The Functional Role and Regulatory Mechanism of FTO m6A RNA Demethylase in Human Uterine Leiomyosarcoma
Abstract
1. Introduction
2. Results
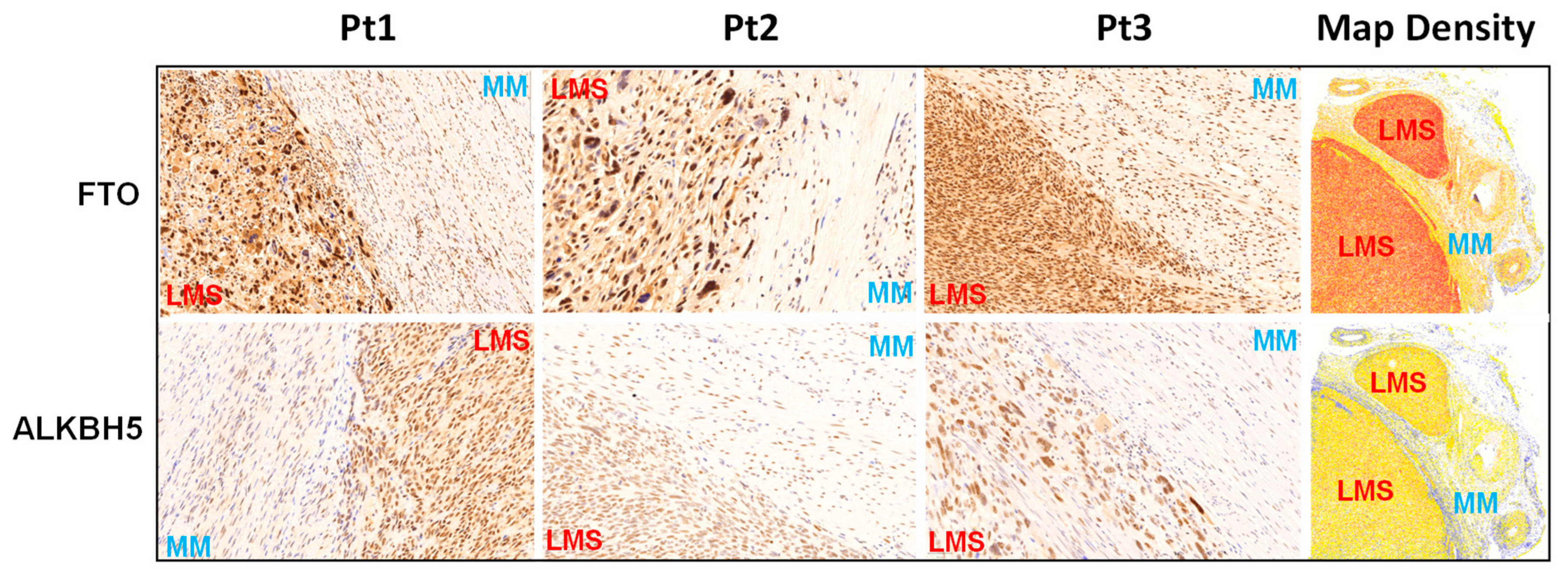
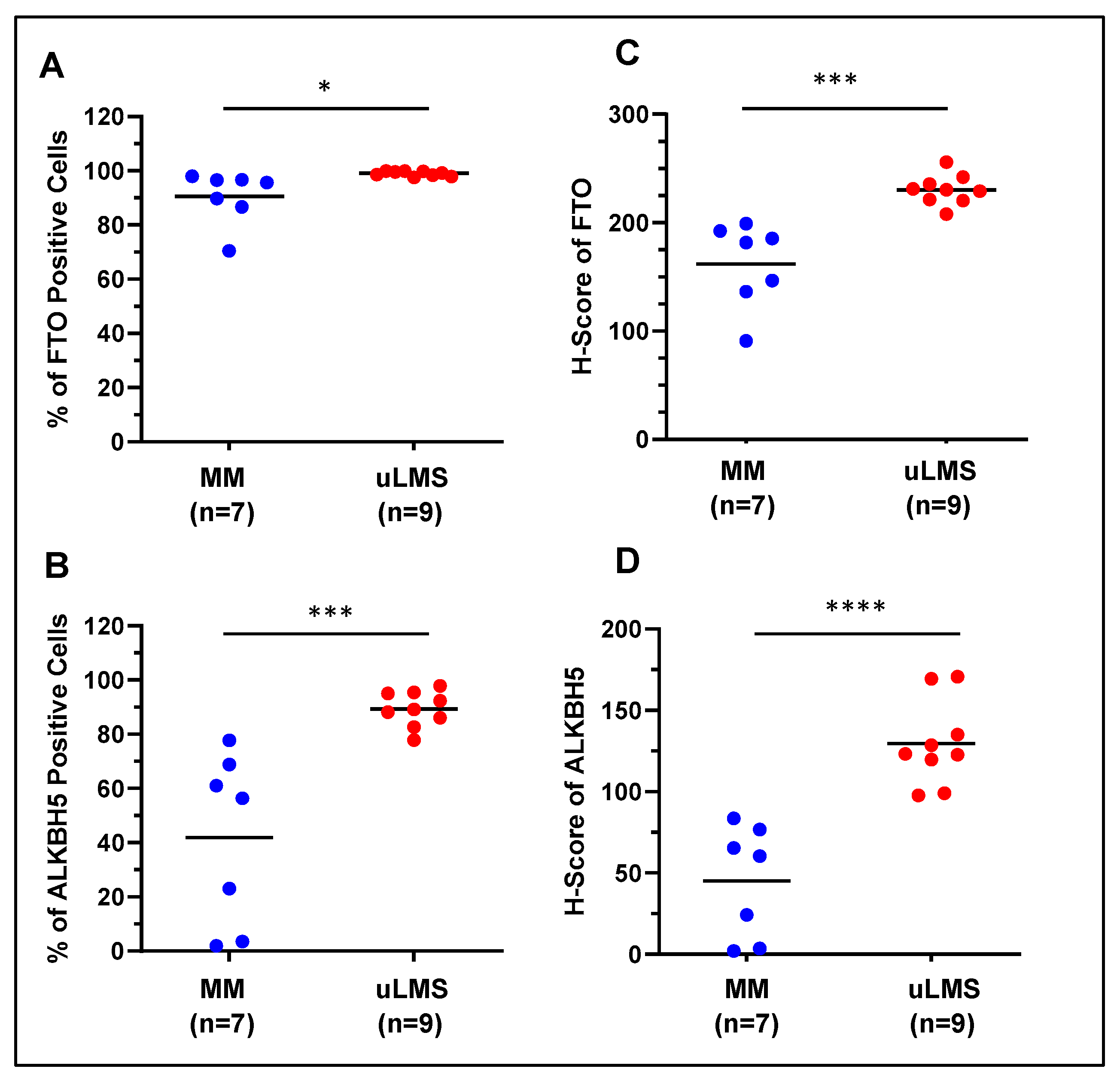
2.1. The Expression Levels of FTO and ALKBH5 m6A RNA Demethylases Are Upregulated in uLMS Tissues Compared to Adjacent Myometrium from Women with uLMS
2.2. Inhibition of FTO Decreased the Cell Proliferation in uLMS Cells
2.3. Inhibition of FTO Induces Cell Cycle Arrest in uLMS Cells
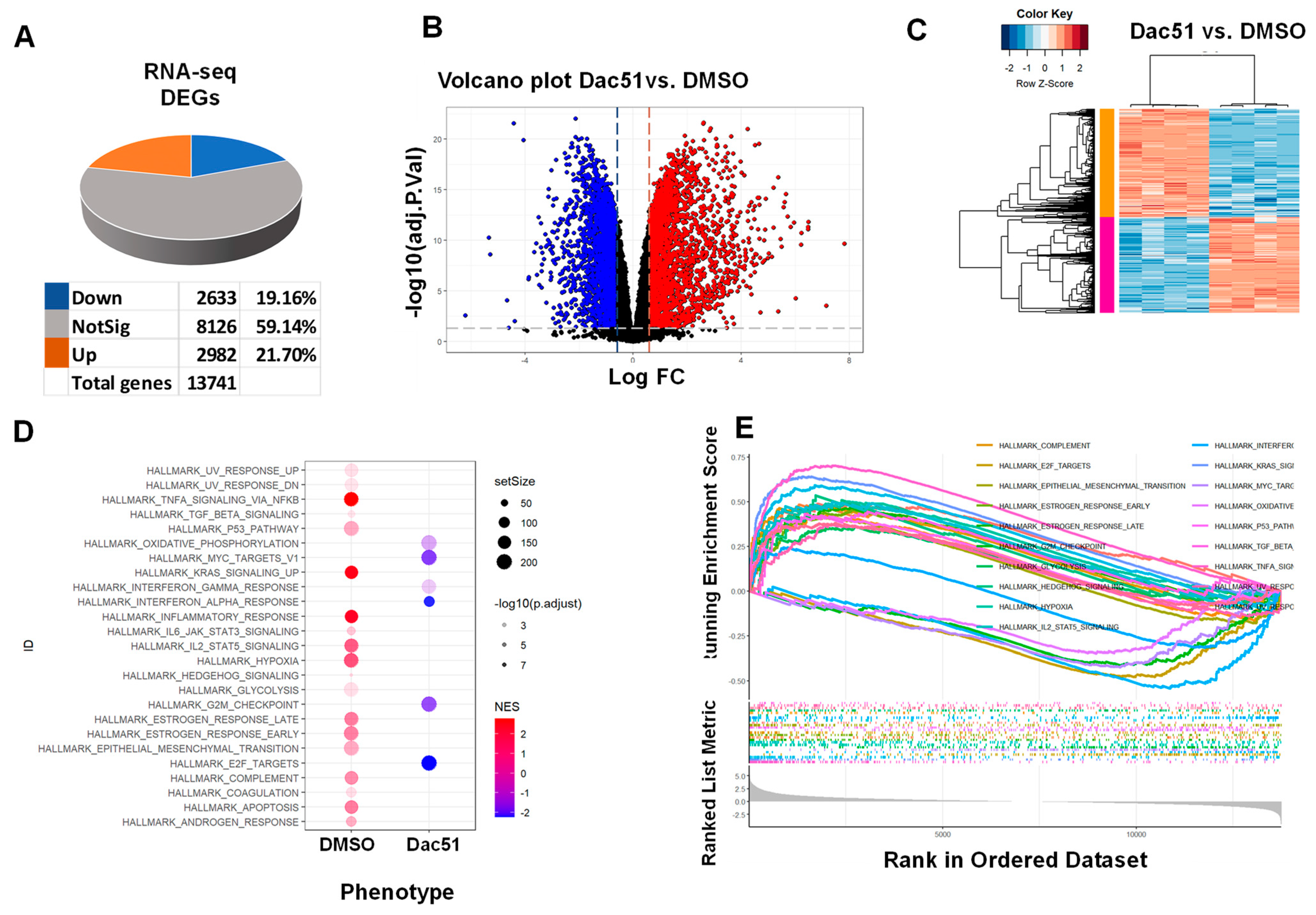
2.4. Dac51 Alters the Transcriptome of uLMS Cells
2.4.1. Enrichment Pathway Analysis
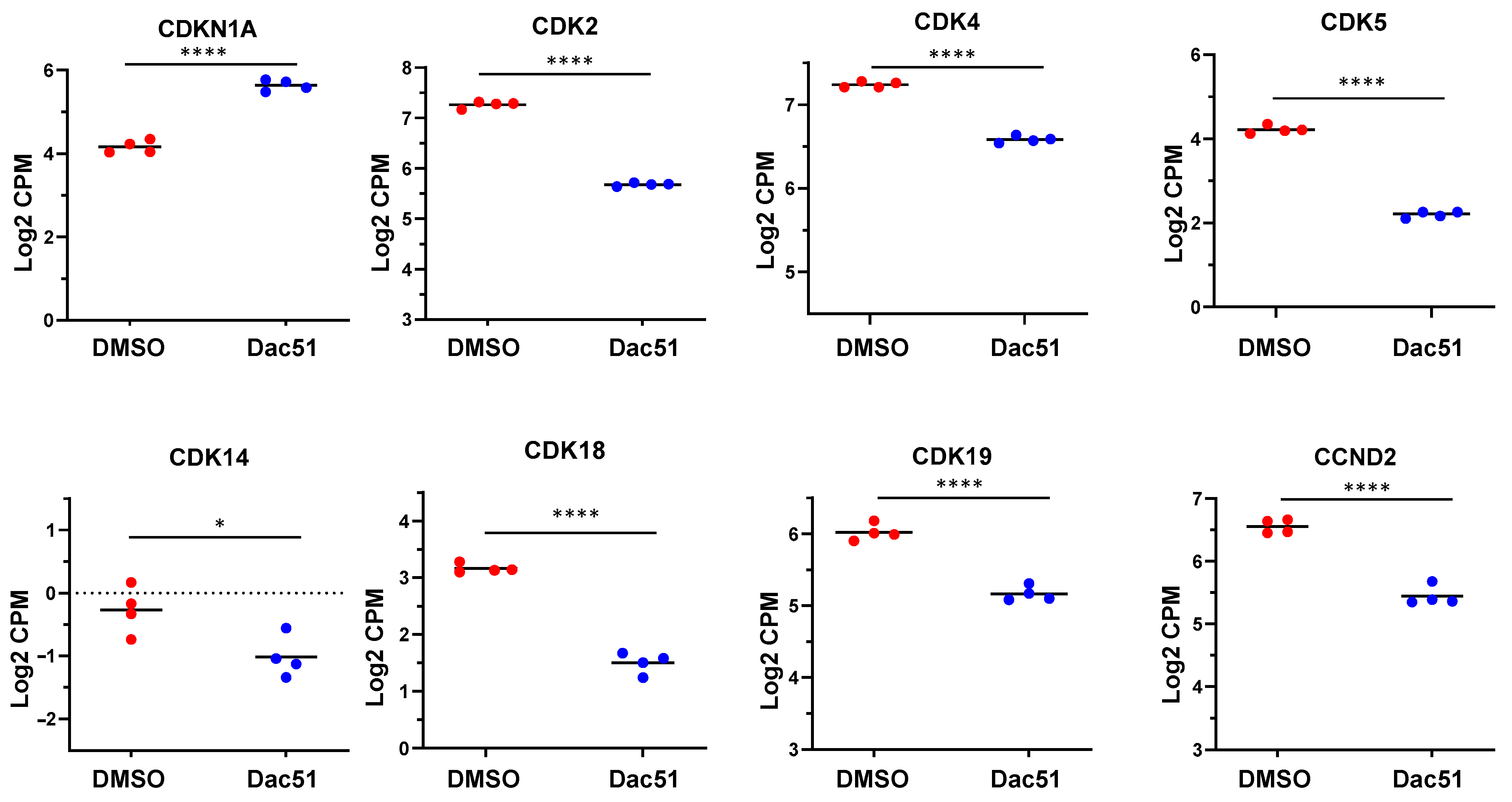
2.4.2. The Expression of Cell Cycle-Related Genes Is Altered upon Dac51 Treatment
2.4.3. Dac51 Altered the Gene Expression Associated with Transcriptional Factors
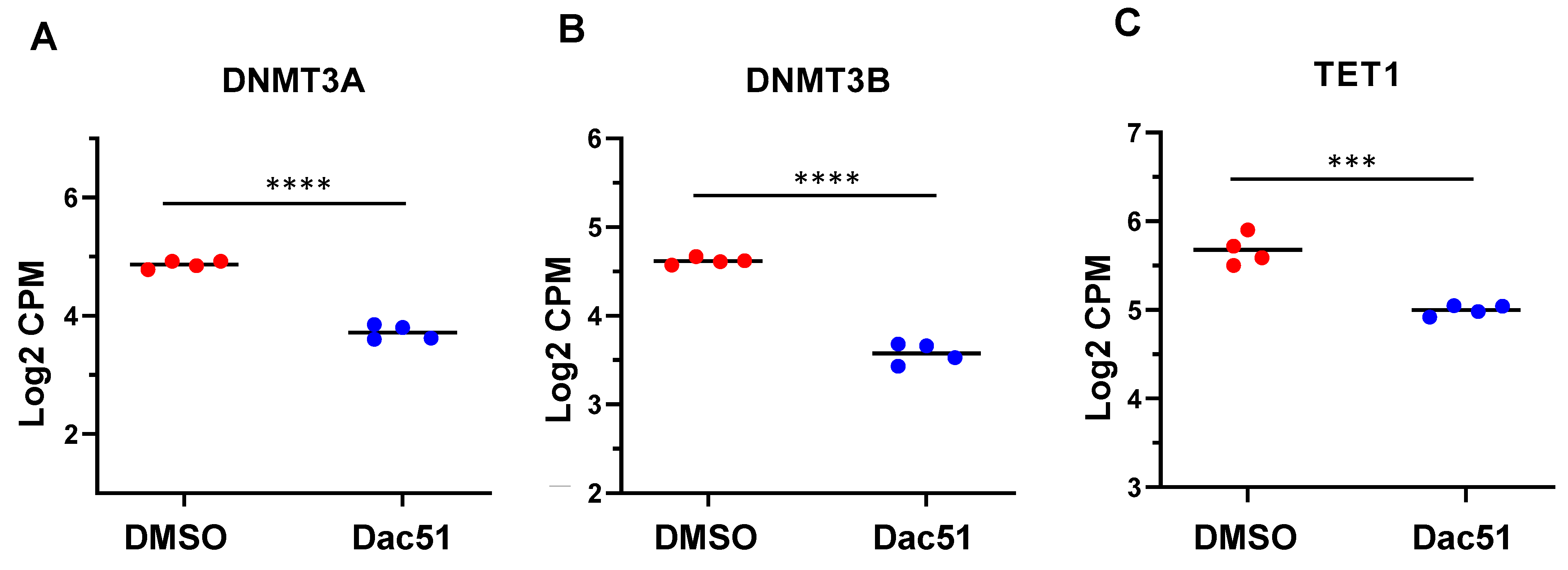
2.4.4. Dac51 Treatment Altered the Expression of Epigenetic Regulators
2.4.5. Dac51 Altered the Gene Expression Correlating with microRNA Regulation
3. Discussion
4. Materials and Methods
4.1. Uterine Leiomyosarcoma Samples
4.2. Immunohistochemistry
4.3. Cells and Reagents
4.4. Proliferation Assay
4.5. Measurement of Cell Cycle Phase Distribution
4.6. RNA-Sequencing
4.7. Transcriptome Profiles Analysis
4.7.1. Transcriptome Data Analysis
4.7.2. Differential Gene Expression Analysis
4.7.3. Gene List Enrichment Analysis
4.8. cDNA Synthesis and Quantitative Real-Time Polymerase Chain Reaction
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Angelo, E.; Prat, J. Uterine sarcomas: A review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Seagle, B.-L.L.; Sobecki-Rausch, J.; Strohl, A.E.; Shilpi, A.; Grace, A.; Shahabi, S. Prognosis and treatment of uterine leiomyosarcoma: A National Cancer Database study. Gynecol. Oncol. 2017, 145, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Blessing, J.A.; Mannel, R.; Rose, P.G. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol. Oncol. 2008, 109, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Landoni, F.; Sartori, E.; Zola, P.; Maggino, T.; Lissoni, A.; Bazzurini, L.; Arisio, R.; Romagnolo, C.; Cristofani, R. Uterine Leiomyosarcoma: Analysis of Treatment Failures and Survival. Gynecol. Oncol. 1996, 62, 25–32. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, B.C.; dos Anjos, L.G.; Dobroff, A.S.; Baracat, E.C.; Yang, Q.; Al-Hendy, A.; Carvalho, K.C. Epigenetic Features in Uterine Leiomyosarcoma and Endometrial Stromal Sarcomas: An Overview of the Literature. Biomedicines 2022, 10, 2567. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ciebiera, M.; Bariani, M.V.