Staphylococcal Resistance Patterns, blaZ and SCCmec Cassette Genes in the Nasopharyngeal Microbiota of Pregnant Women
Abstract
:1. Introduction
2. Results
2.1. Determination of Drug Susceptibility Profile by Disc-Diffusion Method
2.2. Determination of blaZ, mecA and mecC Gene Prevalence among Tested Staphylococcus spp. Isolates
2.3. Determination of SCCmec Gene Cassette Type
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates and Culture
4.2. Disk Diffusion Method
4.3. DNA Extraction and PCR Reaction
4.3.1. Specific PCR Reactions
4.3.2. SCCmec Cassette Detection and Typing
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Recommendations for Prevention and Treatment of Maternal Peripartum Infections; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Żukowska, A.; Hryniewicz, W. Recommendations for Diagnosis, Antibiotic Therapy and Prevention Hospital-Acquired Infections; National Medicines Institute: Warsaw, Poland, 2020. [Google Scholar]
- Różańska, A.; Pac, A.; Jachowicz, E.; Jaślan, D.; Siewierska, M.; Wójkowska-Mach, J. Outpatient Antibiotic Prescriptions in Pregnant Women in Małopolska Province. Antibiotics 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstet. Gynecol. 2020, 135, e51–e72. [CrossRef] [PubMed]
- Schafer, R.; Phillippi, J.C. Group B Streptococcal Bacteriuria in Pregnancy: An Evidence-Based, Patient-Centered Approach to Care. J. Midwifery Womens Health 2020, 65, 376–381. [Google Scholar] [CrossRef]
- Pan, H.; Cui, B.; Huang, Y.; Yang, J.; Ba-Thein, W. Nasal Carriage of Common Bacterial Pathogens among Healthy Kindergarten Children in Chaoshan Region, Southern China: A Cross-Sectional Study. BMC Pediatr. 2016, 16, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zhang, J.; He, Y.; Lv, Z.; Liang, Z.; Chen, J.; Li, P.; Liu, J.; Yang, H.; Tao, A.; et al. Exploring the Role of Staphylococcus aureus in Inflammatory Diseases. Toxins 2022, 14, 464. [Google Scholar] [CrossRef]
- Michels, R.; Last, K.; Becker, S.L.; Papan, C. Update on Coagulase-Negative Staphylococci—What the Clinician Should Know. Microorganisms 2021, 9, 830. [Google Scholar] [CrossRef]
