version]
- Yibirin, M.; De Oliveira, D.; Valera, R.; Plitt, A.E.; Lutgen, S. Adverse Effects Associated with Proton Pump Inhibitor Use. Cureus 2021, 13, e12759. [Google Scholar] [CrossRef]
- Mandel, K.G.; Daggy, B.P.; Brodie, D.A.; Jacoby, H.I. Review Article: Alginate-Raft Formulations in the Treatment of Heartburn and Acid Reflux. Aliment. Pharmacol. Ther. 2000, 14, 669–690. [Google Scholar] [CrossRef]
- Leiman, D.A.; Riff, B.P.; Morgan, S.; Metz, D.C.; Falk, G.W.; French, B.; Umscheid, C.A.; Lewis, J.D. Alginate Therapy Is Effective Treatment for Gerd Symptoms: A Systematic Review and Meta-Analysis. Dis. Esophagus 2017, 30, 1–9. [Google Scholar] [CrossRef][Green Version]
- Oderda, G.; Dell’Olio, D.; Forni, M.; Farina, L.; Tavassoli, K.; Ansaldi, N. Treatment of Childhood Peptic Oesophagitis with Famotidine or Alginate-Antacid. Ital. J. Gastroenterol. 1990, 22, 346–349. [Google Scholar]
- Poynard, T. Relapse Rate of Patients after Healing of Oesophagitis—A Prospective Study of Alginate as Self-Care Treatment for 6 Months. French Co-Operative Study Group. Aliment. Pharmacol. Ther. 1993, 7, 385–392. [Google Scholar] [CrossRef]
- Sandmark, S.; Zenk, L. Treatment with Gaviscon in Hiatus Hernia. Preliminary Results. Sven. Lakartidn. 1964, 61, 1940–1943. [Google Scholar] [PubMed]
- Strugala, V.; Avis, J.; Jolliffe, I.G.; Johnstone, L.M.; Dettmar, P.W. The Role of an Alginate Suspension on Pepsin and Bile Acids—Key Aggressors in the Gastric Refluxate. Does This Have Implications for the Treatment of Gastro-Oesophageal Reflux Disease? J. Pharm. Pharmacol. 2009, 61, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Zentilin, P.; Dulbecco, P.; Savarino, E.; Parodi, A.; Iiritano, E.; Bilardi, C.; Reglioni, S.; Vigneri, S.; Savarino, V. An Evaluation of the Antireflux Properties of Sodium Alginate by Means of Combined Multichannel Intraluminal Impedance and Ph-Metry. Aliment. Pharmacol. Ther. 2005, 21, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rohof, W.O.; Bennink, R.J.; Smout, A.J.; Thomas, E.; Boeckxstaens, G.E. An Alginate-Antacid Formulation Localizes to the Acid Pocket to Reduce Acid Reflux in Patients with Gastroesophageal Reflux Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 1585–1591; quiz e1590. [Google Scholar] [CrossRef]
- Chater, P.I.; Wilcox, M.D.; Brownlee, I.A.; Pearson, J.P. Alginate as a Protease Inhibitor In Vitro and in a Model Gut System; Selective Inhibition of Pepsin but Not Trypsin. Carbohydr. Polym. 2015, 131, 142–151. [Google Scholar] [CrossRef][Green Version]
- Woodland, P.; Batista-Lima, F.; Lee, C.; Preston, S.L.; Dettmar, P.; Sifrim, D. Topical Protection of Human Esophageal Mucosal Integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G975–G980. [Google Scholar] [CrossRef][Green Version]
- Woodland, P.; Lee, C.; Duraisamy, Y.; Farré, R.; Dettmar, P.; Sifrim, D. Assessment and Protection of Esophageal Mucosal Integrity in Patients with Heartburn without Esophagitis. Am. J. Gastroenterol. 2013, 108, 535–543. [Google Scholar] [CrossRef]
