U0126 Compound Triggers Thermogenic Differentiation in Preadipocytes via ERK-AMPK Signaling Axis
Abstract
1. Introduction
2. Results
2.1. Bioinformatic Strategy Identified Small Molecules for Priming Thermogenic Differentiation in Human Progenitor Cells
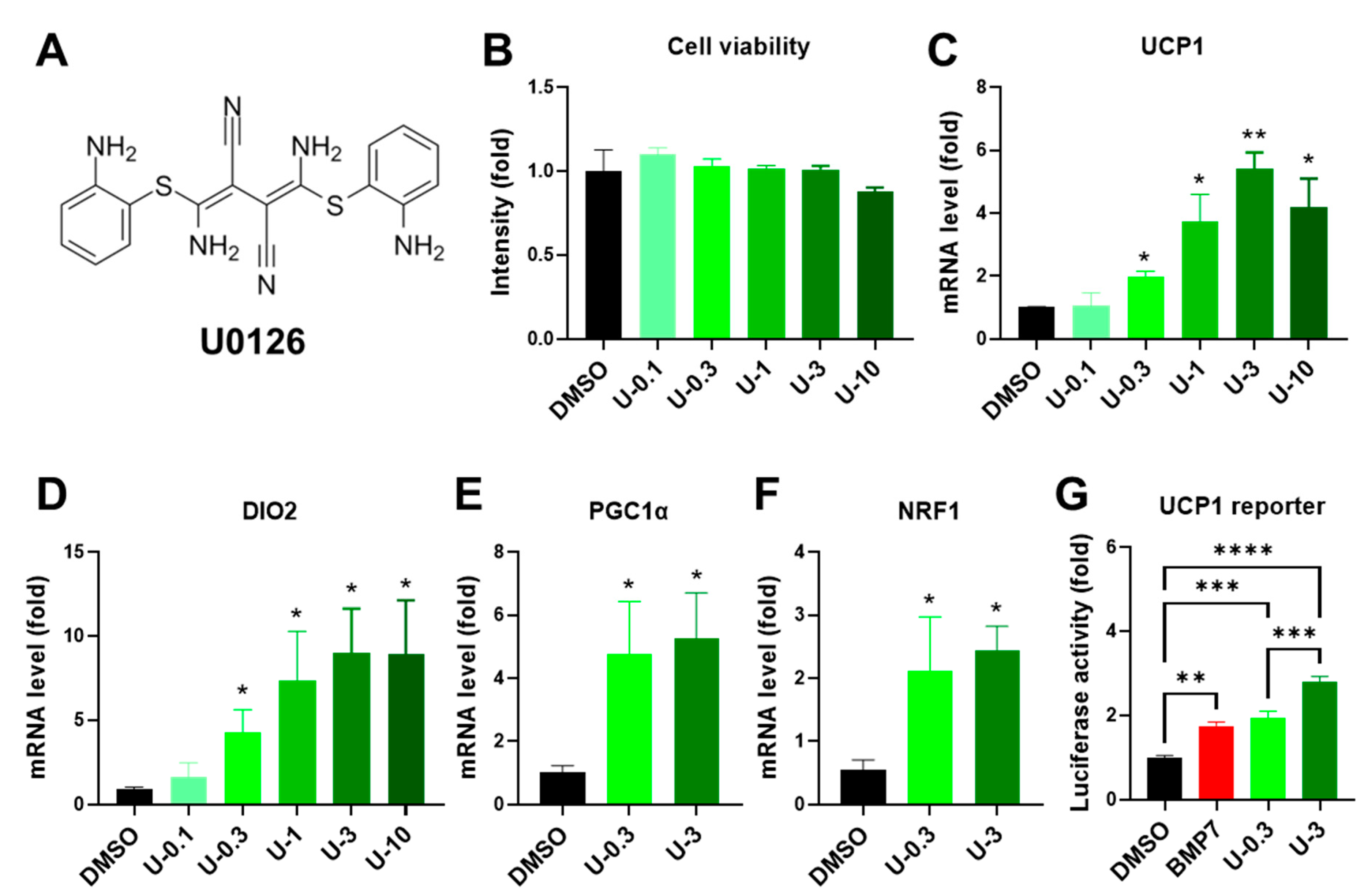
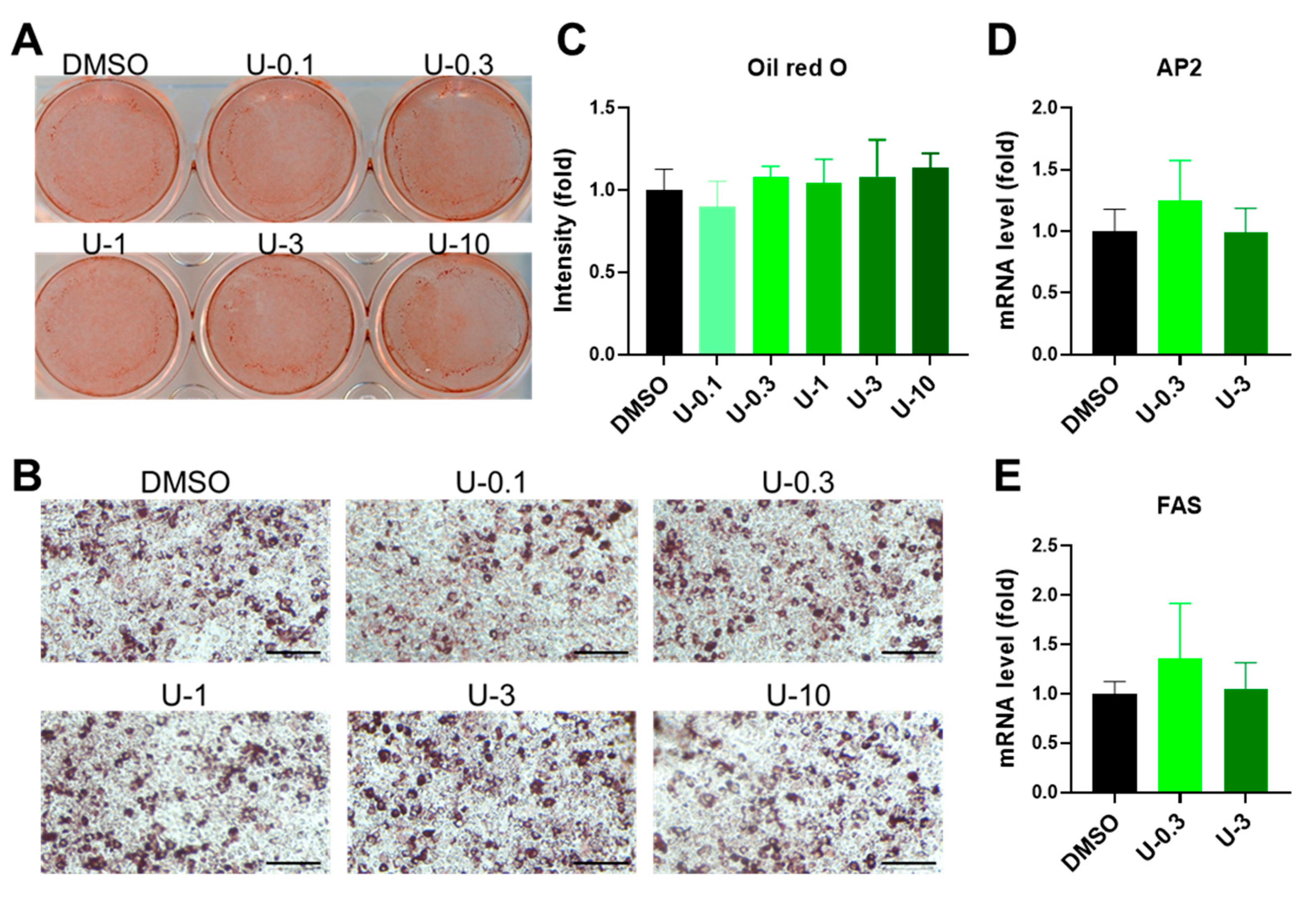
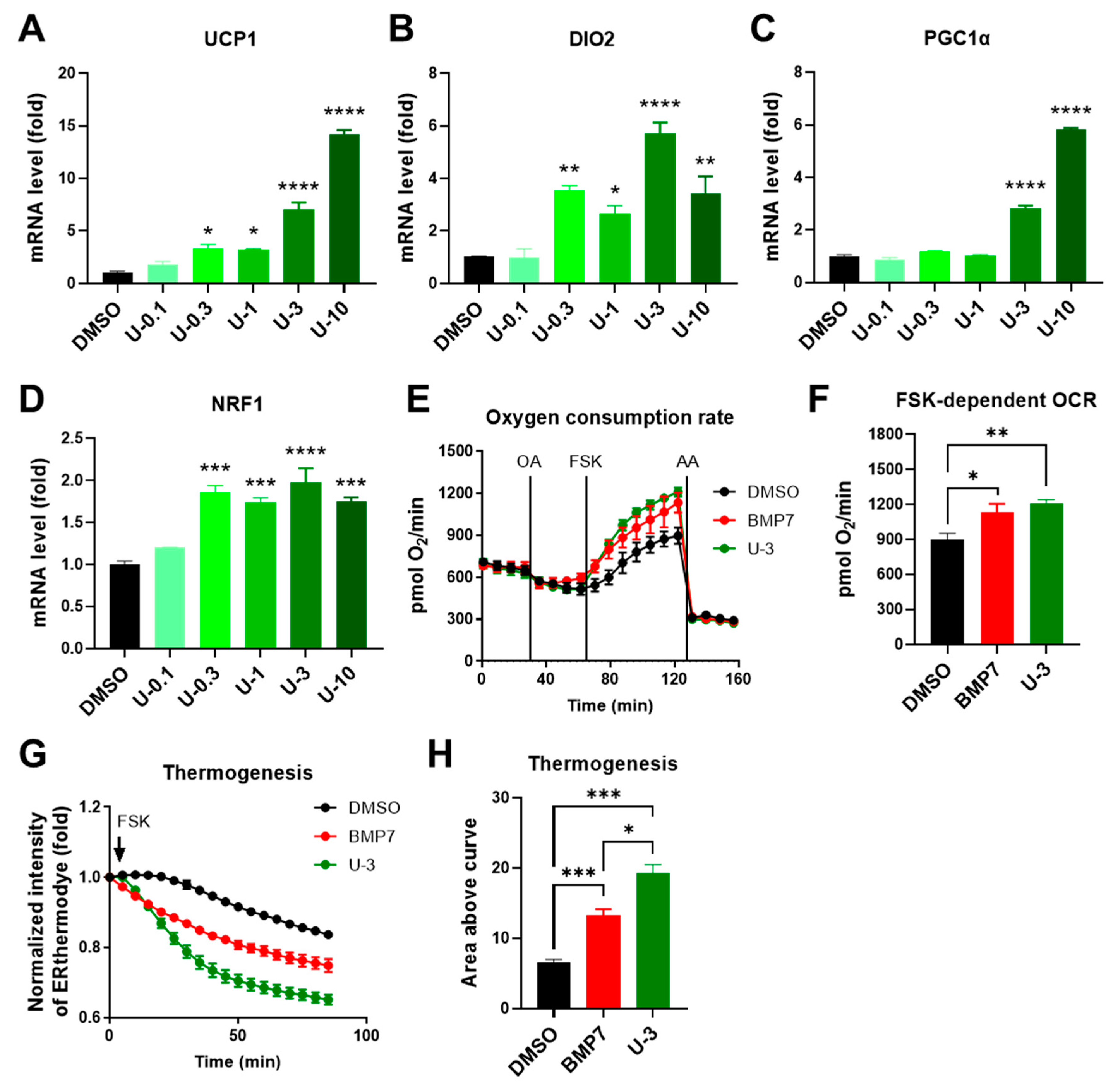
2.2. U0126 Pretreatment Drives the Thermogenic Differentiation of Human White Preadipocytes
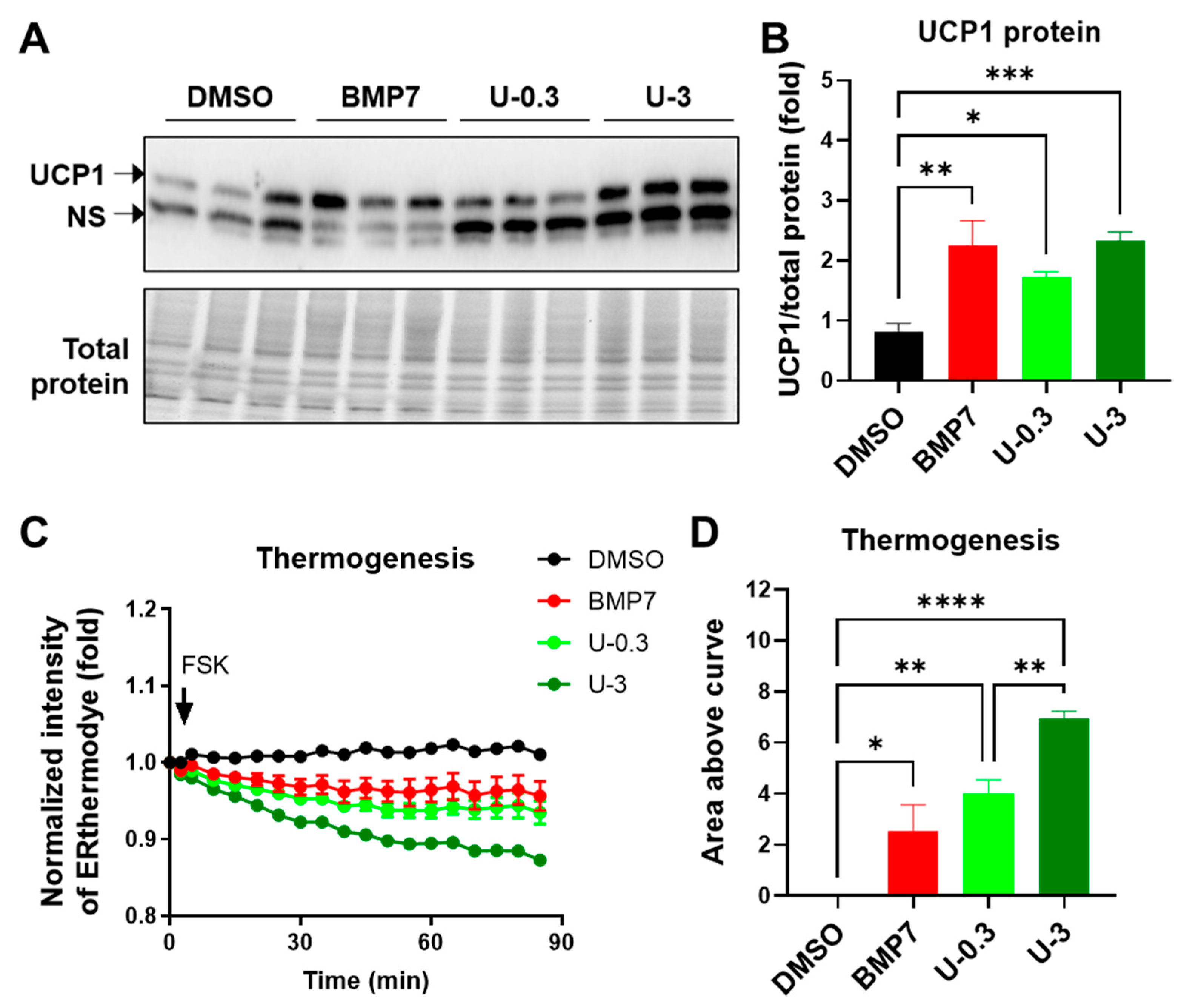
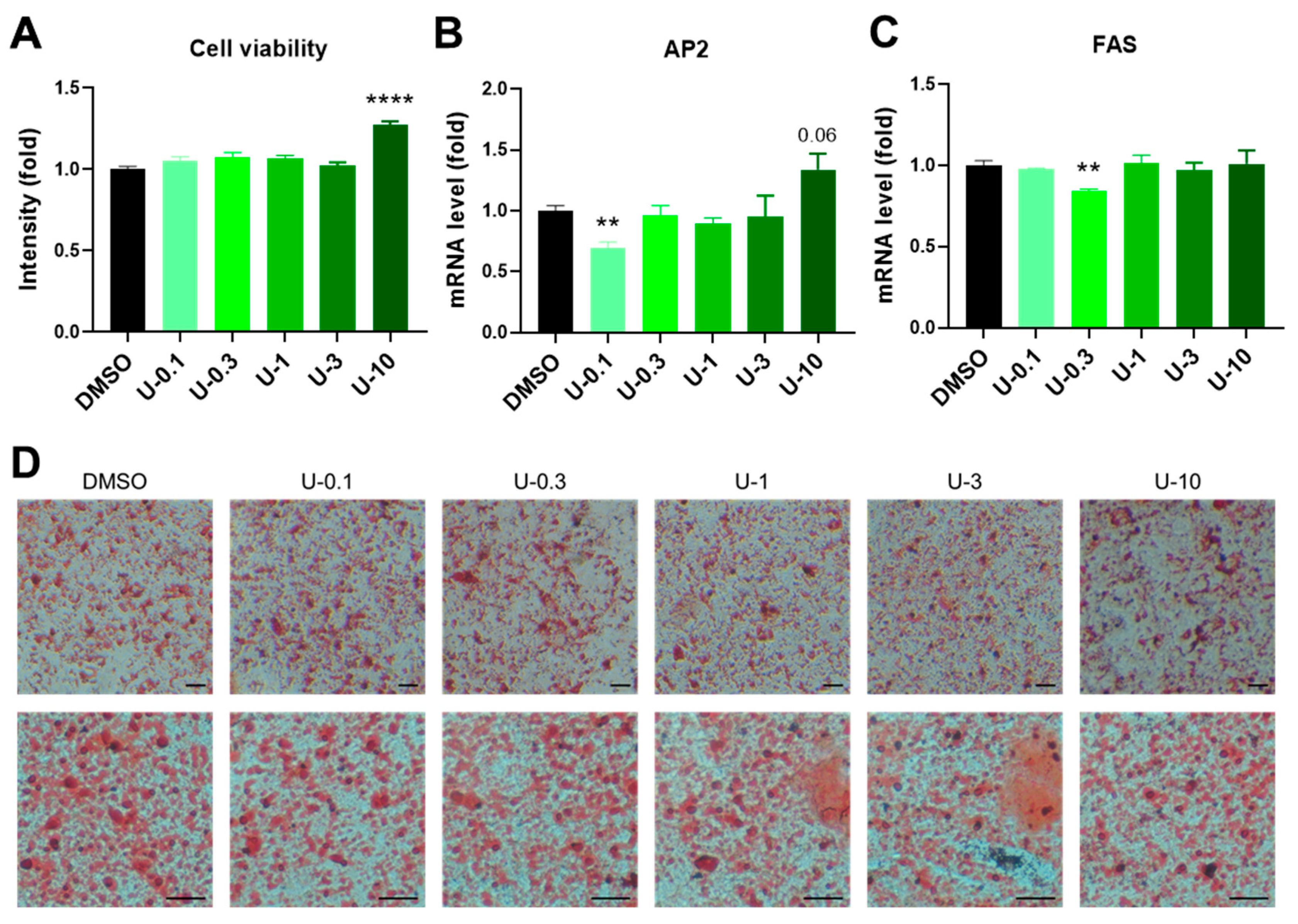
2.3. U0126 Pretreatment Confers Human Brown Preadipocytes to Acquire Enhanced Mitochondrial and Thermogenic Function after Differentiation
2.4. U0126-Mediated AMPK Phosphorylation Involves Thermogenic Activation in Human White Adipogenesis
3. Discussion
4. Materials and Methods
4.1. Identification of Small Molecules as Potential Thermogenic Activators
4.2. Cell Culture
4.3. Viability Assay
4.4. RNA Extraction and Gene Expression Analysis
4.5. UCP1 Reporter Assay
4.6. Oil Red O Staining
4.7. Immunoblotting Assay
4.8. Thermogenesis Assay
4.9. Oxygen Consumption Rate
4.10. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological molecular mechanisms of obesity: A link between MAFLD and NASH with cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Ji, M.; Zhang, S. Global warming and obesity: A systematic review. Obes. Rev. 2018, 19, 150–163. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wei, Y.-H. Therapeutic perspectives of thermogenic adipocytes in obesity and related complications. Int. J. Mol. Sci. 2021, 22, 7177. [Google Scholar] [CrossRef]