- Alharbi, N.S. Screening of Antibiotic-Resistant Staphylococci in the Nasal Cavity of Patients and Healthy Individuals. Saudi J. Biol. Sci. 2020, 27, 100–105. [Google Scholar] [CrossRef]
- Fujiwara, N.; Tsuruda, K.; Iwamoto, Y.; Kato, F.; Odaki, T.; Yamane, N.; Hori, Y.; Harashima, Y.; Sakoda, A.; Tagaya, A.; et al. Significant Increase of Oral Bacteria in the Early Pregnancy Period in Japanese Women. J. Investig. Clin. Dent. 2017, 8, e12189. [Google Scholar] [CrossRef]
- Stratmann, J.A.; Lacko, R.; Ballo, O.; Shaid, S.; Gleiber, W.; Vehreschild, M.J.G.T.; Wichelhaus, T.; Reinheimer, C.; Göttig, S.; Kempf, V.A.J.; et al. Colonization with Multi-Drug-Resistant Organisms Negatively Impacts Survival in Patients with Non-Small Cell Lung Cancer. PLoS ONE 2020, 15, e0242544. [Google Scholar] [CrossRef]
- Aubry, B.; Lemarié, C.; Chenouard, R.; Kempf, M.; Eveillard, M.; Pailhoriès, H. Performance of Penicillinase Detection Tests in Staphylococcus Epidermidis: Comparison of Different Phenotypic Methods. BMC Microbiol. 2020, 20, 240. [Google Scholar] [CrossRef]
- Soares, L.C.; Pereira, I.A.; Pribul, B.R.; Oliva, M.S.; Coelho, S.M.O.; Souza, M.M.S. Antimicrobial Resistance and Detection of MecA and BlaZ Genes in Coagulase-Negative Staphylococcus Isolated from Bovine Mastitis. Pesqui. Veterinária Bras. 2012, 32, 692–696. [Google Scholar] [CrossRef] [Green Version]
- Zong, Z.; Peng, C.; Lü, X. Diversity of SCCmec Elements in Methicillin-Resistant Coagulase-Negative Staphylococci Clinical Isolates. PLoS ONE 2011, 6, e20191. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.E.; Christensen, H.; Aarestrup, F.M. Diversity and Evolution of BlaZ from Staphylococcus aureus and Coagulase-Negative Staphylococci. J. Antimicrob. Chemother. 2006, 57, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Marincola, G.; Liong, O.; Schoen, C.; Abouelfetouh, A.; Hamdy, A.; Wencker, F.D.R.; Marciniak, T.; Becker, K.; Köck, R.; Ziebuhr, W. Antimicrobial Resistance Profiles of Coagulase-Negative Staphylococci in Community-Based Healthy Individuals in Germany. Front. Public Health 2021, 9, 684456. [Google Scholar] [CrossRef] [PubMed]
