The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity
Abstract
1. Introduction
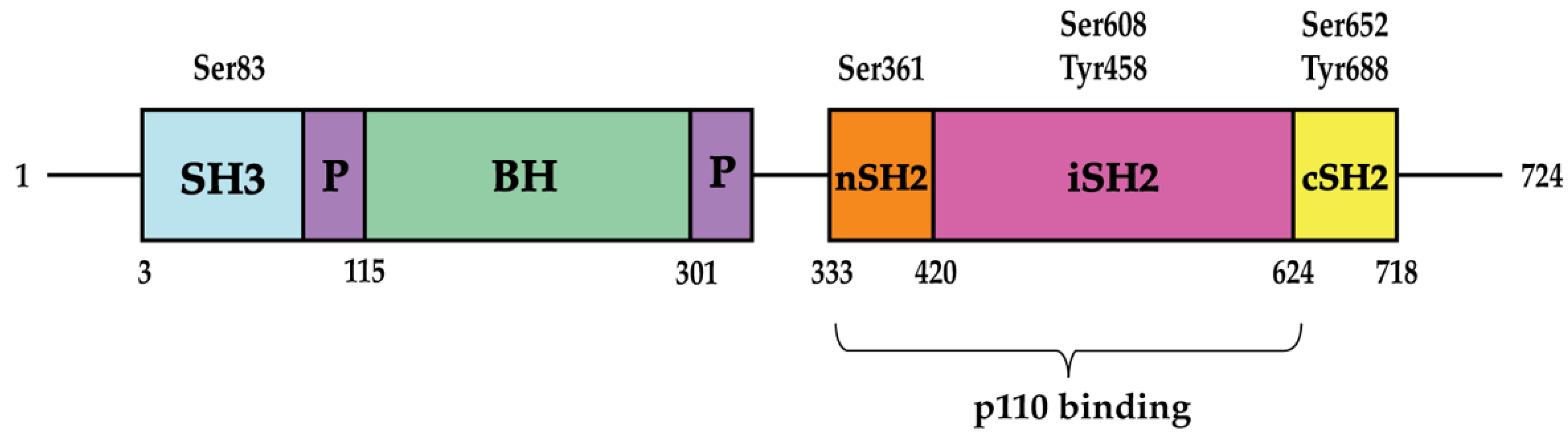
2. Insulin Signaling
3. Metabolic Functions of PIK3R1 In Vivo
3.1. Liver Pik3r1
3.2. Skeletal Muscle Pik3r1
3.3. Adipose Pik3r1
3.4. β Cell Pik3r1
| Mouse Model | Tissue with Pik3r1 Knockout | Characteristics | References |
|---|---|---|---|
| Deletion of all Pik3r1 isoforms | Whole-body homozygous | Causes perinatal lethality and has hepatocyte necrosis, chylous ascites, enlarged skeletal muscle fibers, brown fat necrosis, and calcification of cardiac tissue [40]. Results in a decrease in class 1A PI3K activity in liver and muscle [40]. Is hypoglycemic and has lower insulin levels in fasted and fed state [40]. More glucose tolerant while having lower insulin levels during GTT [40]. | Fruman et al. [40] |
| Pik3r1+/− | Whole-body heterozygous | Maintains whole-body insulin sensitivity in WAT and skeletal muscle under HFD [49]. Reduced WAT macrophage accumulation and proinflammatory gene expression [49]. | McCurdy et al. [49] |
| Pik3r1−/− retaining p55α and p50α isoforms | Whole-body homozygous | Hypoglycemic due to increased glucose transport [41]. Has lower insulin levels in fed and fasted state [41]. Shows isoform switch to p50α in muscle and adipocyte [41]. Exhibit leptin resistance on normal diet. | Terauchi et al. [41] Terauchi et al. [42] |
| Deletion of p55α and p50α | Whole-body homozygous | Lower fasting insulin levels, enhanced insulin sensitivity and increased glucose-stimulated glucose transport [16]. Reduced insulin-stimulated IRS-1 phosphotyrosine-associated PI3K but increased IRS-2-associated PI3K and AKT activation [16]. Adipocytes are more insulin sensitive and have lower lipid content [16]. | Chen et al. [16] |
| L-Pik3r1−/− | Liver | Improved hepatic and whole-body insulin sensitivity [44]. On an HFD, is protected against JNK-induced insulin resistance [47]. | Taniguchi et al. [44] Taniguchi et al. [47] |
| M-Pik3r1−/− | Skeletal and Cardiac Muscle | Reduced insulin-stimulated IRS-1- and IRS-2-mediated PI3K [48]. Under Dexamethasone (Dex) treatment, have lower Dex-induced impaired AKT activity and lower Dex-induced glucose and insulin intolerance [54]. Attenuated Dex-induced muscle atrophy and Dex-induced inhibition of eIF2α and 4E-BP1 phosphorylation [54]. | Luo et al. [48] Chen et al. [54] |
| M-Pik3r1−/−/Pik3r2−/− | Skeletal and Cardiac Muscle | Muscle insulin resistant and has impaired glucose disposal, reduced insulin release and decreased insulin sensitivity [48]. Normal fasting and fed blood glucose and serum insulin levels [48]. On an HFD, does not have exacerbated insulin resistance compared to wild type during ITT [48]. | Luo et al. [48] |
| BAT-Pik3r1−/− | Brown Adipose Tissue | Improved thermogenic functions, reduced HFD-induced adiposity and body weight, insulin resistance, and hepatic steatosis [50]. | Gomez-Hernandez et al. [50] |
| β-Pik3r1−/− | β Cell | Glucose intolerant and impaired glucose-stimulated insulin secretion [51]. | Kaneko et al. [51] |