- Samuels, T.L.; Yan, K.; Patel, N.; Plehhova, K.; Coyle, C.; Hurley, B.P.; Johnston, N. Alginates for Protection against Pepsin-Acid Induced Aerodigestive Epithelial Barrier Disruption. Laryngoscope 2022, 132, 2327–2334. [Google Scholar] [CrossRef]
- Ustaoglu, A.; Nguyen, A.; Spechler, S.; Sifrim, D.; Souza, R.; Woodland, P. Mucosal Pathogenesis in Gastro-Esophageal Reflux Disease. Neurogastroenterol. Motil. 2020, 32, e14022. [Google Scholar] [CrossRef]
- Orlando, R.C. The Integrity of the Esophageal Mucosa. Balance between Offensive and Defensive Mechanisms. Best. Pract. Res. Clin. Gastroenterol. 2010, 24, 873–882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldberg, H.I.; Dodds, W.J.; Gee, S.; Montgomery, C.; Zboralske, F.F. Role of Acid and Pepsin in Acute Experimental Esophagitis. Gastroenterology 1969, 56, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.F.; Harmon, J.W. Experimental Esophagitis in a Rabbit Model. Clinical Relevance. J. Clin. Gastroenterol. 1986, 8 (Suppl. S1), 26–44. [Google Scholar] [CrossRef]
- Tobey, N.A.; Hosseini, S.S.; Caymaz-Bor, C.; Wyatt, H.R.; Orlando, G.S.; Orlando, R.C. The Role of Pepsin in Acid Injury to Esophageal Epithelium. Am. J. Gastroenterol. 2001, 96, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Bortolotti, M.; Fabbri, A.; Areni, A.; Cenacchi, G.; Scialpi, C.; Miglioli, M.; Di Febo, G. Reversibility of Gerd Ultrastructural Alterations and Relief of Symptoms after Omeprazole Treatment. Am. J. Gastroenterol. 2005, 100, 537–542. [Google Scholar] [CrossRef]
- Jovov, B.; Que, J.; Tobey, N.A.; Djukic, Z.; Hogan, B.L.; Orlando, R.C. Role of E-Cadherin in the Pathogenesis of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2011, 106, 1039–1047. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Montiel-Jarquín, Á.J.; Lara-Cisneros, L.G.V.; López-Colombo, A.; Solís-Mendoza, H.A.; Palmer-Márquez, M.L.; Romero-Figueroa, M.S. Expression of Metalloproteinase-9 in Patients with Mild and Severe Forms of Gastroesophageal Reflux Disease. Cir. Cir. 2019, 87, 436–442. [Google Scholar] [CrossRef][Green Version]
- Davelaar, A.L.; Straub, D.; Buttar, N.S.; Fockens, P.; Krishnadath, K.K. Active Matrix Metalloproteases Are Expressed Early on and Are High during the Barrett’s Esophagus Malignancy Sequence. Scand. J. Gastroenterol. 2015, 50, 321–332. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Zhai, R.; Bradbury, P.; Hopkins, J.; Kulke, M.H.; Heist, R.S.; Asomaning, K.; Ma, C.; Xu, W.; Wang, Z.; et al. Single Nucleotide Polymorphisms in the Matrix Metalloproteinase Gene Family and the Frequency and Duration of Gastroesophageal Reflux Disease Influence the Risk of Esophageal Adenocarcinoma. Int. J. Cancer 2012, 131, 2478–2486. [Google Scholar] [CrossRef][Green Version]
- Grimm, M.; Lazariotou, M.; Kircher, S.; Stuermer, L.; Reiber, C.; Höfelmayr, A.; Gattenlöhner, S.; Otto, C.; Germer, C.T.; von Rahden, B.H. Mmp-1 Is a (Pre-)Invasive Factor in Barrett-Associated Esophageal Adenocarcinomas and Is Associated with Positive Lymph Node Status. J. Transl. Med. 2010, 8, 99. [Google Scholar] [CrossRef][Green Version]
