Immunosenescence and Skin: A State of Art of Its Etiopathogenetic Role and Crucial Watershed for Systemic Implications
Abstract
1. Introduction
1.1. Immunosenescence: From a Common Definition to a More Specific One
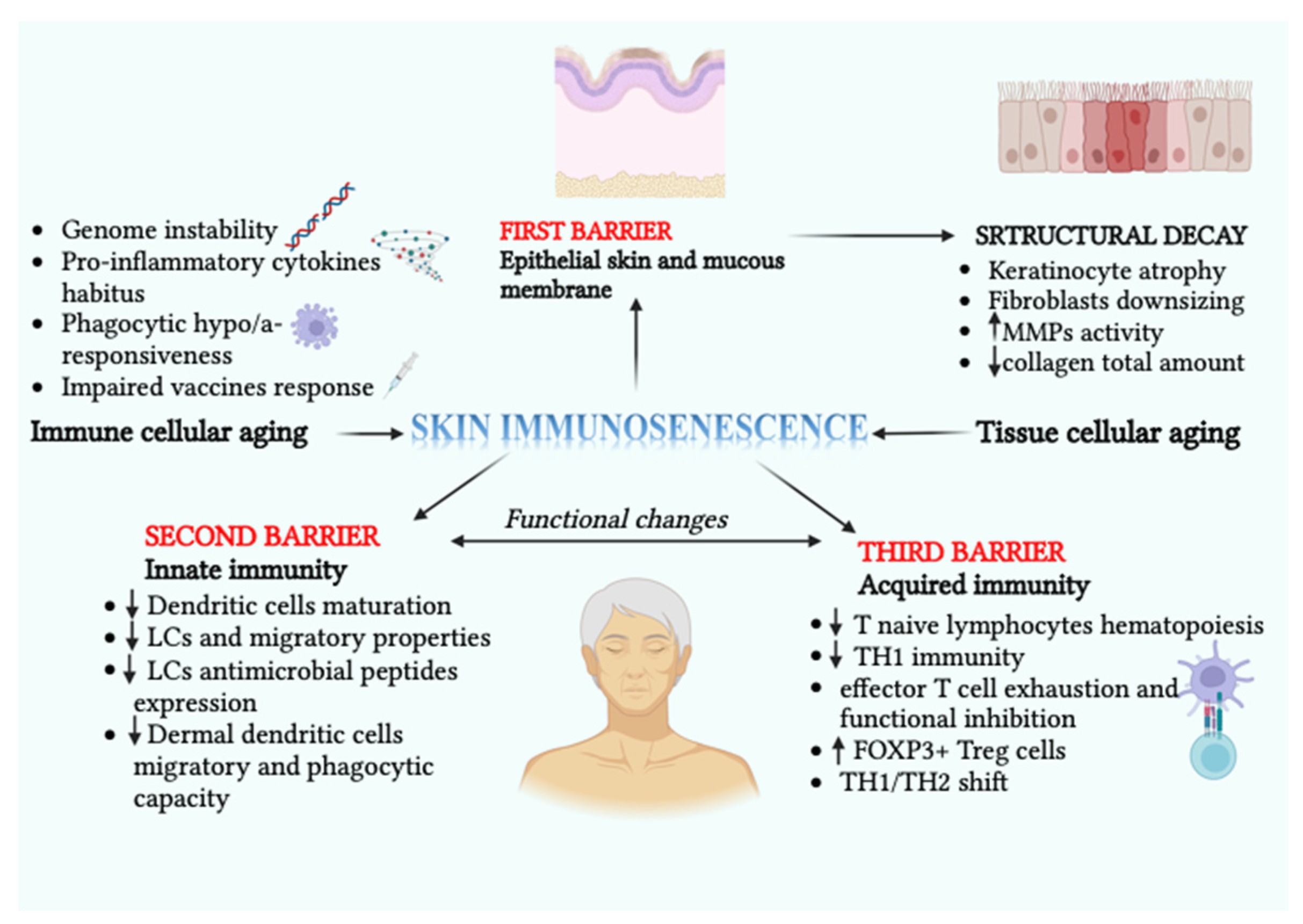
1.2. Skin Immunosenescence: Structural and Functional Hallmarks
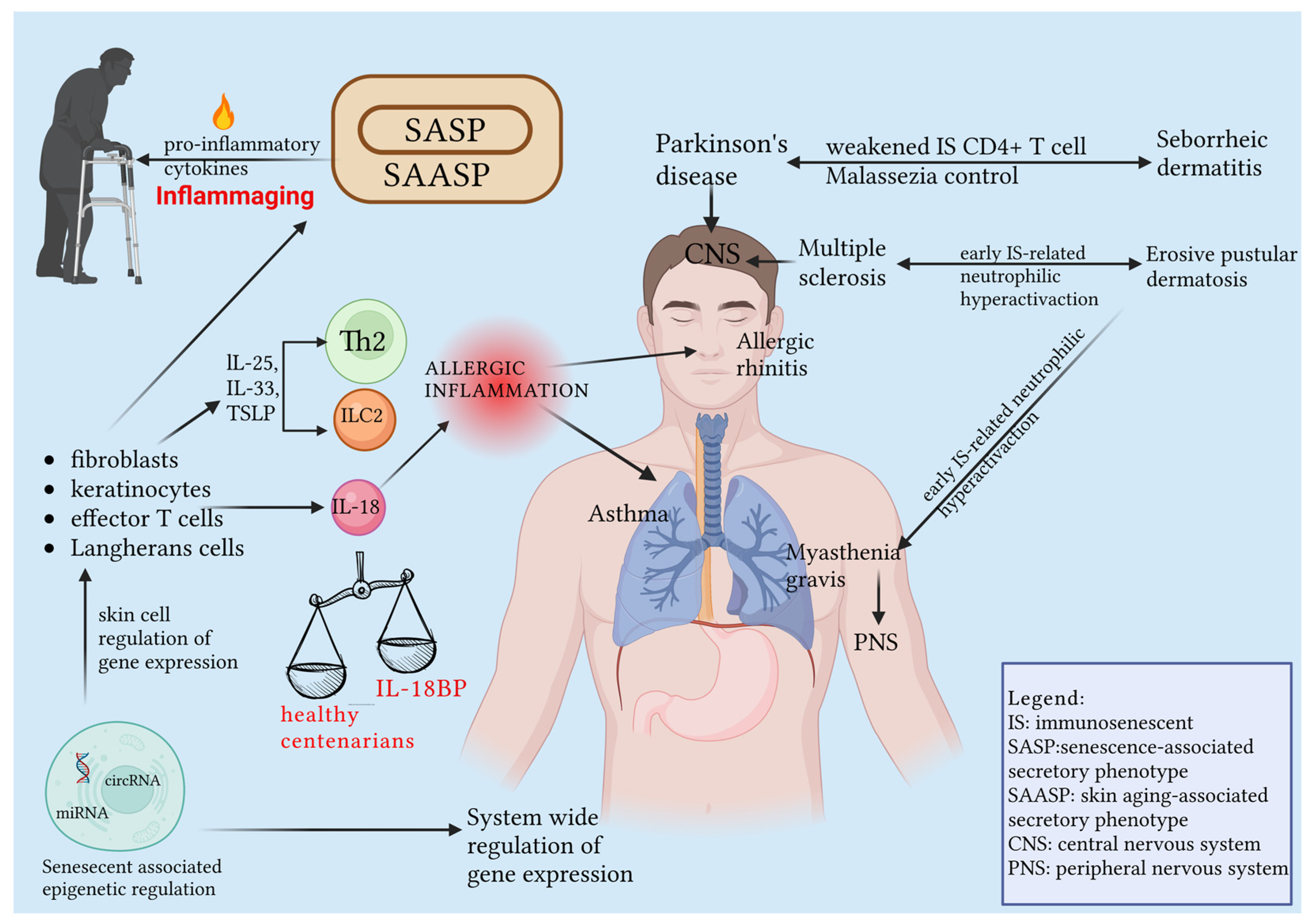
1.3. SASP and SAASP, Two Sides of the Same Coin: Inflammaging
1.4. Epigenetic Influence on Skin Immunosenescence
2. Discussion
2.1. Skin Cancer and Immunosenescence
2.1.1. Melanoma
2.1.2. Cutaneous Squamous Cell Carcinoma
- -
- Immunosenescence causes tissue and cell damage, and immunosurvey escapes, leading to cancer development.
- -
- Cancer and aging can be considered two different manifestations of the same underlying process.
- -
- Loss of antitumor immunity is characterized by naïve T cell reduction, depletion of cancer-specific memory T cells, and high numbers of suppressor cells.