- Onikanni, A.S.; Lawal, B.; Oyinloye, B.E.; Mostafa-Hedeab, G.; Alorabi, M.; Cavalu, S.; Olusola, A.O.; Wang, C.-H.; Batiha, G.E.-S. Therapeutic efficacy of Clompanus pubescens leaves fractions via downregulation of neuronal cholinesterases/Na+-K+ ATPase/IL-1 β, and improving the neurocognitive and antioxidants status of streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2022, 148, 112730. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef]
- Wu, D.; Bang, I.H.; Park, B.-H.; Bae, E.J. Loss of Sirt6 in adipocytes impairs the ability of adipose tissue to adapt to intermittent fasting. Exp. Mol. Med. 2021, 53, 1298–1306. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Liburd, L.C.; Coronado, F. Peer Reviewed: Addressing Racial and Ethnic Disparities in COVID-19 Among School-Aged Children: Are We Doing Enough? Prev. Chronic Dis. 2021, 18, E55. [Google Scholar] [CrossRef]
- Garcia, M.C.; Faul, M.; Massetti, G.; Thomas, C.C.; Hong, Y.; Bauer, U.E.; Iademarco, M.F. Reducing potentially excess deaths from the five leading causes of death in the rural United States. MMWR Surveill. Summ. 2017, 66, 1–7. [Google Scholar] [CrossRef]
- An, R. Health care expenses in relation to obesity and smoking among US adults by gender, race/ethnicity, and age group: 1998–2011. Public Health 2015, 129, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Garvey, W.T.; Ryan, D.H. Challenging obesity: Patient, provider, and expert perspectives on the roles of available and emerging nonsurgical therapies. Obesity 2015, 23, S1–S26. [Google Scholar] [CrossRef]