- Nawrocki-Raby, B.; Gilles, C.; Polette, M.; Bruyneel, E.; Laronze, J.Y.; Bonnet, N.; Foidart, J.M.; Mareel, M.; Birembaut, P. Upregulation of Mmps by Soluble E-Cadherin in Human Lung Tumor Cells. Int. J. Cancer 2003, 105, 790–795. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef][Green Version]
- Sonmez, S.; Coyle, C.; Sifrim, D.; Woodland, P. Duration of Adhesion of Swallowed Alginates to Distal Oesophageal Mucosa: Implications for Topical Therapy of Oesophageal Diseases. Aliment. Pharmacol. Ther. 2020, 52, 442–448. [Google Scholar] [CrossRef]
- Crouch, S.P.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The Use of Atp Bioluminescence as a Measure of Cell Proliferation and Cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar] [CrossRef]
- Russell, C.O.; Hill, L.D.; Holmes, E.R., 3rd; Hull, D.A.; Gannon, R.; Pope, C.E., 2nd. Radionuclide Transit: A Sensitive Screening Test for Esophageal Dysfunction. Gastroenterology 1981, 80, 887–892. [Google Scholar] [CrossRef]
- Smart, J.D.; Dunkley, S.; Tsibouklis, J.; Young, S. An Evaluation of the Adhesion of Solid Oral Dosage Form Coatings to the Oesophagus. Int. J. Pharm. 2015, 496, 299–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, N.; Chen, X.B.; Schreyer, D.J. Influence of Calcium Ions on Cell Survival and Proliferation in the Context of an Alginate Hydrogel. ISRN Chem. Eng. 2012, 2012, 516461. [Google Scholar] [CrossRef][Green Version]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, Physicochemical and Antioxidant Properties of Sodium Alginate Isolated from a Tunisian Brown Seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Heinze, T.; Hipler, U.C. Comparative In Vitro Study on Cytotoxicity, Antimicrobial Activity, and Binding Capacity for Pathophysiological Factors in Chronic Wounds of Alginate and Silver-Containing Alginate. Wound Repair. Regen. 2009, 17, 511–521. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef][Green Version]
- Strugala, V.; Kennington, E.J.; Campbell, R.J.; Skjåk-Braek, G.; Dettmar, P.W. Inhibition of Pepsin Activity by Alginates In Vitro and the Effect of Epimerization. Int. J. Pharm. 2005, 304, 40–50. [Google Scholar] [CrossRef]
- Strugala, V.; Dettmar, P.W.; Thomas, E.C. Evaluation of an Innovative over-the-Counter Treatment for Symptoms of Reflux Disease: Quick-Dissolving Alginate Granules. ISRN Pharm. 2012, 2012, 950162. [Google Scholar] [CrossRef]
- Thangarajah, H.; Wong, A.; Chow, D.C.; Crothers, J.M., Jr.; Forte, J.G. Gastric H-K-Atpase and Acid-Resistant Surface Proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G953–G961. [Google Scholar] [CrossRef][Green Version]