- -
- In melanoma, resistance to ICIs is characterized by the loss of CD27 and CD28 or the expression of Tim-3 and CD57 on T cells.
- -
- Immunosuppression and immunosenescence are risk factors for cSCC development.
- -
- Reduced tumor-infiltrating cytotoxic CD8+ T cells and naive T lymphocytes in the cSCC of OTRs have been detected, along with low numbers of NK cells.
- -
- The percentage of CD8+ T cells expressing CD57 is the strongest immunological predictor of future SCC.
- -
- In cSCC, tumor-infiltrating leucocytes, specifically CD4+ and cytotoxic CD8+ T cells, have reduced density both in intra- and peritumoral tissues in immunosuppressed patients, while Treg levels are increased in the TME.
- -
- The frequency of FOXP3+ Tregs in cSCC is strongly correlated with metastases and poorer clinical outcomes, while high Th1 serum levels and IFN-γ production have been associated with decreased susceptibility to cSCC development.
2.2. Cutaneous Inflammatory Diseases and Immunosenescence
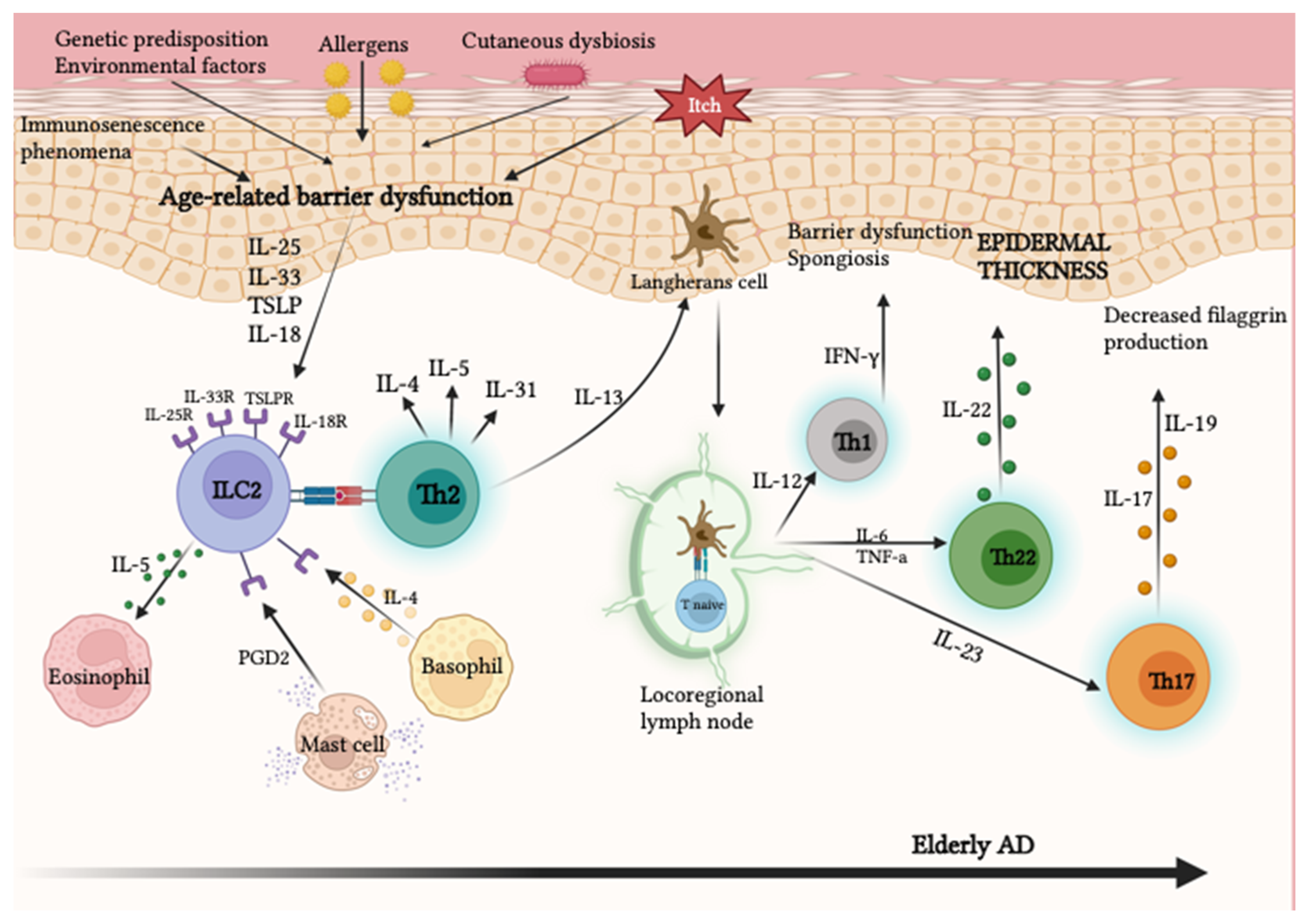
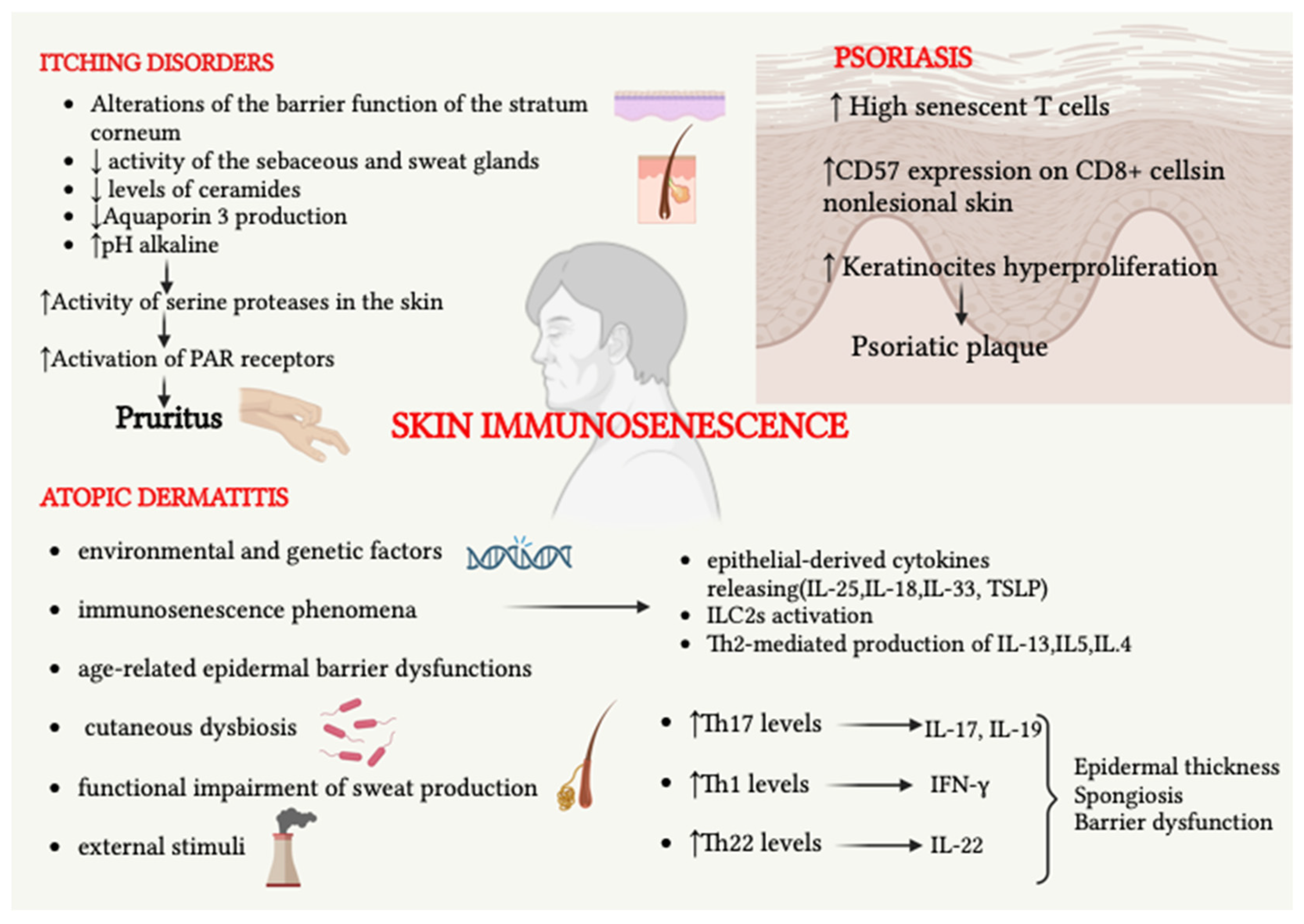
2.2.1. Atopic Dermatitis
2.2.2. Immunosenescence and Itching Diseases
2.2.3. Psoriasis
- -
- AD in elderly patients represents a newly defined subgroup in which immunosenescence phenomena play a central role.