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2021, 43, 678–719. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Zhang, J.; Zhu, J.-S. The role of m6A RNA methylation in human cancer. Mol. Cancer 2019, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhou, Y.; Wu, H.; Lv, X.; Yang, L.; Ren, Z.; Tian, H.; Yu, Q.; Li, J.; Lin, W.; et al. RNA Demethylase FTO Mediated RNA m6A Modification Is Involved in Maintaining Maternal-Fetal Interface in Spontaneous Abortion. Front. Cell Dev. Biol. 2021, 9, 1935. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Mumbach, M.R.; Jovanovic, M.; Wang, T.; Maciag, K.; Kushkin, G.G.; Mertins, P.; Ter-Ovanesyan, D.; Habib, N.; Cacchiarelli, D.; et al. Perturbation of m6A Writers Reveals Two Distinct Classes of mRNA Methylation at Internal and 5′ Sites. Cell Rep. 2014, 8, 284–296. [Google Scholar] [CrossRef]
- Knuckles, P.; Carl, S.H.; Musheev, M.; Niehrs, C.; Wenger, A.; Bühler, M. RNA fate determination through cotranscriptional adenosine methylation and microprocessor binding. Nat. Struct. Mol. Biol. 2017, 24, 561–569. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Lu, Z.; Zhao, B.S.; Ma, H.; Hsu, P.J.; Liu, C.; He, C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017, 27, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wu, X.-N.; Ling, Y.; Rui, Y.; Wu, D.; Zhou, J.; Li, J.; Lin, S.; Peng, Q.; Li, Z.; et al. A novel inhibitor of N6-methyladenosine demethylase FTO induces mRNA methylation and shows anti-cancer activities. Acta Pharm. Sin. B 2021, 12, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Q.; Deng, H.; Xu, B.; Zhou, Y.; Liu, J.; Liu, Y.; Shi, Y.; Zhang, X.; Jiang, J. N6-methyladenosine demethylase FTO promotes growth and metastasis of gastric cancer via m(6)A modification of caveolin-1 and metabolic regulation of mitochondrial dynamics. Cell Death Dis. 2022, 13, 72. [Google Scholar] [CrossRef]
- Wang, D.; Qu, X.; Lu, W.; Wang, Y.; Jin, Y.; Hou, K.; Yang, B.; Li, C.; Qi, J.; Xiao, J.; et al. N6-Methyladenosine RNA Demethylase FTO Promotes Gastric Cancer Metastasis by Down-Regulating the m6A Methylation of ITGB1. Front. Oncol. 2021, 11, 681280. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Li, L.; Yang, W.; Zhang, Z.; Zhang, K.; Ma, K.; Xie, H.; Zhang, Z.; Cai, L.; et al. FTO-mediated autophagy promotes progression of clear cell renal cell carcinoma via regulating SIK2 mRNA stability. Int. J. Biol. Sci. 2022, 18, 5943–5962. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Wu, L.; Zhai, L.L.; Chen, L.J.; Yao, L.C.; Yu, K.H.; Tang, Z.G. N(6)-methyladenosine RNA demethylase FTO regulates extracellular matrix-related genes and promotes pancreatic cancer cell migration and invasion. Cancer Med. 2022, 12, 3731–3743. [Google Scholar] [CrossRef]