- Lanas, A.I.; Blas, J.M.; Ortego, J.; Soria, J.; Sáinz, R. Adaptation of Esophageal Mucosa to Acid- and Pepsin-Induced Damage: Role of Nitric Oxide and Epidermal Growth Factor. Dig. Dis. Sci. 1997, 42, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Hirschowitz, B. Pepsin in the Pathogenesis of Peptic Ulceration. In Mechanisms of Peptic Ulcer Healing; Halter, F., Garner, A., Tytgat, G., Eds.; Kluwer Academic Publishers: Dordrect, The Netherlands, 1990; pp. 183–194. [Google Scholar]
- Niessen, C.M. Tight Junctions/Adherens Junctions: Basic Structure and Function. J. Invest. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qian, X.; Karpova, T.; Sheppard, A.M.; McNally, J.; Lowy, D.R. E-Cadherin-Mediated Adhesion Inhibits Ligand-Dependent Activation of Diverse Receptor Tyrosine Kinases. EMBO J. 2004, 23, 1739–1748. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lal, M.; Caplan, M. Regulated Intramembrane Proteolysis: Signaling Pathways and Biological Functions. Physiology 2011, 26, 34–44. [Google Scholar] [CrossRef][Green Version]
- David, J.M.; Rajasekaran, A.K. Dishonorable Discharge: The Oncogenic Roles of Cleaved E-Cadherin Fragments. Cancer Res. 2012, 72, 2917–2923. [Google Scholar] [CrossRef][Green Version]
- Hu, Q.P.; Kuang, J.Y.; Yang, Q.K.; Bian, X.W.; Yu, S.C. Beyond a Tumor Suppressor: Soluble E-Cadherin Promotes the Progression of Cancer. Int. J. Cancer 2016, 138, 2804–2812. [Google Scholar] [CrossRef][Green Version]
- Ferber, E.C.; Kajita, M.; Wadlow, A.; Tobiansky, L.; Niessen, C.; Ariga, H.; Daniel, J.; Fujita, Y. A Role for the Cleaved Cytoplasmic Domain of E-Cadherin in the Nucleus. J. Biol. Chem. 2008, 283, 12691–12700. [Google Scholar] [CrossRef][Green Version]
- Charalabopoulos, A.; Golias, C. E-Cadherin Expression in Barrett’s Esophagus and Esophageal Carcinoma. Esophagus 2014, 11, 153–161. [Google Scholar] [CrossRef]
- Samuels, T.L.; Altman, K.W.; Gould, J.C.; Kindel, T.; Bosler, M.; MacKinnon, A.; Hagen, C.E.; Johnston, N. Esophageal Pepsin and Proton Pump Synthesis in Barrett’s Esophagus and Esophageal Adenocarcinoma. Laryngoscope 2019, 129, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Wang, L.; Mo, T.T.; Wang, J.; Wang, M.G.; Li, X.P. Pepsin Promotes Il-8 Signaling-Induced Epithelial-Mesenchymal Transition in Laryngeal Carcinoma. Cancer Cell. Int. 2019, 19, 64. [Google Scholar] [CrossRef][Green Version]
- Gill, G.A.; Johnston, N.; Buda, A.; Pignatelli, M.; Pearson, J.; Dettmar, P.W.; Koufman, J. Laryngeal Epithelial Defenses against Laryngopharyngeal Reflux: Investigations of E-Cadherin, Carbonic Anhydrase Isoenzyme Iii, and Pepsin. Ann. Otol. Rhinol. Laryngol. 2005, 114, 913–921. [Google Scholar] [CrossRef]
- Yuksel, H.; Ocalan, M.; Yilmaz, O. E-Cadherin: An Important Functional Molecule at Respiratory Barrier between Defence and Dysfunction. Front. Physiol. 2021, 12, 720227. [Google Scholar] [CrossRef]