- -
- Elderly skin shows greater Th22 polarization, which promotes epidermal hyperplasia and barrier defects.
- -
- Elderly chronic AD lesions show a conversion of polarization towards a Th1 phenotype with the production of IFN-γ and IL-12.
- -
- Immunosenescence causes an increased predisposition to the development of itching.
- -
- Immunosenescence phenomena can also be associated with the development of autoimmune diseases, including pemphigus vulgaris and pemphigoid.
- -
- Terminally differentiated or senescent T cells are present in higher proportions among CD8+ cells in patients with psoriasis.
- -
- T cells of the lesional skin show reduced expression of immunosenescence and replication inhibition markers, unlike what occurs in healthy non-lesional skin. Psoriasis is characterized by a high cell turnover.
- -
- Elderly psoriatic patients are recommended to be carefully screened for possible latent systemic infections before starting biological therapies.
2.3. Role of Immunosenescence in Cutaneous Infectious Diseases
2.4. Cutaneous Microbiome as a Specific Pattern of Frailty
2.5. Immunosenescence: A Subtle Hotline between the Skin and System-Specific Pathologies
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbé-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.M.; Bauer, M.E. The Interplay between Immunosenescence and Age-Related Diseases. Semin. Immunopathol. 2020, 42, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, Aging and Successful Aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef] [PubMed]
- Marrero, Y.T.; Suárez, V.M.; Abraham, C.M.M.; Hernández, I.C.; Ramos, E.H.; Domínguez, G.D.; Pérez, Y.D.; Zamora, M.C.R.; Pita, A.M.S.; Guerra, L.F.H. Peripheral Double Negative T: A Look at Senescent Cubans. Exp. Gerontol. 2023, 171, 112006. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Age and Immunity: What Is “Immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, E.T.; Sorrell, E.M.; Perez, D.R.; Ann Ottinger, M. Immunosenescence and Age-Related Susceptibility to Influenza Virus in Japanese Quail. Dev. Comp. Immunol. 2007, 31, 407–414. [Google Scholar] [CrossRef]
- Corsini, E.; Racchi, M.; Lucchi, L.; Donetti, E.; Bedoni, M.; Viviani, B.; Galli, C.L.; Marinovich, M. Skin Immunosenescence: Decreased Receptor for Activated C Kinase-1 Expression Correlates with Defective Tumour Necrosis Factor-α Production in Epidermal Cells. Br. J. Dermatol. 2009, 160, 16–25. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; Teixeira, V.R.; Alencar-Silva, T.; Simonassi-Paiva, B.; Pereira, R.W.; Pogue, R.; Carvalho, J.L. Hallmarks of Aging and Immunosenescence: Connecting the Dots. Cytokine Growth Factor Rev. 2021, 59, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Großkopf, A.; Simm, A. Alterung Des Immunsystems. Z. Gerontol. Geriatr. 2022, 55, 553–557. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Barron, M.J.; Watson, R.E.B.; Griffiths, C.E.M.; Bulfone-Paus, S. Aged Human Skin Accumulates Mast Cells with Altered Functionality That Localize to Macrophages and Vasoactive Intestinal Peptide-positive Nerve Fibres. Br. J. Dermatol. 2019, 180, 849–858. [Google Scholar] [CrossRef]
- Blackburn, E.; Mur, P.; Jofré, B.; Coll, C.; Jurlow, E. Immunosenescence: Delayed Cutaneous Hypersensitivity Tests in Independently-Living Chilean Elderly Individuals. Rev. Med. Chil. 2000, 128, 379–386. [Google Scholar] [PubMed]
- Vaccaro, M.; Irrera, N.; Cutroneo, G.; Rizzo, G.; Vaccaro, F.; Anastasi, G.; Borgia, F.; Cannavò, S.; Altavilla, D.; Squadrito, F. Differential Expression of Nitric Oxide Synthase Isoforms NNOS and INOS in Patients with Non-Segmental Generalized Vitiligo. Int. J. Mol. Sci. 2017, 18, 2533. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Jin, R.; Zeng, J.; Hua, Y.; Yorek, M.S.; Liu, L.; Mandal, A.; Li, J.; Zheng, H.; Sun, Y.; et al. Consumption of Fish Oil High-Fat Diet Induces Murine Hair Loss via Epidermal Fatty Acid Binding Protein in Skin Macrophages. Cell Rep. 2022, 41, 111804. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jin, R.; Hao, J.; Zeng, J.; Yin, D.; Yi, Y.; Zhu, M.; Mandal, A.; Hua, Y.; Ng, C.K.; et al. Consumption of the Fish Oil High-Fat Diet Uncouples Obesity and Mammary Tumor Growth through Induction of Reactive Oxygen Species in Protumor Macrophages. Cancer Res. 2020, 80, 2564–2574. [Google Scholar] [CrossRef]
- Kim, H.-J.; Barajas, B.; Chan, R.C.-F.; Nel, A.E. Glutathione Depletion Inhibits Dendritic Cell Maturation and Delayed-Type Hypersensitivity: Implications for Systemic Disease and Immunosenescence. J. Allergy Clin. Immunol. 2007, 119, 1225–1233. [Google Scholar] [CrossRef]
- Smithey, M.J.; Uhrlaub, J.L.; Li, G.; Vukmanovic-Stejic, M.; Akbar, A.N.; Nikolich-Zugich, J. Lost in Translation: Mice, Men and Cutaneous Immunity in Old Age. Biogerontology 2015, 16, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Sibaii, H.; El-Zayat, S.R.; Khalil, M. When Wrinkles Appear on the Immune System Can It Be Reversed? Eur. Cytokine Netw. 2020, 31, 1–17. [Google Scholar] [CrossRef]
- Huang, K.; Cai, H.; Bao, J.; Wu, L. Dehydroepiandrosterone and Age-Related Musculoskeletal Diseases: Connections and Therapeutic Implications. Ageing Res. Rev. 2020, 62, 101132. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves de Carvalho, C.M.R.; Ribeiro, S.M.L. Aging, Low-Grade Systemic Inflammation and Vitamin D: A Mini-Review. Eur. J. Clin. Nutr. 2017, 71, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin Barrier Immunity and Ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T Cell Aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes Other than the Gut: Inflammaging and Age-Related Diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef]
- Kinn, P.M.; Holdren, G.O.; Westermeyer, B.A.; Abuissa, M.; Fischer, C.L.; Fairley, J.A.; Brogden, K.A.; Brogden, N.K. Age-Dependent Variation in Cytokines, Chemokines and Biologic Analytes Rinsed from the Surface of Healthy Human Skin. Sci. Rep. 2015, 5, 10472. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.R.; Almeida, P.P.; de Oliveira Santos, L.; Rodrigues, L.P.; de Carvalho, J.L.; Boroni, M. Hallmarks of Aging in Macrophages: Consequences to Skin Inflammaging. Cells 2021, 10, 1323. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Basile, G.; Merendino, R.A.; Minciullo, P.L.; Novick, D.; Rubinstein, M.; Dinarello, C.A.; Lo Balbo, C.; Franceschi, C.; Basili, S.; et al. Increased Circulating Interleukin-18 Levels in Centenarians with No Signs of Vascular Disease: Another Paradox of Longevity? Exp. Gerontol. 2003, 38, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Waaijer, M.E.C.; Goldeck, D.; Gunn, D.A.; Heemst, D.; Westendorp, R.G.J.; Pawelec, G.; Maier, A.B. Are Skin Senescence and Immunosenescence Linked within Individuals? Aging Cell 2019, 18, e12956. [Google Scholar] [CrossRef] [PubMed]
- Neuber, K.; Schmidt, S.; Mensch, A. Telomere Length Measurement and Determination of Immunosenescence-Related Markers (CD28, CD45RO, CD45RA, Interferon-Gamma and Interleukin-4) in Skin-Homing T Cells Expressing the Cutaneous Lymphocyte Antigen: Indication of a Non-Ageing T-Cell Subset. Immunology 2003, 109, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.M.; Olson, L.C.; Momtahen, S. Post-Thymic CD4 Positive Cytotoxic T Cell Infiltrates of the Skin: A Clinical and Histomorphologic Spectrum of the Unique CD4 Positive T Cell of Immunosenescence. Ann. Diagn. Pathol. 2019, 38, 99–105. [Google Scholar] [CrossRef]