- Tan, Z.; Shi, S.; Xu, J.; Liu, X.; Lei, Y.; Zhang, B.; Hua, J.; Meng, Q.; Wang, W.; Yu, X.; et al. RNA N6-methyladenosine demethylase FTO promotes pancreatic cancer progression by inducing the autocrine activity of PDGFC in an m6A-YTHDF2-dependent manner. Oncogene 2022, 41, 2860–2872. [Google Scholar] [CrossRef]
- Duan, X.; Yang, L.; Wang, L.; Liu, Q.; Zhang, K.; Liu, S.; Liu, C.; Gao, Q.; Li, L.; Qin, G.; et al. m6A demethylase FTO promotes tumor progression via regulation of lipid metabolism in esophageal cancer. Cell Biosci. 2022, 12, 60. [Google Scholar] [CrossRef]
- Liu, S.; Huang, M.; Chen, Z.; Chen, J.; Chao, Q.; Yin, X.; Quan, M. FTO promotes cell proliferation and migration in esophageal squamous cell carcinoma through up-regulation of MMP13. Exp. Cell Res. 2020, 389, 111894. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Y.; Sun, M.; Sun, H.; Xie, F.; Chang, H.; Wang, Y.; Song, J.; Lai, S.; Yang, C.; et al. FTO promotes colorectal cancer progression and chemotherapy resistance via demethylating G6PD/PARP1. Clin. Transl. Med. 2022, 12, e772. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhang, J.; Zuo, L.; Yan, H.; Chen, L.; Zhao, F.; Fan, F.; Xu, J.; Zhang, B.; Zhang, Y.; et al. FTO promotes multiple myeloma progression by posttranscriptional activation of HSF1 in an m6A-YTHDF2-dependent manner. Mol. Ther. 2021, 30, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Wu, X.; Sun, Z.; Wang, F.; Wang, B.; Dong, P. The RNA N6-Methyladenosine Demethylase FTO Promotes Head and Neck Squamous Cell Carcinoma Proliferation and Migration by Increasing CTNNB1. Int. J. Gen. Med. 2021, ume 14, 8785–8795. [Google Scholar] [CrossRef]
- Zhou, G.; Yan, K.; Liu, J.; Gao, L.; Jiang, X.; Fan, Y. FTO promotes tumour proliferation in bladder cancer via the FTO/miR-576/CDK6 axis in an m6A-dependent manner. Cell Death Discov. 2021, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Mu, X.; Chen, H.; Jin, D.; Zhang, R.; Zhao, Y.; Fan, J.; Cao, M.; Zhou, Z. FTO modifies the m6A level of MALAT and promotes bladder cancer progression. Clin. Transl. Med. 2021, 11, e310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wan, Y.; Zhang, Z.; Jiang, Y.; Lang, J.; Cheng, W.; Zhu, L. FTO demethylates m6A modifications in HOXB13 mRNA and promotes endometrial cancer metastasis by activating the WNT signalling pathway. RNA Biol. 2021, 18, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, S.; Chen, W.; Zhang, X.; Chen, J.; Jiang, J.; Wang, M.; Zhang, L.; Xuan, Z. Fat mass and obesity-associated protein promotes the tumorigenesis and development of liver cancer. Oncol. Lett. 2020, 20, 1409–1417. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Zhang, H.; Qian, Z.; Jia, W.; Gao, Y.; Zheng, H.; Li, B. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem. Biophys. Res. Commun. 2019, 512, 479–485. [Google Scholar] [CrossRef]
- Liu, J.; Ren, D.; Du, Z.; Wang, H.; Zhang, H.; Jin, Y. m6A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem. Biophys. Res. Commun. 2018, 502, 456–464. [Google Scholar] [CrossRef]
- Niu, Y.; Lin, Z.; Wan, A.; Chen, H.; Liang, H.; Sun, L.; Wang, Y.; Li, X.; Xiong, X.-F.; Wei, B.; et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol. Cancer 2019, 18, 46. [Google Scholar] [CrossRef]
- Su, R.; Dong, L.; Li, Y.; Gao, M.; Han, L.; Wunderlich, M.; Deng, X.; Li, H.; Huang, Y.; Gao, L.; et al. Targeting FTO Suppresses Cancer Stem Cell Maintenance and Immune Evasion. Cancer Cell 2020, 38, 79–96.e11. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Dong, L.; Li, C.; Yin, Z.; Rao, S.; Zhou, Q. The m6A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 2019, 19, 321. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, J.; Zhang, Y.; Lu, D.; Dai, Y. FTO promotes cervical cancer cell proliferation, colony formation, migration and invasion via the regulation of the BMP4/Hippo/YAP1/TAZ pathway. Exp. Cell Res. 2023, 427, 113585. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wan, Y.; Gong, M.; Zhou, S.; Qiu, J.; Cheng, W. RNA demethylase ALKBH5 promotes ovarian carcinogenesis in a simulated tumour microenvironment through stimulating NF-kappaB pathway. J. Cell Mol. Med. 2020, 24, 6137–6148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kong, X.; Zhong, W.; Wang, Y.; Li, P. FTO accelerates ovarian cancer cell growth by promoting proliferation, inhibiting apoptosis, and activating autophagy. Pathol. Res. Pract. 2020, 216, 153042. [Google Scholar] [CrossRef]
- Sun, R.; Yuan, L.; Jiang, Y.; Wan, Y.; Ma, X.; Yang, J.; Sun, G.; Zhou, S.; Wang, H.; Qiu, J. ALKBH5 activates FAK signaling through m6A demethylation in ITGB1 mRNA and enhances tumor-associated lymphangiogenesis and lymph node metastasis in ovarian cancer. Theranostics 2023, 13, 833–848. [Google Scholar] [CrossRef]
- Huang, Y.; Su, R.; Sheng, Y.; Dong, L.; Dong, Z.; Xu, H.; Ni, T.; Zhang, Z.S.; Zhang, T.; Li, C. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell 2019, 35, 677–691.e10. [Google Scholar] [CrossRef]