- Biesbroek, G.; Tsivtsivadze, E.; Sanders, E.A.M.; Montijn, R.; Veenhoven, R.H.; Keijser, B.J.F.; Bogaert, D. Early Respiratory Microbiota Composition Determines Bacterial Succession Patterns and Respiratory Health in Children. Am. J. Respir. Crit. Care Med. 2014, 190, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Bagcigil, A.F.; Taponen, S.; Koort, J.; Bengtsson, B.; Myllyniemi, A.-L.; Pyörälä, S. Genetic Basis of Penicillin Resistance of S. aureus Isolated in Bovine Mastitis. Acta Vet. Scand. 2012, 54, 69. [Google Scholar] [CrossRef] [Green Version]
- Rocha, G.D.; Nogueira, J.F.; Gomes dos Santos, M.V.; Boaventura, J.A.; Nunes Soares, R.A.; José de Simoni Gouveia, J.; Matiuzzi da Costa, M.; Gouveia, G.V. Impact of Polymorphisms in BlaZ, BlaR1 and BlaI Genes and Their Relationship with β-Lactam Resistance in S. Aureus Strains Isolated from Bovine Mastitis. Microb. Pathog. 2022, 165, 105453. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Martins, K.B.; da Silva, V.R.; Mondelli, A.L.; de Lourdes Ribeiro de Souza da Cunha, M. Correlation of Phenotypic Tests with the Presence of the BlaZ Gene for Detection of Beta-Lactamase. Braz. J. Microbiol. 2017, 48, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Singh-Moodley, A.; Strasheim, W.; Mogokotleng, R.; Ismail, H.; Perovic, O. Unconventional SCCmec Types and Low Prevalence of the Panton-Valentine Leukocidin Exotoxin in South African Blood Culture Staphylococcus aureus Surveillance Isolates, 2013–2016. PLoS ONE 2019, 14, e0225726. [Google Scholar] [CrossRef]
- Okiki, P.A.; Eromosele, E.S.; Ade-Ojo, P.; Sobajo, O.A.; Idris, O.O.; Agbana, R.D. Occurrence of MecA and BlaZ Genes in Methicillin-Resistant Staphylococcus aureus Associated with Vaginitis among Pregnant Women in Ado-Ekiti, Nigeria. New Microbes New Infect. 2020, 38, 100772. [Google Scholar] [CrossRef]