- Xu, Y.-P.; Qi, R.-Q.; Chen, W.-B.; Shi, Y.-L.; Cui, Z.-Z.; Gao, X.-H.; Chen, H.-D.; Zhou, L.; Mi, Q.-S. Aging Affects Epidermal Langerhans Cell Development and Function and Alters Their MiRNA Gene Expression Profile. Aging 2012, 4, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Dearman, R.J.; Kimber, I.; Griffiths, C.E.M. Langerhans Cells Express Human β-Defensin 3: Relevance for Immunity during Skin Ageing. Br. J. Dermatol. 2018, 179, 1170–1171. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The Role of MicroRNAs in Organismal and Skin Aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef]
- Allegra, A.; Cicero, N.; Tonacci, A.; Musolino, C.; Gangemi, S. Circular RNA as a Novel Biomarker for Diagnosis and Prognosis and Potential Therapeutic Targets in Multiple Myeloma. Cancers 2022, 14, 1700. [Google Scholar] [CrossRef]
- Cai, H.; Li, Y.; Niringiyumukiza, J.D.; Su, P.; Xiang, W. Circular RNA Involvement in Aging: An Emerging Player with Great Potential. Mech. Ageing Dev. 2019, 178, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Suárez-Fariñas, M.; He, H.; Malik, K.; Wen, H.-C.; Gonzalez, J.; Chan, T.C.-C.; Estrada, Y.; Zheng, X.; Khattri, S.; et al. The Atopic Dermatitis Blood Signature Is Characterized by Increases in Inflammatory and Cardiovascular Risk Proteins. Sci. Rep. 2017, 7, 8707. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Association of Atopic Dermatitis with Allergic, Autoimmune, and Cardiovascular Comorbidities in US Adults. Ann. Allergy Asthma Immunol. 2018, 121, 604–612.e3. [Google Scholar] [CrossRef]
- Ascott, A.; Mulick, A.; Yu, A.M.; Prieto-Merino, D.; Schmidt, M.; Abuabara, K.; Smeeth, L.; Roberts, A.; Langan, S.M. Atopic Eczema and Major Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Population-Based Studies. J. Allergy Clin. Immunol. 2019, 143, 1821–1829. [Google Scholar] [CrossRef]
- Ivert, L.; Johansson, E.; Dal, H.; Lindelöf, B.; Wahlgren, C.; Bradley, M. Association Between Atopic Dermatitis and Cardiovascular Disease: A Nationwide Register-Based Case-Control Study from Sweden. Acta Dermatol. Venereol. 2019, 99, 865–870. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, R.; Choi, S.; Zhou, L.; Pavel, A.; Estrada, Y.D.; Krueger, J.G.; Guttman-Yassky, E. Increased Cardiovascular and Atherosclerosis Markers in Blood of Older Patients with Atopic Dermatitis. Ann. Allergy Asthma Immunol. 2020, 124, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Lee, D.H.; Park, M.Y.; Ahn, J. Cardiovascular Comorbidities of Atopic Dermatitis: Using National Health Insurance Data in Korea. Allergy Asthma Clin. Immunol. 2021, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gallego, N.; Castillo-González, R.; Méndez-Barbero, N.; López-Sanz, C.; Obeso, D.; Villaseñor, A.; Escribese, M.M.; López-Melgar, B.; Salamanca, J.; Benedicto-Buendía, A.; et al. The Impact of Type 2 Immunity and Allergic Diseases in Atherosclerosis. Allergy 2022, 77, 3249–3266. [Google Scholar] [CrossRef] [PubMed]
- Chester, J.; Kaleci, S.; Liberati, S.; Alicandro, T.; Rivi, M.; Bonzano, L. Atopic Dermatitis Associated with Autoimmune, Cardiovascular and Mental Health Comorbidities: A Systematic Review and Meta-Analysis. Eur. J. Dermatol. 2022, 32, 34–48. [Google Scholar] [CrossRef]
- Tomihara, K.; Curiel, T.J.; Zhang, B. Optimization of Immunotherapy in Elderly Cancer Patients. Crit. Rev. Oncog. 2013, 18, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.T.; Casciaro, M.; Gangemi, S.; Buquicchio, R. Immunosenescence in Aging: Between Immune Cells Depletion and Cytokines up-Regulation. Clin. Mol. Allergy 2017, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, X.; Feng, Y.; Wu, L.; Ma, W.; Ding, G.; Wei, Y.; Sun, L. The Impact of Immunosenescence on the Efficacy of Immune Checkpoint Inhibitors in Melanoma Patients: A Meta-Analysis. Onco. Targets Ther. 2018, 11, 7521–7527. [Google Scholar] [CrossRef]
- Kendal, W.S. Dying with Cancer. Cancer 2008, 112, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Cristani, M.; Speciale, A.; Saija, A.; Gangemi, S.; Minciullo, P.; Cimino, F. Circulating Advanced Oxidation Protein Products as Oxidative Stress Biomarkers and Progression Mediators in Pathological Conditions Related to Inflammation and Immune Dysregulation. Curr. Med. Chem. 2016, 23, 3862–3882. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.; Saddawi-Konefka, R.; Vermi, W.; Koebel, C.M.; Arthur, C.; White, J.M.; Uppaluri, R.; Andrews, D.M.; Ngiow, S.F.; Teng, M.W.L.; et al. Cancer Immunoediting by the Innate Immune System in the Absence of Adaptive Immunity. J. Exp. Med. 2012, 209, 1869–1882. [Google Scholar] [CrossRef]
- Li Pomi, F.; Borgia, F.; Custurone, P.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Role of HMGB1 in Cutaneous Melanoma: State of the Art. Int. J. Mol. Sci. 2022, 23, 9327. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Tonacci, A.; Bertino, L.; Casciaro, M.; Borgia, F.; Gangemi, S. The Role of Oxidative Stress in the Biology of Melanoma: A Systematic Review. Pathol. Res. Pract. 2019, 215, 21–28. [Google Scholar] [CrossRef]
- Segal, N.H.; Parsons, D.W.; Peggs, K.S.; Velculescu, V.; Kinzler, K.W.; Vogelstein, B.; Allison, J.P. Epitope Landscape in Breast and Colorectal Cancer. Cancer Res. 2008, 68, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Campoli, M.; Chang, C.-C.; Ferrone, S. HLA Class I Antigen Loss, Tumor Immune Escape and Immune Selection. Vaccine 2002, 20, A40–A45. [Google Scholar] [CrossRef]
- Rolinski, J.; Hus, I. Breaking Immunotolerance of Tumors: A New Perspective for Dendritic Cell Therapy. J. Immunotoxicol. 2014, 11, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Does Patient Age Influence Anti-Cancer Immunity? Semin. Immunopathol. 2019, 41, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Daste, A.; Domblides, C.; Gross-goupil, M.; Chakiba, C.; Quivy, A.; Cochin, V.; de Mones, E.; Larmonier, N.; Soubeyran, P.; Ravaud, A. Immune Checkpoint Inhibitors and Elderly People: A Review. Eur. J. Cancer 2017, 82, 155–166. [Google Scholar] [CrossRef]
- Kim, C.M.; Lee, J.B.; Shin, S.J.; Ahn, J.B.; Lee, M.; Kim, H.S. The Efficacy of Immune Checkpoint Inhibitors in Elderly Patients: A Meta-Analysis and Meta-Regression. ESMO Open 2022, 7, 100577. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.P.; Atwal, D.; Ravilla, R.; Tao, J.; Su, J.; Makhoul, I.; Hutchins, L.F.; Mahmoud, F.A. Outcomes of Immunotherapy in Advanced Melanoma in Relation to Age. J. Clin. Oncol. 2018, 36, 187. [Google Scholar] [CrossRef]