- Huff, S.; Kummetha, I.R.; Zhang, L.; Wang, L.; Bray, W.; Yin, J.; Kelly, V.; Wang, Y.; Rana, T.M. Rational Design and Optimization of m6A-RNA Demethylase FTO Inhibitors as Anticancer Agents. J. Med. Chem. 2022, 65, 10920–10937. [Google Scholar] [CrossRef]
- Qin, B.; Bai, Q.; Yan, D.; Yin, F.; Zhu, Z.; Xia, C.; Yang, Y.; Zhao, Y. Discovery of novel mRNA demethylase FTO inhibitors against esophageal cancer. J. Enzym. Inhib. Med. Chem. 2022, 37, 1995–2003. [Google Scholar] [CrossRef]
- You, Y.; Fu, Y.; Huang, M.; Shen, D.; Zhao, B.; Liu, H.; Zheng, Y.; Huang, L. Recent Advances of m6A Demethylases Inhibitors and Their Biological Functions in Human Diseases. Int. J. Mol. Sci. 2022, 23, 5815. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, G.; Xu, H.; Dong, W.; Dong, Z.; Qiu, Z.; Zhang, Z.; Li, F.; Huang, Y.; Li, Y.; et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 2021, 33, 1221–1233.e11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Ryu, T.; Jung, C.R.; Lee, M.S.; Lim, J.H.; Park, K.; Kim, D.S.; Son, M.Y.; Hamamoto, R.; et al. EHMT1 knockdown induces apoptosis and cell cycle arrest in lung cancer cells by increasing CDKN1A expression. Mol. Oncol. 2021, 15, 2989–3002. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, A.; You, Z.; Xu, J.; Zhu, S. Epigenetic silencing of CDKN1A and CDKN2B by SNHG1 promotes the cell cycle, migration and epithelial-mesenchymal transition progression of hepatocellular carcinoma. Cell Death Dis. 2020, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, H.; Liu, Y.; Li, X.; Zhang, C.; Qin, Q.; Pang, Q. miR-106b-5p promotes cell proliferation and cell cycle progression by directly targeting CDKN1A in osteosarcoma. Exp. Ther. Med. 2020, 19, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Bradner, J.E.; Hnisz, D.; Young, R.A. Transcriptional Addiction in Cancer. Cell 2017, 168, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, L.; Lin, R.Y.-T.; Müschen, M.; Koeffler, H.P. Core transcriptional regulatory circuitries in cancer. Oncogene 2020, 39, 6633–6646. [Google Scholar] [CrossRef] [PubMed]
- Lasman, L.; Hanna, J.H.; Novershtern, N. Role of m6A in Embryonic Stem Cell Differentiation and in Gametogenesis. Epigenomes 2020, 4, 5. [Google Scholar] [CrossRef]
- Fiorenzano, A.; Pascale, E.; Gagliardi, M.; Terreri, S.; Papa, M.; Andolfi, G.; Galasso, M.; Tagliazucchi, G.M.; Taccioli, C.; Patriarca, E.J.; et al. An Ultraconserved Element Containing lncRNA Preserves Transcriptional Dynamics and Maintains ESC Self-Renewal. Stem Cell Rep. 2018, 10, 1102–1114. [Google Scholar] [CrossRef]