- Maretzky, T.; Reiss, K.; Ludwig, A.; Buchholz, J.; Scholz, F.; Proksch, E.; de Strooper, B.; Hartmann, D.; Saftig, P. Adam10 Mediates E-Cadherin Shedding and Regulates Epithelial Cell-Cell Adhesion, Migration, and Beta-Catenin Translocation. Proc. Natl. Acad. Sci. USA 2005, 102, 9182–9187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bleibaum, F.; Sommer, A.; Veit, M.; Rabe, B.; Andrä, J.; Kunzelmann, K.; Nehls, C.; Correa, W.; Gutsmann, T.; Grötzinger, J.; et al. Adam10 Sheddase Activation Is Controlled by Cell Membrane Asymmetry. J. Mol. Cell. Biol. 2019, 11, 979–993. [Google Scholar] [CrossRef][Green Version]
- Tousseyn, T.; Thathiah, A.; Jorissen, E.; Raemaekers, T.; Konietzko, U.; Reiss, K.; Maes, E.; Snellinx, A.; Serneels, L.; Nyabi, O.; et al. Adam10, the Rate-Limiting Protease of Regulated Intramembrane Proteolysis of Notch and Other Proteins, Is Processed by Adams-9, Adams-15, and the Gamma-Secretase. J. Biol. Chem. 2009, 284, 11738–11747. [Google Scholar] [CrossRef][Green Version]
- Endsley, M.A.; Somasunderam, A.D.; Li, G.; Oezguen, N.; Thiviyanathan, V.; Murray, J.L.; Rubin, D.H.; Hodge, T.W.; O’Brien, W.A.; Lewis, B.; et al. Nuclear Trafficking of the Hiv-1 Pre-Integration Complex Depends on the Adam10 Intracellular Domain. Virology 2014, 454–455, 60–66. [Google Scholar] [CrossRef]
- Carey, R.M.; Blusztajn, J.K.; Slack, B.E. Surface Expression and Limited Proteolysis of Adam10 Are Increased by a Dominant Negative Inhibitor of Dynamin. BMC Cell. Biol. 2011, 12, 20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kauttu, T.; Mustonen, H.; Vainionpää, S.; Krogerus, L.; Ilonen, I.; Räsänen, J.; Salo, J.; Puolakkainen, P. Disintegrin and Metalloproteinases (Adams) Expression in Gastroesophageal Reflux Disease and in Esophageal Adenocarcinoma. Clin. Transl. Oncol. 2017, 19, 58–66. [Google Scholar] [CrossRef][Green Version]
- Kim, B.; Lee, H.J.; Im, N.R.; Lee, D.Y.; Kang, C.Y.; Park, I.H.; Lee, S.H.; Lee, S.H.; Baek, S.K.; Kim, T.H. Effect of Matrix Metalloproteinase Inhibitor on Disrupted E-Cadherin after Acid Exposure in the Human Nasal Epithelium. Laryngoscope 2018, 128, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Im, N.R.; Lee, D.Y.; Kim, B.; Kim, J.; Jung, K.Y.; Kim, T.H.; Baek, S.K. Role of Matrix Metalloproteinases 7 in the Pathogenesis of Laryngopharyngeal Reflux: Decreased E-Cadherin in Acid Exposed Primary Human Pharyngeal Epithelial Cells. Int. J. Mol. Sci. 2019, 20, 5276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Im, N.R.; Kim, B.; Jung, K.Y.; Baek, S.K. Matrix Metalloproteinase-7 Induces E-Cadherin Cleavage in Acid-Exposed Primary Human Pharyngeal Epithelial Cells via the Ros/Erk/C-Jun Pathway. J. Mol. Med. 2022, 100, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; van Malenstein, H.; De Vos, R.; Geboes, K.; Depoortere, I.; Vanden Berghe, P.; Fornari, F.; Blondeau, K.; Mertens, V.; Tack, J.; et al. Short Exposure of Oesophageal Mucosa to Bile Acids, Both in Acidic and Weakly Acidic Conditions, Can Impair Mucosal Integrity and Provoke Dilated Intercellular Spaces. Gut 2008, 57, 1366–1374. [Google Scholar] [CrossRef]
- Schmidt, T.P.; Perna, A.M.; Fugmann, T.; Böhm, M.; Jan, H.; Haller, S.; Götz, C.; Tegtmeyer, N.; Hoy, B.; Rau, T.T.; et al. Identification of E-Cadherin Signature Motifs Functioning as Cleavage Sites for Helicobacter Pylori Htra. Sci. Rep. 2016, 6, 23264. [Google Scholar] [CrossRef][Green Version]