- Moreira, A.; Gross, S.; Kirchberger, M.C.; Erdmann, M.; Schuler, G.; Heinzerling, L. Senescence Markers: Predictive for Response to Checkpoint Inhibitors. Int. J. Cancer 2019, 144, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Azin, M.; Demehri, S. Cutaneous Squamous Cell Carcinoma: The Frontier of Cancer Immunoprevention. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, L.; Vaccaro, F.; Irrera, N.; Borgia, F.; Li Pomi, F.; Squadrito, F.; Vaccaro, M. Wnt Signaling Pathways: From Inflammation to Non-Melanoma Skin Cancers. Int. J. Mol. Sci. 2023, 24, 1575. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Thomson, J.; Harwood, C.; Leigh, I. The Role of the Immune System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2009. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Hansen, S.; Møller, B.; Leivestad, T.; Pfeffer, P.; Geiran, O.; Fauchald, P.; Simonsen, S. Skin Cancer in Kidney and Heart Transplant Recipients and Different Long-Term Immunosuppressive Therapy Regimens. J. Am. Acad. Dermatol. 1999, 40, 177–186. [Google Scholar] [CrossRef]
- Lanz, J.; Bouwes Bavinck, J.N.; Westhuis, M.; Quint, K.D.; Harwood, C.A.; Nasir, S.; Van-de-Velde, V.; Proby, C.M.; Ferrándiz, C.; Genders, R.E.; et al. Aggressive Squamous Cell Carcinoma in Organ Transplant Recipients. JAMA Dermatol. 2019, 155, 66. [Google Scholar] [CrossRef]
- Madeleine, M.M.; Patel, N.S.; Plasmeijer, E.I.; Engels, E.A.; Bouwes Bavinck, J.N.; Toland, A.E.; Green, A.C. Epidemiology of Keratinocyte Carcinomas after Organ Transplantation. Br. J. Dermatol. 2017, 177, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Otley, C.C.; Berg, D.; Ulrich, C.; Stasko, T.; Murphy, G.M.; Salasche, S.J.; Christenson, L.J.; Sengelmann, R.; Loss, G.E.; Garces, J. Reduction of Immunosuppression for Transplant-Associated Skin Cancer: Expert Consensus Survey. Br. J. Dermatol. 2006, 154, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Agnew, K.L.; Ruchlemer, R.; Catovsky, D.; Matutes, E.; Bunker, C.B. Cutaneous Findings in Chronic Lymphocytic Leukaemia. Br. J. Dermatol. 2004, 150, 1129–1135. [Google Scholar] [CrossRef]
- Onajin, O.; Brewer, J.D. Skin Cancer in Patients with Chronic Lymphocytic Leukemia and Non-Hodgkin Lymphoma. Clin. Adv. Hematol. Oncol. 2012, 10, 571–576. [Google Scholar]
- Silverberg, M.J.; Leyden, W.; Warton, E.M.; Quesenberry, C.P.; Engels, E.A.; Asgari, M.M. HIV Infection Status, Immunodeficiency, and the Incidence of Non-Melanoma Skin Cancer. JNCI J. Natl. Cancer Inst. 2013, 105, 350–360. [Google Scholar] [CrossRef]
- Asgari, M.M.; Ray, G.T.; Quesenberry, C.P.; Katz, K.A.; Silverberg, M.J. Association of Multiple Primary Skin Cancers with Human Immunodeficiency Virus Infection, CD4 Count, and Viral Load. JAMA Dermatol. 2017, 153, 892. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.P.; Segundo, D.S.; Hollowood, K.; Marafioti, T.; Clark, T.G.; Harden, P.N.; Wood, K.J. Immune Phenotype Predicts Risk for Posttransplantation Squamous Cell Carcinoma. J. Am. Soc. Nephrol. 2010, 21, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Sherston, S.N.; Vogt, K.; Schlickeiser, S.; Sawitzki, B.; Harden, P.N.; Wood, K.J. Demethylation of the TSDR Is a Marker of Squamous Cell Carcinoma in Transplant Recipients. Am. J. Transplant. 2014, 14, 2617–2622. [Google Scholar] [CrossRef]
- Crespo, E.; Fernandez, L.; Lúcia, M.; Melilli, E.; Lauzurica, R.; Penin, R.M.; Quer, A.; Luque, S.; Quero, M.; Manonelles, A.; et al. Effector Antitumor and Regulatory T Cell Responses Influence the Development of Nonmelanoma Skin Cancer in Kidney Transplant Patients. Transplantation 2017, 101, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Frazzette, N.; Khodadadi-Jamayran, A.; Doudican, N.; Santana, A.; Felsen, D.; Pavlick, A.C.; Tsirigos, A.; Carucci, J.A. Decreased Cytotoxic T Cells and TCR Clonality in Organ Transplant Recipients with Squamous Cell Carcinoma. NPJ Precis. Oncol. 2020, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Harden, P.N.; Wood, K.J. CD8+ Immunosenescence Predicts Post-Transplant Cutaneous Squamous Cell Carcinoma in High-Risk Patients. J. Am. Soc. Nephrol. 2016, 27, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Akagi, J.; Baba, H. Prognostic Value of CD57+ T Lymphocytes in the Peripheral Blood of Patients with Advanced Gastric Cancer. Int. J. Clin. Oncol. 2008, 13, 528–535. [Google Scholar] [CrossRef]
- Characiejus, D.; Pasukoniene, V.; Jonusauskaite, R.; Azlauskaite, N.; Aleknavicius, E.; Mauricas, M.; Otter, W. Den Peripheral Blood CD8highCD57+ Lymphocyte Levels May Predict Outcome in Melanoma Patients Treated with Adjuvant Interferon-Alpha. Anticancer Res. 2008, 28, 1139–1142. [Google Scholar]
- Characiejus, D.; Pasukoniene, V.; Kazlauskaite, N.; Valuckas, K.P.; Petraitis, T.; Mauricas, M.; Den Otter, W. Predictive Value of CD8highCD57+ Lymphocyte Subset in Interferon Therapy of Patients with Renal Cell Carcinoma. Anticancer Res. 2002, 22, 3679–3683. [Google Scholar]
- Klebanoff, C.A.; Gattinoni, L.; Torabi-Parizi, P.; Kerstann, K.; Cardones, A.R.; Finkelstein, S.E.; Palmer, D.C.; Antony, P.A.; Hwang, S.T.; Rosenberg, S.A.; et al. Central Memory Self/Tumor-Reactive CD8+ T Cells Confer Superior Antitumor Immunity Compared with Effector Memory T Cells. Proc. Natl. Acad. Sci. USA 2005, 102, 9571–9576. [Google Scholar] [CrossRef] [PubMed]
- Krynitz, B.; Edgren, G.; Lindelöf, B.; Baecklund, E.; Brattström, C.; Wilczek, H.; Smedby, K.E. Risk of Skin Cancer and Other Malignancies in Kidney, Liver, Heart and Lung Transplant Recipients 1970 to 2008—A Swedish Population-Based Study. Int. J. Cancer 2013, 132, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Strobel, S.B.; Safferling, K.; Lahrmann, B.; Hoffmann, J.H.; Enk, A.H.; Hadaschik, E.N.; Grabe, N.; Lonsdorf, A.S. Altered Density, Composition and Microanatomical Distribution of Infiltrating Immune Cells in Cutaneous Squamous Cell Carcinoma of Organ Transplant Recipients. Br. J. Dermatol. 2018, 179, 405–412. [Google Scholar] [CrossRef]
- Zhang, S.; Fujita, H.; Mitsui, H.; Yanofsky, V.R.; Fuentes-Duculan, J.; Pettersen, J.S.; Suárez-Fariñas, M.; Gonzalez, J.; Wang, C.Q.F.; Krueger, J.G.; et al. Increased Tc22 and Treg/CD8 Ratio Contribute to Aggressive Growth of Transplant Associated Squamous Cell Carcinoma. PLoS ONE 2013, 8, e62154. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; August, S.; Albibas, A.; Behar, R.; Cho, S.-Y.; Polak, M.E.; Theaker, J.; MacLeod, A.S.; French, R.R.; Glennie, M.J.; et al. OX40+ Regulatory T Cells in Cutaneous Squamous Cell Carcinoma Suppress Effector T-Cell Responses and Associate with Metastatic Potential. Clin. Cancer Res. 2016, 22, 4236–4248. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.; Liu, Y. Prognostic Value of Tumor-Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Nindl, I.; Gottschling, M.; Stockfleth, E. Human Papillomaviruses and Non-Melanoma Skin Cancer: Basic Virology and Clinical Manifestations. Dis. Markers 2007, 23, 247–259. [Google Scholar] [CrossRef]
- Wang, J.; Aldabagh, B.; Yu, J.; Arron, S.T. Role of Human Papillomavirus in Cutaneous Squamous Cell Carcinoma: A Meta-Analysis. J. Am. Acad. Dermatol. 2014, 70, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Arron, S.T.; Ruby, J.G.; Dybbro, E.; Ganem, D.; Derisi, J.L. Transcriptome Sequencing Demonstrates That Human Papillomavirus Is Not Active in Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2011, 131, 1745–1753. [Google Scholar] [CrossRef]
- Badalamenti, G.; Incorvaia, L.; Algeri, L.; Carreca, I.U.; Brando, C.; Madonia, G.; Peri, M.; Cucinella, A.; Perez, A.; Barraco, N.; et al. Immunometabolic Predictive Factors in Merkel Cell Carcinoma (MCC) Patients Treated with Avelumab. J. Clin. Oncol. 2022, 40, e21525. [Google Scholar] [CrossRef]