- Uehara, Y. Current Status of Staphylococcal Cassette Chromosome Mec (SCCmec). Antibiotics 2022, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- International Working Group on the Staphylococcal Cassette Chromosome Elements (IWG-SCC). Available online: https://www.sccmec.org/index.php/en/ (accessed on 5 December 2022).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0; The European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2022. [Google Scholar]
- Bucka-Kolendo, J.; Sokołowska, B.; Winiarczyk, S. Influence of High Hydrostatic Pressure on the Identification of Lactobacillus by MALDI-TOF MS- Preliminary Study. Microorganisms 2020, 8, 813. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of Multiplex PCRs for Staphylococcal Cassette Chromosome Mec Type Assignment: Rapid Identification System for Mec, Ccr, and Major Differences in Junkyard Regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Daley, A.J.; Istivan, T.S.; Garland, S.M.; Deighton, M.A. Antibiotic Susceptibility of Coagulase-Negative Staphylococci Isolated from Very Low Birth Weight Babies: Comprehensive Comparisons of Bacteria at Different Stages of Biofilm Formation. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xu, Y.; Zhao, H.; Wang, X.; Rao, L.; Guo, Y.; Yi, X.; Hu, L.; Chen, S.; Han, L.; et al. Methicillin-Resistant Staphylococcus aureus in China: A Multicentre Longitudinal Study and Whole-Genome Sequencing. Emerg. Microbes Infect. 2022, 11, 532–542. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Supplement M100le; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2022. [Google Scholar]
- Shifa Meharaj, S.H.; Jayanthi, S.; Danisvijay, D.; Sujhithra, A.; Perumal, J. Multiplex PCR Based Detection of MecA, MecC and PVL Gene in Analysis of Prevalence, Circulation, Transmission of MSSA/MRSA Strains in a Tertiary Care Hospital. IP Int. J. Med. Microbiol. Trop. Dis. 2019, 5, 155–159. [Google Scholar]