- Cypess, A.M. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Rodríguez-Rodríguez, R.; Betz, M.J.; Rensen, P.C. current challenges for targeting brown fat thermogenesis to combat obesity. Front. Endocrinol. 2020, 11, 600341. [Google Scholar] [CrossRef]
- Hussain, M.F.; Roesler, A.; Kazak, L. Regulation of adipocyte thermogenesis: Mechanisms controlling obesity. FEBS J. 2020, 287, 3370–3385. [Google Scholar] [CrossRef]
- Shamsi, F.; Wang, C.H.; Tseng, Y.H. The evolving view of thermogenic adipocytes—Ontogeny, niche and function. Nat. Rev. Endocrinol. 2021, 17, 726–744. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Mena, H.A.; Sansbury, B.E.; Kobayashi, S.; Tsuji, T.; Wang, C.H.; Yin, X.; Huang, T.L.; Kusuyama, J.; Kodani, S.D.; et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat. Metab. 2022, 4, 775–790. [Google Scholar] [CrossRef]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary factors promoting brown and beige fat development and thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Takahashi, N.; Goto, T.; Kawada, T. Dietary factors evoke thermogenesis in adipose tissues. Obes. Res. Clin. Pract. 2014, 8, e533–e539. [Google Scholar] [CrossRef]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.D.; Smith, B.K.; Green, A.E.; Ducommun, S.; Henriksen, T.I.; Rebalka, I.A.; Razi, A.; Sakamoto, K. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab. 2016, 24, 118–129. [Google Scholar] [CrossRef]