- Fiorenzano, A.; Pascale, E.; Patriarca, E.J.; Minchiotti, G.; Fico, A. LncRNAs and PRC2: Coupled Partners in Embryonic Stem Cells. Epigenomes 2019, 3, 14. [Google Scholar] [CrossRef]
- Han, F.; Cheng, C.; Xu, Q.; Chen, J.; Yang, Z.; Liu, J. DEPDC1B promotes colorectal cancer via facilitating cell proliferation and migration while inhibiting apoptosis. Cell Cycle 2022, 22, 131–143. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, W.J.; Zhou, Y.H.; Chen, Z.M.; Liu, K. Andrographolide inhibits non-small cell lung cancer cell proliferation through the activation of the mitochondrial apoptosis pathway and by reprogramming host glucose metabolism. Ann. Transl. Med. 2021, 9, 1701. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Fan, H.; Ma, X.; Yang, C.; He, Y.; Ge, Y.; Jiang, M.; Xu, Z.; Yang, L. miR-1301-3p Promotes Cell Proliferation and Facilitates Cell Cycle Progression via Targeting SIRT1 in Gastric Cancer. Front. Oncol. 2021, 11, 664242. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Feng, H.; Wang, C.; Song, Z.; Zhang, Y.; Liu, K.; Cheng, X.; Zhao, R. The role of E26 transformation-specific variant transcription factor 5 in colorectal cancer cell proliferation and cell cycle progression. Cell Death Dis. 2021, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Falahati, A.; Khosh, A.; Mohammed, H.; Kang, W.; Corachán, A.; Bariani, M.V.; Boyer, T.G.; Al-Hendy, A. Targeting Class I Histone Deacetylases in Human Uterine Leiomyosarcoma. Cells 2022, 11, 3801. [Google Scholar] [CrossRef]
- Liu, Z.; Duan, Z.; Zhang, D.; Xiao, P.; Zhang, T.; Xu, H.; Wang, C.H.; Rao, G.W.; Gan, J.; Huang, Y.; et al. Structure–Activity Relationships and Antileukemia Effects of the Tricyclic Benzoic Acid FTO Inhibitors. J. Med. Chem. 2022, 65, 10638–10654. [Google Scholar] [CrossRef]
- Hu, F.; Yan, H.J.; Gao, C.X.; Sun, W.W.; Long, Y.S. Inhibition of Hypothalamic FTO Activates STAT3 Signal through ERK1/2 Associated with Reductions in Food Intake and Body Weight. Neuroendocrinology 2023, 113, 80–91. [Google Scholar] [CrossRef]
- Bushweller, J.H. Targeting transcription factors in cancer from undruggable to reality. Nat. Rev. Cancer 2019, 19, 611–624. [Google Scholar] [CrossRef]
- Ala, M. Target c-Myc to treat pancreatic cancer. Cancer Biol. Ther. 2022, 23, 34–50. [Google Scholar] [CrossRef]
- Donati, G.; Amati, B. MYC and therapy resistance in cancer: Risks and opportunities. Mol. Oncol. 2022, 16, 3828–3854. [Google Scholar] [CrossRef]
- Fatma, H.; Maurya, S.K.; Siddique, H.R. Epigenetic modifications of c-MYC: Role in cancer cell reprogramming, progression and chemoresistance. Semin. Cancer Biol. 2022, 83, 166–176. [Google Scholar] [CrossRef]