- Samuels, T.L.; Zimmermann, M.T.; Zeighami, A.; Demos, W.; Southwood, J.E.; Blumin, J.H.; Bock, J.M.; Johnston, N. RNA Sequencing Reveals Cancer-Associated Changes in Laryngeal Cells Exposed to Non-Acid Pepsin. Laryngoscope 2021, 131, 121–129. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Martins de Brito, O.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; et al. Opa1 Controls Apoptotic Cristae Remodeling Independently from Mitochondrial Fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef][Green Version]
- Stabenau, K.A.; Samuels, T.L.; Lam, T.K.; Mathison, A.J.; Wells, C.; Altman, K.W.; Battle, M.A.; Johnston, N. Pepsinogen/Proton Pump Co-Expression in Barrett’s Esophageal Cells Induces Cancer-Associated Changes. Laryngoscope 2022, 133, 59–69. [Google Scholar] [CrossRef]
- Johnston, N.; Yan, J.C.; Hoekzema, C.R.; Samuels, T.L.; Stoner, G.D.; Blumin, J.H.; Bock, J.M. Pepsin Promotes Proliferation of Laryngeal and Pharyngeal Epithelial Cells. Laryngoscope 2012, 122, 1317–1325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelly, E.A.; Samuels, T.L.; Johnston, N. Chronic Pepsin Exposure Promotes Anchorage-Independent Growth and Migration of a Hypopharyngeal Squamous Cell Line. Otolaryngol. Head. Neck Surg. 2014, 150, 618–624. [Google Scholar] [CrossRef][Green Version]
- Le Bras, G.F.; Allison, G.L.; Richards, N.F.; Ansari, S.S.; Washington, M.K.; Andl, C.D. Cd44 Upregulation in E-Cadherin-Negative Esophageal Cancers Results in Cell Invasion. PLoS ONE 2011, 6, e27063. [Google Scholar] [CrossRef][Green Version]
- Sun, X.; Martin, R.C.G.; Zheng, Q.; Farmer, R.; Pandit, H.; Li, X.; Jacob, K.; Suo, J.; Li, Y. Drug-Induced Expression of Epcam Contributes to Therapy Resistance in Esophageal Adenocarcinoma. Cell. Oncol. 2018, 41, 651–662. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Chen, J.; Capobianco, A.J. The Notch Signaling Pathway in Esophageal Adenocarcinoma. Cell. Mol. Biol. 2015, 61, 24–32. [Google Scholar] [PubMed]
- Niu, K.; Guo, C.; Teng, S.; Zhou, D.; Yu, S.; Yin, W.; Wang, P.; Zhu, W.; Duan, M. Pepsin Promotes Laryngopharyngeal Neoplasia by Modulating Signaling Pathways to Induce Cell Proliferation. PLoS ONE 2020, 15, e0227408. [Google Scholar] [CrossRef]
- Doukas, P.G.; Vageli, D.P.; Sasaki, C.T.; Judson, B.L. Pepsin Promotes Activation of Epidermal Growth Factor Receptor and Downstream Oncogenic Pathways, at Slightly Acidic and Neutral Ph, in Exposed Hypopharyngeal Cells. Int. J. Mol. Sci. 2021, 22, 4275. [Google Scholar] [CrossRef] [PubMed]
- Samuels, T.; Hoekzema, C.; Gould, J.; Goldblatt, M.; Frelich, M.; Bosler, M.; Lee, S.H.; Johnston, N. Local Synthesis of Pepsin in Barrett’s Esophagus and the Role of Pepsin in Esophageal Adenocarcinoma. Ann. Otol. Rhinol. Laryngol. 2015, 124, 893–902. [Google Scholar] [CrossRef][Green Version]