- Yan, M.; Audet-Walsh, É.; Manteghi, S.; Dufour, C.R.; Walker, B.; Baba, M.; St-Pierre, J.; Giguère, V.; Pause, A. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1α/ERRα. Genes Dev. 2016, 30, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Xue, R.; Lynes, M.D.; Dreyfuss, J.M.; Shamsi, F.; Schulz, T.J.; Zhang, H.; Huang, T.L.; Townsend, K.L.; Li, Y.; Takahashi, H.; et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat. Med. 2015, 21, 760–768. [Google Scholar] [CrossRef]
- Hanssen, M.J.; Hoeks, J.; Brans, B.; van der Lans, A.A.; Schaart, G.; van den Driessche, J.J.; Jorgensen, J.A.; Boekschoten, M.V.; Hesselink, M.K.; Havekes, B.; et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015, 21, 863–865. [Google Scholar] [CrossRef]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Finlin, B.S.; Memetimin, H.; Zhu, B.; Confides, A.L.; Vekaria, H.J.; El Khouli, R.H.; Johnson, Z.R.; Westgate, P.M.; Chen, J.; Morris, A.J.; et al. The beta3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Investig. 2020. [Google Scholar] [CrossRef]
- Lamb, J. The Connectivity Map: A new tool for biomedical research. Nat. Rev. Cancer 2007, 7, 54–60. [Google Scholar] [CrossRef]
- Favata, M.F.; Horiuchi, K.Y.; Manos, E.J.; Daulerio, A.J.; Stradley, D.A.; Feeser, W.S.; Van Dyk, D.E.; Pitts, W.J.; Earl, R.A.; Hobbs, F.; et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998, 273, 18623–18632. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Niu, Y.; Zhang, J.; Huang, S.; Ding, P.; Sun, F.; Wang, X. U0126: Not only a MAPK kinase inhibitor. Front. Pharmacol. 