- Mazziotta, C.; Lanzillotti, C.; Govoni, M.; Pellielo, G.; Mazzoni, E.; Tognon, M.; Martini, F.; Rotondo, J.C. Decreased IgG Antibody Response to Viral Protein Mimotopes of Oncogenic Merkel Cell Polyomavirus in Sera From Healthy Elderly Subjects. Front. Immunol. 2021, 12, 4206. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A.; Del Rosario, M.; Codreanu, I.; Hyams, D.M.; Plaxe, S.C. Inferior Outcomes in Immunocompromised Merkel Cell Carcinoma Patients: Can They Be Overcome by the Use of PD1/PDL1 Inhibitors? J. Oncol. Pharm. Pract. 2019, 25, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Lavery, A.; Solman, L.; Grindlay, D.J.C.; Rogers, N.K.; Thomas, K.S.; Harman, K.E. What’s New in Atopic Eczema? An Analysis of Systematic Reviews Published in 2016. Part 2: Epidemiology, Aetiology and Risk Factors. Clin. Exp. Dermatol. 2019, 44, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic Atopic Dermatitis Shows Similar TH2 and Higher TH17 Immune Activation Compared with Extrinsic Atopic Dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Immunological Differences between Intrinsic and Extrinsic Types of Atopic Dermatitis. Clin. Exp. Allergy 2003, 33, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Leonard, A.; Pavel, A.B.; Malik, K.; Raja, A.; Glickman, J.; Estrada, Y.D.; Peng, X.; del Duca, E.; Sanz-Cabanillas, J.; et al. Age-Specific Changes in the Molecular Phenotype of Patients with Moderate-to-Severe Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 144, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Bozek, A.; Fisher, A.; Filipowska, B.; Mazur, B.; Jarzab, J. Clinical Features and Immunological Markers of Atopic Dermatitis in Elderly Patients. Int. Arch. Allergy Immunol. 2012, 157, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Tanei, R.; Hasegawa, Y. Atopic Dermatitis in Older Adults: A Viewpoint from Geriatric Dermatology. Geriatr. Gerontol. Int. 2016, 16, 75–86. [Google Scholar] [CrossRef]
- Milgrom, H.; Huang, H. Allergic Disorders at a Venerable Age: A Mini-Review. Gerontology 2014, 60, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental Biodiversity, Human Microbiota, and Allergy Are Interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.-C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.J.; et al. A Role for IL-25 and IL-33–Driven Type-2 Innate Lymphoid Cells in Atopic Dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef]
- Leyva-Castillo, J.M.; Galand, C.; Mashiko, S.; Bissonnette, R.; McGurk, A.; Ziegler, S.F.; Dong, C.; McKenzie, A.N.J.; Sarfati, M.; Geha, R.S. ILC2 Activation by Keratinocyte-Derived IL-25 Drives IL-13 Production at Sites of Allergic Skin Inflammation. J. Allergy Clin. Immunol. 2020, 145, 1606–1614.e4. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y. Interleukin-33 in Atopic Dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, S.; Mehta, H.; Bissonnette, R.; Sarfati, M. Increased Frequencies of Basophils, Type 2 Innate Lymphoid Cells and Th2 Cells in Skin of Patients with Atopic Dermatitis but Not Psoriasis. J. Dermatol. Sci. 2017, 88, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Custurone, P.; Peterle, L.; Pioggia, G.; Gangemi, S. Role of Epithelium-Derived Cytokines in Atopic Dermatitis and Psoriasis: Evidence and Therapeutic Perspectives. Biomolecules 2021, 11, 1843. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Li Pomi, F.; Alessandrello, C.; Vaccaro, M.; Gangemi, S. Potential Role of Innate Lymphoid Cells in the Pathogenesis and Treatment of Skin Diseases. J. Clin. Med. 2023, 12, 3043. [Google Scholar] [CrossRef]
- Borgia, F.; Custurone, P.; Li Pomi, F.; Cordiano, R.; Alessandrello, C.; Gangemi, S. IL-31: State of the Art for an Inflammation-Oriented Interleukin. Int. J. Mol. Sci. 2022, 23, 6507. [Google Scholar] [CrossRef]
- Yoon, J.; Leyva-Castillo, J.M.; Wang, G.; Galand, C.; Oyoshi, M.K.; Kumar, L.; Hoff, S.; He, R.; Chervonsky, A.; Oppenheim, J.J.; et al. IL-23 Induced in Keratinocytes by Endogenous TLR4 Ligands Polarizes Dendritic Cells to Drive IL-22 Responses to Skin Immunization. J. Exp. Med. 2016, 213, 2147–2166. [Google Scholar] [CrossRef]
- Jin, M.; Yoon, J. From Bench to Clinic: The Potential of Therapeutic Targeting of the IL-22 Signaling Pathway in Atopic Dermatitis. Immune Netw. 2018, 18, e42. [Google Scholar] [CrossRef]
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J. Immunol. 2017, 198, 2543–2555. [Google Scholar] [CrossRef]
- Gutowska-Owsiak, D.; Ogg, G.S. Cytokine Regulation of the Epidermal Barrier. Clin. Exp. Allergy 2012, 43, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Gutowska-Owsiak, D.; Schaupp, A.L.; Salimi, M.; Taylor, S.; Ogg, G.S. Interleukin-22 Downregulates Filaggrin Expression and Affects Expression of Profilaggrin Processing Enzymes. Br. J. Dermatol. 2011, 165, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight Junction Defects in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e7. [Google Scholar] [CrossRef] [PubMed]
- Kisich, K.O.; Carspecken, C.W.; Fiéve, S.; Boguniewicz, M.; Leung, D.Y.M. Defective Killing of Staphylococcus Aureus in Atopic Dermatitis Is Associated with Reduced Mobilization of Human β-Defensin-3. J. Allergy Clin. Immunol. 2008, 122, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Canter, T.; Han, J.; Lefferdink, R.; Erickson, T.; Rangel, S.; Kameyama, N.; Kim, H.J.; Pavel, A.B.; et al. Evolution of Pathologic T-Cell Subsets in Patients with Atopic Dermatitis from Infancy to Adulthood. J. Allergy Clin. Immunol. 2020, 145, 215–228. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Waldman, A.; Ahluwalia, J.; Ong, P.Y.; Eichenfield, L. Atopic Dermatitis: Pathogenesis. Semin. Cutan. Med. Surg. 2017, 36, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian Atopic Dermatitis Phenotype Combines Features of Atopic Dermatitis and Psoriasis with Increased TH17 Polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Kabashima-Kubo, R.; Nakamura, M.; Sakabe, J.; Sugita, K.; Hino, R.; Mori, T.; Kobayashi, M.; Bito, T.; Kabashima, K.; Ogasawara, K.; et al. A Group of Atopic Dermatitis without IgE Elevation or Barrier Impairment Shows a High Th1 Frequency: Possible Immunological State of the Intrinsic Type. J. Dermatol. Sci. 2012, 67, 37–43. [Google Scholar] [CrossRef]
- Oriss, T.B.; McCarthy, S.A.; Morel, B.F.; Campana, M.A.; Morel, P.A. Crossregulation between T Helper Cell (Th)1 and Th2: Inhibition of Th2 Proliferation by IFN-Gamma Involves Interference with IL-1. J. Immunol. 1997, 158, 3666–3672. [Google Scholar] [CrossRef]
- Gutowska-Owsiak, D.; Schaupp, A.L.; Salimi, M.; Selvakumar, T.A.; McPherson, T.; Taylor, S.; Ogg, G.S. IL-17 Downregulates Filaggrin and Affects Keratinocyte Expression of Genes Associated with Cellular Adhesion. Exp. Dermatol. 2012, 21, 104–110. [Google Scholar] [CrossRef]
- Leslie, T.A. Itch Management in the Elderly. Itch-Manag. Clin. Pract. 2016, 50, 192–201. [Google Scholar] [CrossRef]
- Fourzali, K.M.; Yosipovitch, G. Management of Itch in the Elderly: A Review. Dermatol. Ther. 2019, 9, 639–653. [Google Scholar] [CrossRef]
- Valdes-Rodriguez, R.; Stull, C.; Yosipovitch, G. Chronic Pruritus in the Elderly: Pathophysiology, Diagnosis and Management. Drugs Aging 2015, 32, 201–215. [Google Scholar] [CrossRef]