- Feagins, L.A.; Zhang, H.Y.; Hormi-Carver, K.; Quinones, M.H.; Thomas, D.; Zhang, X.; Terada, L.S.; Spechler, S.J.; Ramirez, R.D.; Souza, R.F. Acid Has Antiproliferative Effects in Nonneoplastic Barrett’s Epithelial Cells. Am. J. Gastroenterol. 2007, 102, 10–20. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhang, X.; Hormi-Carver, K.; Feagins, L.A.; Spechler, S.J.; Souza, R.F. In Non-Neoplastic Barrett’s Epithelial Cells, Acid Exerts Early Antiproliferative Effects through Activation of the Chk2 Pathway. Cancer Res. 2007, 67, 8580–8587. [Google Scholar] [CrossRef][Green Version]
- Ylitalo, R.; Baugh, A.; Li, W.; Thibeault, S. Effect of Acid and Pepsin on Gene Expression in Laryngeal Fibroblasts. Ann. Otol. Rhinol. Laryngol. 2004, 113, 866–871. [Google Scholar] [CrossRef]
- Choi, Y.S.; Na, H.G.; Bae, C.H.; Song, S.Y.; Kim, Y.D. Pepsin Exposure in a Non-Acidic Environment Upregulates Mucin 5ac (Muc5ac) Expression Via Matrix Metalloproteinase 9 (Mmp9)/Nuclear Factor Κb (Nf-Κb) in Human Airway Epithelial Cells. Int. Forum Allergy Rhinol. 2021, 11, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, J.A.; Johnstone, L.M.; Sykes, J.; Strugala, V.; Dettmar, P.W. The Value of a Liquid Alginate Suspension (Gaviscon Advance) in the Management of Laryngopharyngeal Reflux. Eur. Arch. Otorhinolaryngol. 2009, 266, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Samuels, T.L.; Blaine-Sauer, S.; Yan, K.; Johnston, N. Amprenavir Protects against Pepsin-Mediated Laryngeal E-Cadherin Cleavage and Matrix Metalloproteinase Induction In Vitro. Submitted to Laryngoscope 2023, submitted.
- Balan, K.K.; Jones, A.T.; Roberts, N.B.; Pearson, J.P.; Critchley, M.; Jenkins, S.A. The Effects of Helicobacter Pylori Colonization on Gastric Function and the Incidence of Portal Hypertensive Gastropathy in Patients with Cirrhosis of the Liver. Am. J. Gastroenterol. 1996, 91, 1400–1406. [Google Scholar]
- Fletcher, J.; Wirz, A.; Young, J.; Vallance, R.; McColl, K.E. Unbuffered Highly Acidic Gastric Juice Exists at the Gastroesophageal Junction after a Meal. Gastroenterology 2001, 121, 775–783. [Google Scholar] [CrossRef]
- Boeckxstaens, G.E.; Smout, A. Systematic Review: Role of Acid, Weakly Acidic and Weakly Alkaline Reflux in Gastro-Oesophageal Reflux Disease. Aliment. Pharmacol. Ther. 2010, 32, 334–343. [Google Scholar] [CrossRef]
- Zerbib, F.; Roman, S.; Ropert, A.; des Varannes, S.B.; Pouderoux, P.; Chaput, U.; Mion, F.; Vérin, E.; Galmiche, J.P.; Sifrim, D. Esophageal Ph-Impedance Monitoring and Symptom Analysis in Gerd: A Study in Patients Off and on Therapy. Am. J. Gastroenterol. 2006, 101, 1956–1963. [Google Scholar] [CrossRef]
- Herbella, F.A.; Vicentine, F.P.; Silva, L.C.; Patti, M.G. Postprandial Proximal Gastric Acid Pocket and Gastroesophageal Reflux Disease. Dis. Esophagus 2012, 25, 652–655. [Google Scholar] [CrossRef]