2022, 13, 927083. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Elsen, M.; Raschke, S.; Tennagels, N.; Schwahn, U.; Jelenik, T.; Roden, M.; Romacho, T.; Eckel, J. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am. J. Physiol.-Cell Physiol. 2014, 306, C431–C440. [Google Scholar] [CrossRef] [PubMed]
- Kriszt, R.; Arai, S.; Itoh, H.; Lee, M.H.; Goralczyk, A.G.; Ang, X.M.; Cypess, A.M.; White, A.P.; Shamsi, F.; Xue, R.; et al. Optical visualisation of thermogenesis in stimulated single-cell brown adipocytes. Sci. Rep. 2017, 7, 1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lundh, M.; Fu, A.; Kriszt, R.; Huang, T.L.; Lynes, M.D.; Leiria, L.O.; Shamsi, F.; Darcy, J.; Greenwood, B.P.; et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. 2020, 12, eaaz8664. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.K.; Park, J.I. MEK1/2 Inhibitors: Molecular Activity and Resistance Mechanisms. Semin. Oncol. 2015, 42, 849–862. [Google Scholar] [CrossRef]
- Hotokezaka, H.; Sakai, E.; Kanaoka, K.; Saito, K.; Matsuo, K.; Kitaura, H.; Yoshida, N.; Nakayama, K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J. Biol. Chem. 2002, 277, 47366–47372. [Google Scholar] [CrossRef]
- Dokladda, K.; Green, K.A.; Pan, D.A.; Hardie, D.G. PD98059 and U0126 activate AMP-activated protein kinase by increasing the cellular AMP:ATP ratio and not via inhibition of the MAP kinase pathway. FEBS Lett. 2005, 579, 236–240. [Google Scholar] [CrossRef]