- Shevchenko, A.; Valdes-Rodriguez, R.; Yosipovitch, G. Causes, Pathophysiology, and Treatment of Pruritus in the Mature Patient. Clin. Dermatol. 2018, 36, 140–151. [Google Scholar] [CrossRef]
- Beauregard, S.; Gilchrest, B.A. A Survey of Skin Problems and Skin Care Regimens in the Elderly. Arch. Dermatol. 1987, 123, 1638–1643. [Google Scholar] [CrossRef]
- Polat, M.; Yalçın, B.; Çalışkan, D.; Allı, N. Complete Dermatological Examination in the Elderly: An Exploratory Study from an Outpatient Clinic in Turkey. Gerontology 2009, 55, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Maumus-Robert, S.; Mazereeuw-Hautier, J.; Guyen, C.N.; Saudez, X.; Schmitt, A.M. Prevalence and Risk Factors for Xerosis in the Elderly: A Cross-Sectional Epidemiological Study in Primary Care. Dermatology 2011, 223, 260–265. [Google Scholar] [CrossRef]
- White-Chu, E.F.; Reddy, M. Dry Skin in the Elderly: Complexities of a Common Problem. Clin. Dermatol. 2011, 29, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, F.; Schliemann, S.; Antonov, D.; Elsner, P. Dry Skin, Barrier Function, and Irritant Contact Dermatitis in the Elderly. Clin. Dermatol. 2011, 29, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.; Minondo, A.-M.; Camus, C.; Fiat, F.; Corcuff, P.; Schmidt, R.; Simon, M.; Serre, G. Persistence of Both Peripheral and Non-Peripheral Corneodesmosomes in the Upper Stratum Corneum of Winter Xerosis Skin Versus Only Peripheral in Normal Skin. J. Investig. Dermatol. 2001, 116, 23–30. [Google Scholar] [CrossRef]
- Elias, P.M.; Ghadially, R. The Aged Epidermal Permeability Barrier. Clin. Geriatr. Med. 2002, 18, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.-M.; Forl, M.; Winoto-Morbach, S.; Seite, S.; Schunck, M.; Proksch, E.; Schutze, S. Acid and Neutral Sphingomyelinase, Ceramide Synthase, and Acid Ceramidase Activities in Cutaneous Aging. Exp. Dermatol. 2005, 14, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hara, M.; Sougrat, R.; Verbavatz, J.-M.; Verkman, A.S. Impaired Stratum Corneum Hydration in Mice Lacking Epidermal Water Channel Aquaporin-3. J. Biol. Chem. 2002, 277, 17147–17153. [Google Scholar] [CrossRef]
- Li, J.; Tang, H.; Hu, X.; Chen, M.; Xie, H. Aquaporin-3 Gene and Protein Expression in Sun-Protected Human Skin Decreases with Skin Ageing. Australas. J. Dermatol. 2010, 51, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Age-Related Changes in Male Skin: Quantitative Evaluation of One Hundred and Fifty Male Subjects. Ski. Pharmacol. Physiol. 2014, 27, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Xiong, G.L.; Haus, E.; Sackett-Lundeen, L.; Ashkenazi, I.; Maibach, H.I. Time-Dependent Variations of the Skin Barrier Function in Humans: Transepidermal Water Loss, Stratum Corneum Hydration, Skin Surface PH, and Skin Temperature. J. Investig. Dermatol. 1998, 110, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-H.; Man, M.-Q.; Xu, P.; Xin, S.; Liu, Z.; Crumrine, D.A.; Jiang, Y.J.; Fluhr, J.W.; Feingold, K.R.; Elias, P.M.; et al. Stratum Corneum Acidification Is Impaired in Moderately Aged Human and Murine Skin. J. Investig. Dermatol. 2007, 127, 2847–2856. [Google Scholar] [CrossRef]
- Ali, S.; Yosipovitch, G. Skin PH: From Basic SciencE to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Elias, P.M. Role of Lipids in the Formation and Maintenance of the Cutaneous Permeability Barrier. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 280–294. [Google Scholar] [CrossRef]
- Xu, A.Z.; Tripathi, S.V.; Kau, A.L.; Schaffer, A.; Kim, B.S. Immune Dysregulation Underlies a Subset of Patients with Chronic Idiopathic Pruritus. J. Am. Acad. Dermatol. 2016, 74, 1017–1020. [Google Scholar] [CrossRef]
- Schmidt, T.; Sitaru, C.; Amber, K.; Hertl, M. BP180- and BP230-Specific IgG Autoantibodies in Pruritic Disorders of the Elderly: A Preclinical Stage of Bullous Pemphigoid? Br. J. Dermatol. 2014, 171, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Brégégère, F.; Soroka, Y.; Frusic-Zlotkin, M.; Milner, Y. Replicative Senescence Enhances Apoptosis Induced by Pemphigus Autoimmune Antibodies in Human Keratinocytes. FEBS Lett. 2004, 567, 281–286. [Google Scholar] [CrossRef]
- Wang, X.; Brégégère, F.; Soroka, Y.; Kayat, A.; Redziniak, G.; Milner, Y. Enhancement of Fas-Mediated Apoptosis in Ageing Human Keratinocytes. Mech. Ageing Dev. 2004, 125, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Ciodaro, F.; Guarneri, F.; Bartolotta, A.; Papaianni, V.; Guarneri, C.; Catalano, N.; Galletti, F.; Cannavò, S. Auditory System Involvement in Psoriasis. Acta Derm. Venereol. 2018, 98, 655–659. [Google Scholar] [CrossRef]
- Kwon, H.H.; Kwon, I.H.; Youn, J., II. Clinical Study of Psoriasis Occurring over the Age of 60° Years: Is Elderly-Onset Psoriasis a Distinct Subtype? Int. J. Dermatol. 2012, 51, 53–58. [Google Scholar] [CrossRef]
- Fernandez-Torres, R.M.; Paradela, S.; Fonseca, E. Psoriasis in Patients Older than 65 Years. A Comparative Study with Younger Adult Psoriatic Patients. J. Nutr. Health Aging 2012, 16, 586–591. [Google Scholar] [CrossRef]
- Sampogna, F.; Chren, M.M.; Melchi, C.F.; Pasquini, P.; Tabolli, S.; Abeni, D. Age, Gender, Quality of Life and Psychological Distress in Patients Hospitalized with Psoriasis. Br. J. Dermatol. 2006, 154, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Šahmatova, L.; Sügis, E.; Šunina, M.; Hermann, H.; Prans, E.; Pihlap, M.; Abram, K.; Rebane, A.; Peterson, H.; Peterson, P.; et al. Signs of Innate Immune Activation and Premature Immunosenescence in Psoriasis Patients. Sci. Rep. 2017, 7, 7553. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.D.; Tincati, C.; Milush, J.M.; Ho, E.L.; Ndhlovu, L.C.; York, V.A.; Kallas, E.G.; Kalil, J.; Keating, S.M.; Norris, P.J.; et al. CD57 Expression and Cytokine Production by T Cells in Lesional and Unaffected Skin from Patients with Psoriasis. PLoS ONE 2013, 8, e52144. [Google Scholar] [CrossRef] [PubMed]
- Motolese, A.; Ceccarelli, M.; Macca, L.; Li Pomi, F.; Ingrasciotta, Y.; Nunnari, G.; Guarneri, C. Novel Therapeutic Approaches to Psoriasis and Risk of Infectious Disease. Biomedicines 2022, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Neuhaus, I.M.; Koo, J.Y.M. An Evidence-Based Review of Skin Cancer Rates on Biologic Therapies. J. Dermatol. Treat. 2012, 23, 305–315. [Google Scholar] [CrossRef]
- Sandhu, V.K.; Ighani, A.; Fleming, P.; Lynde, C.W. Biologic Treatment in Elderly Patients with Psoriasis: A Systematic Review. J. Cutan. Med. Surg. 2020, 24, 174–186. [Google Scholar] [CrossRef]
- Ongrádi, J.; Stercz, B.; Kövesdi, V.; Vértes, L. Immunosenescence and Vaccination of the Elderly I. Age-Related Immune Impairment. Acta Microbiol. Immunol. Hung. 2009, 56, 199–210. [Google Scholar] [CrossRef]
- Anderson, D.J.; Kaye, K.S. Skin and Soft Tissue Infections in Older Adults. Clin. Geriatr. Med. 2007, 23, 595–613. [Google Scholar] [CrossRef]
- Shintouo, C.M.; Shey, R.A.; Mets, T.; Vanhamme, L.; Souopgui, J.; Ghogomu, S.M.; Njemini, R. Onchocerciasis Fingerprints in the Geriatric Population: Does Host Immunity Play a Role? Trop Med. Infect. Dis. 2021, 6, 153. [Google Scholar] [CrossRef]
- Loureiro Salgado, C.; Mendéz Corea, A.F.; Covre, L.P.; De Matos Guedes, H.L.; Falqueto, A.; Gomes, D.C.O. Ageing Impairs Protective Immunity and Promotes Susceptibility to Murine Visceral Leishmaniasis. Parasitology 2022, 149, 1249–1256. [Google Scholar] [CrossRef]