- Jaiswal, K.R.; Morales, C.P.; Feagins, L.A.; Gandia, K.G.; Zhang, X.; Zhang, H.Y.; Hormi-Carver, K.; Shen, Y.; Elder, F.; Ramirez, R.D.; et al. Characterization of Telomerase-Immortalized, Non-Neoplastic, Human Barrett’s Cell Line (Bar-T). Dis. Esophagus 2007, 20, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Grobelny, D.; Poncz, L.; Galardy, R.E. Inhibition of Human Skin Fibroblast Collagenase, Thermolysin, and Pseudomonas Aeruginosa Elastase by Peptide Hydroxamic Acids. Biochemistry 1992, 31, 7152–7154. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wu, W.; Yang, G.; Li, J.; Li, J.; Ye, Q. Tetrahydroisoquinoline Based Sulfonamide Hydroxamates as Potent Matrix Metalloproteinase Inhibitors. Bioorg Med. Chem. Lett. 2004, 14, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Uttamchandani, M.; Wang, J.; Li, J.; Hu, M.; Sun, H.; Chen, K.Y.; Liu, K.; Yao, S.Q. Inhibitor Fingerprinting of Matrix Metalloproteases Using a Combinatorial Peptide Hydroxamate Library. J. Am. Chem. Soc. 2007, 129, 7848–7858. [Google Scholar] [CrossRef] [PubMed]
- Brummer, T.; Pigoni, M.; Rossello, A.; Wang, H.; Noy, P.J.; Tomlinson, M.G.; Blobel, C.P.; Lichtenthaler, S.F. The Metalloprotease Adam10 (a Disintegrin and Metalloprotease 10) Undergoes Rapid, Postlysis Autocatalytic Degradation. FASEB J. 2018, 32, 3560–3573. [Google Scholar] [CrossRef][Green Version]
- Lee, S.H.; Samuels, T.; Bock, J.M.; Blumin, J.H.; Johnston, N. Establishment of an Immortalized Laryngeal Posterior Commissure Cell Line as a Tool for Reflux Research. Laryngoscope 2015, 125, E73–E77. [Google Scholar] [CrossRef]
- Rubie, C.; Kempf, K.; Hans, J.; Su, T.; Tilton, B.; Georg, T.; Brittner, B.; Ludwig, B.; Schilling, M. Housekeeping Gene Variability in Normal and Cancerous Colorectal, Pancreatic, Esophageal, Gastric and Hepatic Tissues. Mol. Cell. Probes 2005, 19, 101–109. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuels, T.L.; Blaine-Sauer, S.; Yan, K.; Plehhova, K.; Coyle, C.; Johnston, N. Topical Alginate Protection against Pepsin-Mediated Esophageal Damage: E-Cadherin Proteolysis and Matrix Metalloproteinase Induction. Int. J. Mol. Sci. 2023, 24, 7932. https://doi.org/10.3390/ijms24097932
Samuels TL, Blaine-Sauer S, Yan K, Plehhova K, Coyle C, Johnston N. Topical Alginate Protection against Pepsin-Mediated Esophageal Damage: E-Cadherin Proteolysis and Matrix Metalloproteinase Induction. International Journal of Molecular Sciences. 2023; 24(9):7932. https://doi.org/10.3390/ijms24097932
Chicago/Turabian StyleSamuels, Tina L., Simon Blaine-Sauer, Ke Yan, Kate Plehhova, Cathal Coyle, and Nikki Johnston. 2023. "Topical Alginate Protection against Pepsin-Mediated Esophageal Damage: E-Cadherin Proteolysis and Matrix Metalloproteinase Induction" International Journal of Molecular Sciences 24, no. 9: 7932. https://doi.org/10.3390/ijms24097932
APA StyleSamuels, T. L., Blaine-Sauer, S., Yan, K., Plehhova, K., Coyle, C., & Johnston, N. (2023). Topical Alginate Protection against Pepsin-Mediated Esophageal Damage: E-Cadherin Proteolysis and Matrix Metalloproteinase Induction. International Journal of Molecular Sciences, 24(9), 7932. https://doi.org/10.3390/ijms24097932