- Kawashima, I.; Mitsumori, T.; Nozaki, Y.; Yamamoto, T.; Shobu-Sueki, Y.; Nakajima, K.; Kirito, K. Negative regulation of the LKB1/AMPK pathway by ERK in human acute myeloid leukemia cells. Exp. Hematol. 2015, 43, 524–533.e1. [Google Scholar] [CrossRef]
- Mauro, A.; Ciccarelli, C.; De Cesaris, P.; Scoglio, A.; Bouche, M.; Molinaro, M.; Aquino, A.; Zani, B.M. PKCalpha-mediated ERK, JNK and p38 activation regulates the myogenic program in human rhabdomyosarcoma cells. J. Cell Sci. 2002, 115, 3587–3599. [Google Scholar] [CrossRef]
- Mund, R.A.; Frishman, W.H. Brown adipose tissue thermogenesis: β3-adrenoreceptors as a potential target for the treatment of obesity in humans. Cardiol. Rev. 2013, 21, 265–269. [Google Scholar] [CrossRef]
- Sugimoto, S.; Nakajima, H.; Kodo, K.; Mori, J.; Matsuo, K.; Kosaka, K.; Aoi, W.; Yoshimoto, K.; Ikegaya, H.; Hosoi, H. Miglitol increases energy expenditure by upregulating uncoupling protein 1 of brown adipose tissue and reduces obesity in dietary-induced obese mice. Nutr. Metab. 2014, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Wu, Z.; Choi, C.H.J.; Nguyen, L.; Tegegne, S.; Ackerman, S.E.; Crane, A.; Marchildon, F.; Tessier-Lavigne, M.; Cohen, P. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 2018, 27, 226–236.e3. [Google Scholar] [CrossRef]
- Rui, L. Brown and Beige Adipose Tissues in Health and Disease. Compr. Physiol. 2017, 7, 1281–1306. [Google Scholar] [CrossRef] [PubMed]
- Ost, M.; Keipert, S.; Klaus, S. Targeted mitochondrial uncoupling beyond UCP1–the fine line between death and metabolic health. Biochimie 2017, 134, 77–85. [Google Scholar] [CrossRef]