- Chen, L.; Mediavilla, J.R.; Oliveira, D.C.; Willey, B.M.; de Lencastre, H.; Kreiswirth, B.N. Multiplex Real-Time PCR for Rapid Staphylococcal Cassette Chromosome Mec Typing. J. Clin. Microbiol. 2009, 47, 3692–3706. [Google Scholar] [CrossRef] [Green Version]
- Sigudu, T.T.; Oguttu, J.W.; Qekwana, D.N. Prevalence of Staphylococcus spp. from Human Specimens Submitted to Diagnostic Laboratories in South Africa, 2012–2017. S. Afr. J. Infect. Dis. 2023, 38, 477. [Google Scholar] [CrossRef]
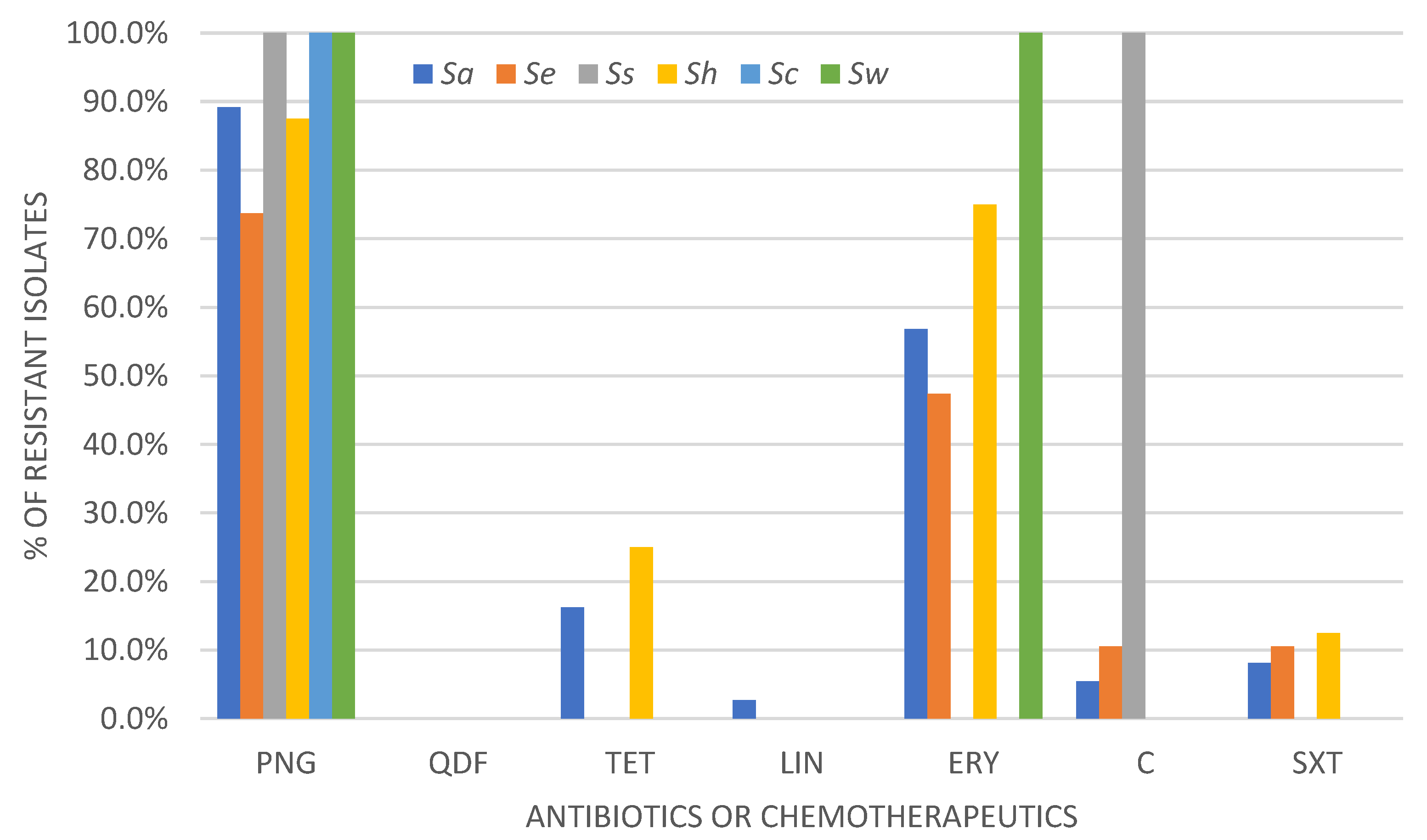
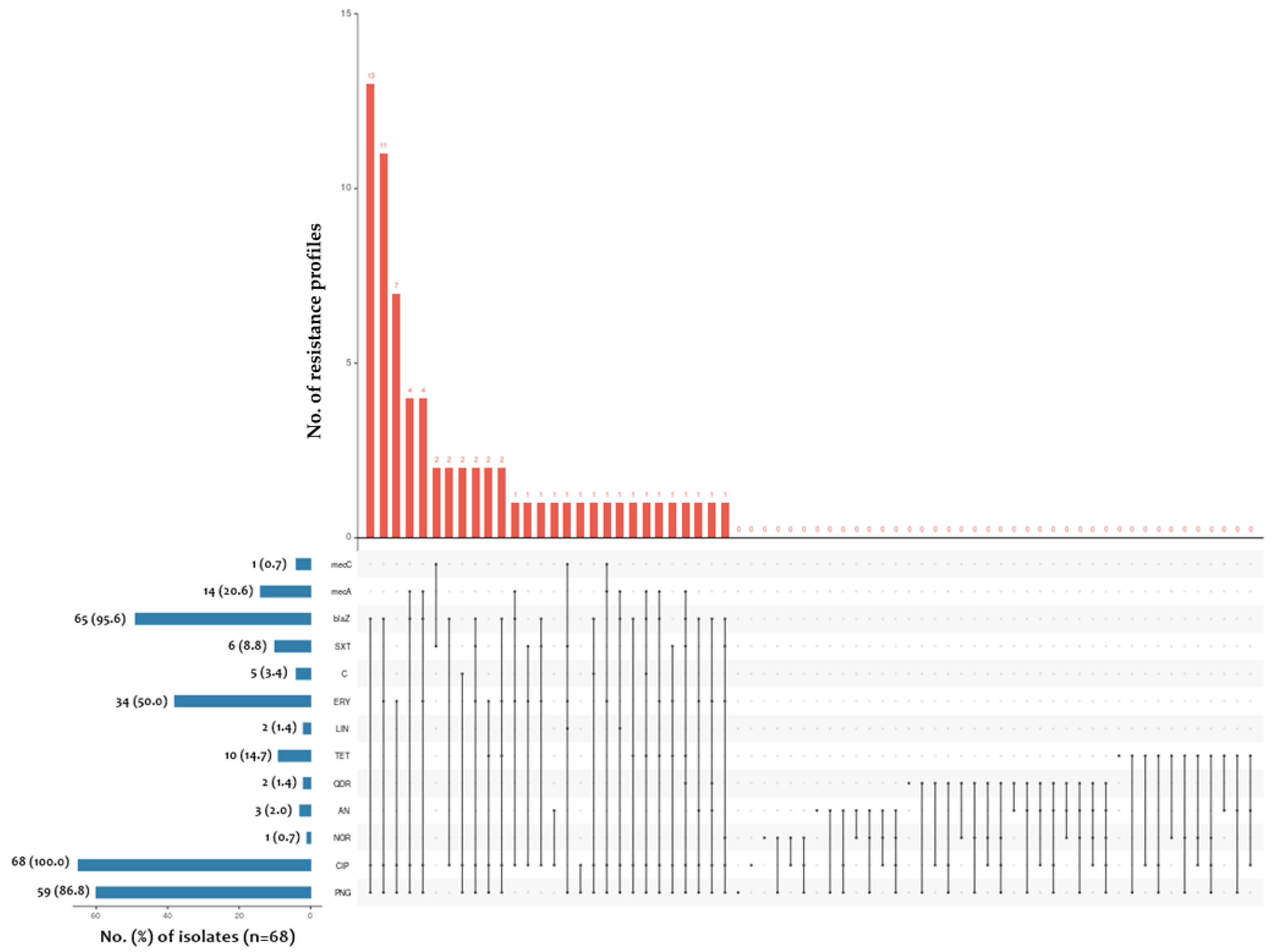
| Antimicrobial Drug | % (Number) of Resistant/Susceptible, Increased Exposure Isolates (n = 68) | |||||
|---|---|---|---|---|---|---|
| SA (n = 37) | CoNSE (n = 19) | nonSE-CoNS (n = 12) | ||||
| MSSA (n = 21) | MRSA (n = 16) | MRCoNSE (n = 5) | MSCoNSE (n = 14) | nonSE-MRCoNS (n = 5) | nonSE-MSCoNS (n = 7) | |
| PNG | 13.6 (20) | 9.5 (14) | 3.4 (5) | 6.1 (9) | 2.7 (4) | 4.8 (7) |
| CIP | 1.4 (2)/12.3 (19) | 3.4 (5)/7.5 (11) | 0.0 (0)/3.4 (5) | 0.0 (0)/9.5 (14) | 0.0 (0)/3.4 (5) | 0.0 (0)/4.8 (7) |
| NOR | 0.0 (0) | 0.7 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| AN | 0.0 (0) | 0.7 (1) | 0.0 (0) | 1.4 (2) | 0.0 (0) | 0.0 (0) |
| QDR | 0.0 (0)/0.7 (1) | 0.0 (0)/0.7 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| TET | 2.7 (4)/1.4 (2) | 1.4 (2) | 0.0 (0) | 0.0 (0) | 1.4 (2) | 0.0 (0) |
| LIN | 0.0 (0) | 0.7 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| ERY | 7.5 (11) | 6.8 (10) | 0.7 (1) | 5.4 (8) | 2.7 (4) | 0.0 (0) |
| C | 1.4 (2) | 0.0 (0) | 1.4 (2) | 0.0 (0) | 0.0 (0) | 0.7 (1) |
| SXT | 0.7 (1) | 1.4 (2) | 0.0 (0) | 1.4 (2) | 0.7 (1) | 0.0 (0) |
| Species | No. (%) of Isolates Phenotypically Beta-Lactam Resistant | No. (%) of Isolates with Each Resistance Gene Occurring | ||||
|---|---|---|---|---|---|---|
| blaZ Gene | mecA Gene | mecC Gene | ||||
| SA | S. aureus | 3 (4.4%) | 35 (51.5%) | 9 (13.2%) | 1 (1.5%) | |
| CoNSE | S. epidermidis | 5 (7.4%) | 19 (27.9%) | 2 (2.9%) | 0 | |
| nonSE-CoNS | S. hominis | 0 | 7 (10.3%) | 3 (4.4%) | 0 | |
| S. capitis | 0 | 2 (2.9%) | 0 | 0 | ||
| S. warneri | 0 | 1 (1.5%) | 0 | 0 | ||