- Roworth, A.P.; Ghari, F.; La Thangue, N.B. To live or let die–complexity within the E2F1 pathway. Mol. Cell. Oncol. 2014, 2, e970480. [Google Scholar] [CrossRef] [PubMed]
- Fouad, S.; Hauton, D.; D’Angiolella, V. E2F1: Cause and Consequence of DNA Replication Stress. Front. Mol. Biosci. 2021, 7, 599332. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Lin, M.; Li, C.; Liu, H.; Gong, C. A comprehensive review of the roles of E2F1 in colon cancer. Am. J. Cancer Res. 2020, 10, 757–768. [Google Scholar] [PubMed]
- Huang, Y.; Chen, R.; Zhou, J. E2F1 and NF-kappaB: Key Mediators of Inflammation-associated Cancers and Potential Therapeutic Targets. Curr. Cancer Drug Targets 2016, 16, 765–772. [Google Scholar] [CrossRef]
- Sun, H.; Ma, H.; Zhang, H.; Ji, M. Up-regulation of MELK by E2F1 promotes the proliferation in cervical cancer cells. Int. J. Biol. Sci. 2021, 17, 3875–3888. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.-Y.; Chen, Y.; Tham, V.Y.; Lin, R.Y.; Dakle, P.; Nacro, K.; Puhaindran, M.E.; Houghton, P.; Pang, A.; Lee, V.K.; et al. MNK1 and MNK2 enforce expression of E2F1, FOXM1, and WEE1 to drive soft tissue sarcoma. Oncogene 2021, 40, 1851–1867. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, L.; Li, Y.; Cao, D.; Quan, S.; Guo, Y.; Yang, X.; Yang, T. Human Papillomavirus E7 Oncoprotein Promotes Proliferation and Migration through the Transcription Factor E2F1 in Cervical Cancer Cells. Anti-Cancer Agents Med. Chem. 2021, 21, 1689–1696. [Google Scholar] [CrossRef]
- Hemming, M.L.; Fan, C.; Raut, C.P.; Demetri, G.D.; Armstrong, S.A.; Sicinska, E.; George, S. Oncogenic Gene-Expression Programs in Leiomyosarcoma and Characterization of Conventional, Inflammatory, and Uterogenic Subtypes. Mol. Cancer Res. 2020, 18, 1302–1314. [Google Scholar] [CrossRef]
- Black, A.R.; Black, J.D.; Azizkhan-Clifford, J. Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell. Physiol. 2001, 188, 143–160. [Google Scholar] [CrossRef]
- Deng, Y.R.; Chen, X.J.; Chen, W.; Wu, L.F.; Jiang, H.P.; Kin, D.; Wang, L.J.; Wang, W.; Guo., S.Q. Sp1 contributes to radioresistance of cervical cancer through targeting G2/M cell cycle checkpoint CDK1. Cancer Manag. Res. 2019, 11, 5835–5844. [Google Scholar] [CrossRef]
- Grinstein, E.; Jundt, F.; Weinert, I.; Wernet, P.; Royer, H.-D. Sp1 as G1 cell cycle phase specific transcription factor in epithelial cells. Oncogene 2002, 21, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Opitz, O.G.; Rustgi, A.K. Interaction between Sp1 and cell cycle regulatory proteins is important in transactivation of a differentiation-related gene. Cancer Res. 2000, 60, 2825–2830. [Google Scholar] [PubMed]