- Droebner, K.; Pleschka, S.; Ludwig, S.; Planz, O. Antiviral activity of the MEK-inhibitor U0126 against pandemic H1N1v and highly pathogenic avian influenza virus in vitro and in vivo. Antivir. Res. 2011, 92, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ahnstedt, H.; Mostajeran, M.; Blixt, F.W.; Warfvinge, K.; Ansar, S.; Krause, D.N.; Edvinsson, L. U0126 Attenuates Cerebral Vasoconstriction and Improves Long-Term Neurologic Outcome after Stroke in Female Rats. J. Cereb. Blood Flow Metab. 2015, 35, 454–460. [Google Scholar] [CrossRef]
- Bessard, A.; Frémin, C.; Ezan, F.; Fautrel, A.; Gailhouste, L.; Baffet, G. RNAi-mediated ERK2 knockdown inhibits growth of tumor cells in vitro and in vivo. Oncogene 2008, 27, 5315–5325. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Peng, C.; Tan, C.; Sun, H.; Liu, H.; Zhang, Y.; Wu, P.; Cui, C.; Liu, C.; et al. The phytochemical hyperforin triggers thermogenesis in adipose tissue via a Dlat-AMPK signaling axis to curb obesity. Cell Metab. 2021, 33, 565–580.e7. [Google Scholar] [CrossRef]
- Banks, A.S.; McAllister, F.E.; Camporez, J.P.; Zushin, P.J.; Jurczak, M.J.; Laznik-Bogoslavski, D.; Shulman, G.I.; Gygi, S.P.; Spiegelman, B.M. An ERK/Cdk5 axis controls the diabetogenic actions of PPARgamma. Nature 2015, 517, 391–395. [Google Scholar] [CrossRef]
- Kosari, S.; Camera, D.M.; Hawley, J.A.; Stebbing, M.; Badoer, E. ERK1/2 in the brain mediates the effects of central resistin on reducing thermogenesis in brown adipose tissue. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 184–189. [Google Scholar]