| S. saprophyticus | 0 | 1 (1.5%) | 0 | 0 | ||
| Total | 65 (95.6%) | 14 (20.6%) | 1 (1.5%) | |||
| Type of ccr Complex According to [24,26] | % (Number) of Isolates (n = 68) |
|---|---|
| Type I | 2.9 (2) |
| Type II | 0.0 (0) |
| Type III | 0.0 (0) |
| Type IV | 5.9 (4) |
| Type V | 4.4 (3) |
| Type IV + type V | 1.5 (1) |
| Not defined | 8.8 (6) |
| Total | 23.5 (16) |
| Strain | Type of ccr Complex | Class of mec Gene Complex | SCCmec Cassette Type According to [27] | Type According to [14] |
|---|---|---|---|---|
| Staphylococcus aureus | ||||
| 1. | V | - | ND | - |
| 2. | IV | - | ND | - |
| 3. | - | - | ND | - |
| 4. | IV | - | ND | - |
| 5. | - | - | ND | - |
| 6. | - | - | ND | - |
| 7. | - | - | ND | - |
| 8. | V | - | ND | - |
| 9. | I | A | ND | UT5v |
| Staphylococcus epidermidis | ||||
| 10. | IV | B | VI | VI |
| 11. | IV + V | - | ND | - |
| Staphylococcus hominis | ||||
| 12. | - | A | ND | - |
| 13. | I | A | ND | UT5v |
| blaZ | mecA | |
|---|---|---|
| Specificity | 43.75% | 18.18% |
| Sensitivity | 94.23% | 100.00% |
| PPV | 82.76% | 23.73% |
| NPV | 70.00% | 100.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrzejczuk, S.; Cygan, M.; Dłuski, D.; Stępień-Pyśniak, D.; Kosikowska, U. Staphylococcal Resistance Patterns, blaZ and SCCmec Cassette Genes in the Nasopharyngeal Microbiota of Pregnant Women. Int. J. Mol. Sci. 2023, 24, 7980. https://doi.org/10.3390/ijms24097980
Andrzejczuk S, Cygan M, Dłuski D, Stępień-Pyśniak D, Kosikowska U. Staphylococcal Resistance Patterns, blaZ and SCCmec Cassette Genes in the Nasopharyngeal Microbiota of Pregnant Women. International Journal of Molecular Sciences. 2023; 24(9):7980. https://doi.org/10.3390/ijms24097980
Chicago/Turabian StyleAndrzejczuk, Sylwia, Monika Cygan, Dominik Dłuski, Dagmara Stępień-Pyśniak, and Urszula Kosikowska. 2023. "Staphylococcal Resistance Patterns, blaZ and SCCmec Cassette Genes in the Nasopharyngeal Microbiota of Pregnant Women" International Journal of Molecular Sciences 24, no. 9: 7980. https://doi.org/10.3390/ijms24097980