- Bourassa, M.W.; Ratan, R.R. The interplay between microRNAs and histone deacetylases in neurological diseases. Neurochem. Int. 2014, 77, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Baltan, S.; Sandau, U.S.; Brunet, S.; Bastian, C.; Tripathi, A.; Nguyen, H.; Liu, H.; Saugstad, J.A.; Zarnegarnia, Y.; Dutta, R. Identification of miRNAs That Mediate Protective Functions of Anti-Cancer Drugs During White Matter Ischemic Injury. ASN Neuro 2021, 13, 17590914211042220. [Google Scholar] [CrossRef]
- Klieser, E.; Urbas, R.; Swierczynski, S.; Stättner, S.; Primavesi, F.; Jäger, T.; Mayr, C.; Kiesslich, T.; Di Fazio, P.; Helm, K.; et al. HDAC-Linked “Proliferative” miRNA Expression Pattern in Pancreatic Neuroendocrine Tumors. Int. J. Mol. Sci. 2018, 19, 2781. [Google Scholar] [CrossRef]
- Liu, J.; Dou, X.; Chen, C.Y.; Chen, C.; Liu, C.; Xu, M.M.; Zhao, S.; Shen, B.; Gao, Y.; Han, D.; et al. N6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 2020, 367, 580–586. [Google Scholar] [CrossRef]
- Yang, Q.; Bariani, M.V.; Falahati, A.; Khosh, A.; Lastra, R.R.; Siblini, H.; Boyer, T.G.; Al-Hendy, A. The Functional Role and Regulatory Mechanism of Bromodomain-Containing Protein 9 in Human Uterine Leiomyosarcoma. Cells 2022, 11, 2160. [Google Scholar] [CrossRef]
- Ram, S.; Vizcarra, P.; Whalen, P.; Deng, S.; Painter, C.L.; Jackson-Fisher, A.; Pirie-Shepherd, S.; Xia, X.; Powell, E.L. Pixelwise H-score: A novel digital image analysis-based metric to quantify membrane biomarker expression from immunohistochemistry images. PLoS ONE 2021, 16, e0245638. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Al-Hendy, A. The Functional Role and Regulatory Mechanism of FTO m6A RNA Demethylase in Human Uterine Leiomyosarcoma. Int. J. Mol. Sci. 2023, 24, 7957. https://doi.org/10.3390/ijms24097957
Yang Q, Al-Hendy A. The Functional Role and Regulatory Mechanism of FTO m6A RNA Demethylase in Human Uterine Leiomyosarcoma. International Journal of Molecular Sciences. 2023; 24(9):7957. https://doi.org/10.3390/ijms24097957
Chicago/Turabian StyleYang, Qiwei, and Ayman Al-Hendy. 2023. "The Functional Role and Regulatory Mechanism of FTO m6A RNA Demethylase in Human Uterine Leiomyosarcoma" International Journal of Molecular Sciences 24, no. 9: 7957. https://doi.org/10.3390/ijms24097957
APA StyleYang, Q., & Al-Hendy, A. (2023). The Functional Role and Regulatory Mechanism of FTO m6A RNA Demethylase in Human Uterine Leiomyosarcoma. International Journal of Molecular Sciences, 24(9), 7957. https://doi.org/10.3390/ijms24097957