- Quinn, K.M.; Linterman, M.A. Senescence Blurs the Line between Innate and Adaptive Immune Cells. Immunol. Cell Biol. 2020, 98, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Covre, L.P.; Martins, R.F.; Devine, O.P.; Chambers, E.S.; Vukmanovic-Stejic, M.; Silva, J.A.; Dietze, R.; Rodrigues, R.R.; de Matos Guedes, H.L.; Falqueto, A.; et al. Circulating Senescent T Cells Are Linked to Systemic Inflammation and Lesion Size During Human Cutaneous Leishmaniasis. Front. Immunol. 2019, 9, 3001. [Google Scholar] [CrossRef] [PubMed]
- Fantecelle, C.H.; Covre, L.P.; Garcia de Moura, R.; Guedes, H.L.d.M.; Amorim, C.F.; Scott, P.; Mosser, D.; Falqueto, A.; Akbar, A.N.; Gomes, D.C.O. Transcriptomic Landscape of Skin Lesions in Cutaneous Leishmaniasis Reveals a Strong CD8+ T Cell Immunosenescence Signature Linked to Immunopathology. Immunology 2021, 164, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Moura, R.; Covre, L.P.; Fantecelle, C.H.; Gajardo, V.A.T.; Cunha, C.B.; Stringari, L.L.; Belew, A.T.; Daniel, C.B.; Zeidler, S.V.V.; Tadokoro, C.E.; et al. PD-1 Blockade Modulates Functional Activities of Exhausted-Like T Cell in Patients With Cutaneous Leishmaniasis. Front. Immunol. 2021, 12, 632667. [Google Scholar] [CrossRef] [PubMed]
- Dojcinov, S.D.; Venkataraman, G.; Raffeld, M.; Pittaluga, S.; Jaffe, E.S. EBV Positive Mucocutaneous Ulcer—A Study of 26 Cases Associated With Various Sources of Immunosuppression. Am. J. Surg. Pathol. 2010, 34, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Bedier, H.; Lin, J.; Julien, L.-A.; Routy, J.-P. Concurrent Development of HIV-Negative Kaposi’s Sarcoma and Mycosis Fungoides in an Elderly Inuit from Canada. BMJ Case Rep. 2021, 14, e238644. [Google Scholar] [CrossRef]
- Stowe, R.P.; Peek, M.K.; Cutchin, M.P.; Goodwin, J.S. Reactivation of Herpes Simplex Virus Type 1 Is Associated with Cytomegalovirus and Age. J. Med. Virol. 2012, 84, 1797–1802. [Google Scholar] [CrossRef]
- Kim, J.-A.; Park, S.-K.; Kumar, M.; Lee, C.-H.; Shin, O.S. Insights into the Role of Immunosenescence during Varicella Zoster Virus Infection (Shingles) in the Aging Cell Model. Oncotarget 2015, 6, 35324–35343. [Google Scholar] [CrossRef]
- Lewthwaite, P.; Parsons, H.K.; Bates, C.J.; McKendrick, M.W.; Dockrell, D.H. Group G Streptococcal Bacteraemia: An Opportunistic Infection Associated with Immune Senescence. Scand. J. Infect. Dis. 2002, 34, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Nguyen, K.C.T.; Nguyen, C.T.; Jang, I.; Han, J.M.; Fabian, C.; Lee, S.E.; Rhee, J.H.; Cho, K.A. Flagellin-dependent TLR5/Caveolin-1 as a Promising Immune Activator in Immunosenescence. Aging Cell 2015, 14, 907–915. [Google Scholar] [CrossRef]
- Ault, R.; Dwivedi, V.; Koivisto, E.; Nagy, J.; Miller, K.; Nagendran, K.; Chalana, I.; Pan, X.; Wang, S.-H.; Turner, J. Altered Monocyte Phenotypes but Not Impaired Peripheral T Cell Immunity May Explain Susceptibility of the Elderly to Develop Tuberculosis. Exp. Gerontol. 2018, 111, 35–44. [Google Scholar] [CrossRef]
- da Silva, P.H.L.; de Castro, K.K.G.; Mendes, M.A.; Leal-Calvo, T.; Leal, J.M.P.; Nery, J.A.d.C.; Sarno, E.N.; Lourenço, R.A.; Moraes, M.O.; Lara, F.A.; et al. Presence of Senescent and Memory CD8+ Leukocytes as Immunocenescence Markers in Skin Lesions of Elderly Leprosy Patients. Front. Immunol. 2021, 12, 647385. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Spacova, I.; Patusco, R.; Lebeer, S.; Jensen, M.G. Influence of Biotic Interventions on the Immune Response to Vaccines in Young and Older Adults. Clin. Nutr. 2023, 42, 216–226. [Google Scholar] [CrossRef]
- Larson, P.J.; Zhou, W.; Santiago, A.; Driscoll, S.; Fleming, E.; Voigt, A.Y.; Chun, O.K.; Grady, J.J.; Kuchel, G.A.; Robison, J.T.; et al. Associations of the Skin, Oral and Gut Microbiome with Aging, Frailty and Infection Risk Reservoirs in Older Adults. Nat. Aging 2022, 2, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.Y.; Emiola, A.; Johnson, J.S.; Fleming, E.S.; Nguyen, H.; Zhou, W.; Tsai, K.Y.; Fink, C.; Oh, J. Skin Microbiome Variation with Cancer Progression in Human Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2022, 142, 2773–2782.e16. [Google Scholar] [CrossRef] [PubMed]
- Karimova, M.; Moyes, D.; Ide, M.; Setterfield, J.F. The Human Microbiome in Immunobullous Disorders and Lichen Planus. Clin. Exp. Dermatol. 2022, 47, 522–528. [Google Scholar] [CrossRef]
- De Spiegeleer, A.; Descamps, A.; Govindarajan, S.; Coudenys, J.; Van der borght, K.; Hirmz, H.; Van Den Noortgate, N.; Elewaut, D.; De Spiegeleer, B.; Wynendaele, E. Bacterial Quorum-Sensing Peptides as Immune Modulators Present in Systemic Circulation. Biomolecules 2023, 13, 296. [Google Scholar] [CrossRef]
- Scichilone, N.; Callari, A.; Augugliaro, G.; Marchese, M.; Togias, A.; Bellia, V. The Impact of Age on Prevalence of Positive Skin Prick Tests and Specific IgE Tests. Respir. Med. 2011, 105, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Di Bona, D.; Belluzzo, F.; Macchia, L. Immunological and Non-Immunological Mechanisms of Allergic Diseases in the Elderly: Biological and Clinical Characteristics. Immun. Ageing 2017, 14, 23. [Google Scholar] [CrossRef]
- Chen, B.; Yang, J.; Song, Y.; Zhang, D.; Hao, F. Skin Immunosenescence and Type 2 Inflammation: A Mini-Review With an Inflammaging Perspective. Front. Cell Dev. Biol. 2022, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Laurence, M.; Benito-León, J.; Calon, F. Malassezia and Parkinson’s Disease. Front. Neurol. 2019, 10, 758. [Google Scholar] [CrossRef]
- Molle, M.F.; Burroni, A.G.; Herzum, A.; Parodi, A. Erosive Pustular Dermatosis of the Scalp and Multiple Sclerosis: Just a Coincidence? Dermatol. Rep. 2022, 14, 9445. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, D.W. Senotherapeutics and Their Molecular Mechanism for Improving Aging. Biomol. Ther. 2022, 30, 490–500. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, V.; Li Pomi, F.; Borgia, F.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Immunosenescence and Skin: A State of Art of Its Etiopathogenetic Role and Crucial Watershed for Systemic Implications. Int. J. Mol. Sci. 2023, 24, 7956. https://doi.org/10.3390/ijms24097956
Papa V, Li Pomi F, Borgia F, Vaccaro M, Pioggia G, Gangemi S. Immunosenescence and Skin: A State of Art of Its Etiopathogenetic Role and Crucial Watershed for Systemic Implications. International Journal of Molecular Sciences. 2023; 24(9):7956. https://doi.org/10.3390/ijms24097956
Chicago/Turabian StylePapa, Vincenzo, Federica Li Pomi, Francesco Borgia, Mario Vaccaro, Giovanni Pioggia, and Sebastiano Gangemi. 2023. "Immunosenescence and Skin: A State of Art of Its Etiopathogenetic Role and Crucial Watershed for Systemic Implications" International Journal of Molecular Sciences 24, no. 9: 7956. https://doi.org/10.3390/ijms24097956
APA StylePapa, V., Li Pomi, F., Borgia, F., Vaccaro, M., Pioggia, G., & Gangemi, S. (2023). Immunosenescence and Skin: A State of Art of Its Etiopathogenetic Role and Crucial Watershed for Systemic Implications. International Journal of Molecular Sciences, 24(9), 7956. https://doi.org/10.3390/ijms24097956