- Li, H.; Tang, S. Baicalin attenuates diet-induced obesity partially through promoting thermogenesis in adipose tissue. Obes. Res. Clin. Pract. 2021, 15, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Henley-Smith, C.J.; De Canha, M.N.; Oosthuizen, C.B.; Berrington, D. Viability Reagent, PrestoBlue, in Comparison with Other Available Reagents, Utilized in Cytotoxicity and Antimicrobial Assays. Int. J. Microbiol. 2013, 2013, 420601. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onikanni, S.A.; Yang, C.-Y.; Noriega, L.; Wang, C.-H. U0126 Compound Triggers Thermogenic Differentiation in Preadipocytes via ERK-AMPK Signaling Axis. Int. J. Mol. Sci. 2023, 24, 7987. https://doi.org/10.3390/ijms24097987
Onikanni SA, Yang C-Y, Noriega L, Wang C-H. U0126 Compound Triggers Thermogenic Differentiation in Preadipocytes via ERK-AMPK Signaling Axis. International Journal of Molecular Sciences. 2023; 24(9):7987. https://doi.org/10.3390/ijms24097987
Chicago/Turabian StyleOnikanni, Sunday Amos, Cheng-Ying Yang, Lloyd Noriega, and Chih-Hao Wang. 2023. "U0126 Compound Triggers Thermogenic Differentiation in Preadipocytes via ERK-AMPK Signaling Axis" International Journal of Molecular Sciences 24, no. 9: 7987. https://doi.org/10.3390/ijms24097987
APA StyleOnikanni, S. A., Yang, C.-Y., Noriega, L., & Wang, C.-H. (2023). U0126 Compound Triggers Thermogenic Differentiation in Preadipocytes via ERK-AMPK Signaling Axis. International Journal of Molecular Sciences, 24(9), 7987. https://doi.org/10.3390/ijms24097987