| β-Pik3r1−/−/Pik3r2−/−- | β Cell | Exacerbated glucose intolerance and defect in insulin secretion compared to β-Pik3r1−/− mice [51]. Loss of synchronicity in β cell insulin secretion and impaired exocytosis of insulin caused by reduced expression of SNARE complex genes [51]. | Kaneko et al. [51] |
| Akita+/−/β-Pik3r1−/− | β Cell deletion in Akita+/− mice | Compared to Akita+/− mice, do not have hyperglycemia or reduced plasma insulin levels [52]. Have normal ER structure and many insulin secretory granules as well as reduced apoptotic rates, ER stress, and oxidative stress [52]. | Winnay et al. [52] |
| Pik3r1+/R649W (Arg649Trp mutation) | Whole-body heterozygous | Show phenotypes similar with SHORT syndrome [55]. Are insulin resistant, glucose intolerant, and hyperinsulinemic in fed and fasted states [55]. Have impaired insulin secretion and GLP-1 action [55]. Reduced insulin-stimulated IRS-1 tyrosine phosphorylation and AKT phosphorylation [55]. On an HFD, have lower adiposity but are more hyperglycemic and insulin resistant [55]. | Winnay et al. [55] |
| Pik3r1+/R649W-ob/ob | Whole-body heterozygous | Protected from obesity and hepatic steatosis but are hyperglycemic [56]. | Solheim et al. [56] |
| A-Pik3r1−/− | Adipose | Reduced levels of Dex-induced phospho-Hsl, phospho-Plin1, catalytic and regulatory subunits of PKA [57]. Improved Dex-induced hepatic steatosis and hypertriglyceridemia [57]. | Kuo et al. [57] |
4. GWAS Studies in PIK3R1
5. PIK3R1-Interacting Proteins and PI3K-Independent Function of PIK3R1

| PIK3R1-Interacting Proteins | Location of Interaction on p85α | Effect of p85α Interaction | References |
|---|---|---|---|
| BRD7 | iSH2 domain | BRD7 interaction leads to nuclear translocation of p85α [70]. Can reduce p85α levels in the cytosol and lead to reduced PI3K downstream signaling [70,71]. Enhances p85α binding to XBP-1s [72]. | Chiu et al. [70] Park et al. [71] |
| PAK4 | SH3 domain | PAK4 interaction relieves p110 catalytic subunit of PI3K. Reducing PAK4 levels in cells results in reduced phosphorylation of AKT [73]. | King et al. [73] |
| CBL, CBL-B | SH3 domain | CBL and CBL-B interaction leads to p85α ubiquitination [65,66]. | Bulut et al. [65] Fang et al. [66] |
| p42 (short isoform of EBP1) | cSH2 domain | p42 interaction leads to p85α degradation by HSP70/CHIP complex [67]. Inhibits PI3K activity [67]. | Ko et al. [67] |
| TRAF6 | iSH2 domain | TRAF6 polyubiquitinates p85α in its iSH2 domain, leading to formation of the TβR1 and p85α complex that activates PI3K and AKT [68]. | Hamidi et al. [68] |
| KBTBD2 | iSH2 domain | KBTBD2 interaction with p85α and CUL3 leads to p85α degradation [69]. KBTBD2 deletion in adipocytes leads to monomeric p85α accumulation which disrupts insulin signaling [69]. | Zhang et al. [69] |
| CDC42 | BH domain | CDC42 interaction is involved in cell migration through increasing filopodia formation, N-WASP-mediated decrease in actin stress fibers, and lowering focal adhesion complexes [76]. | Jiménez et al. [76] |
| XBP-1s | BH domain | p85α interaction with XBP-1s leads to increased XBP-1s stability and nuclear translocation [53,77]. Depletion of p85α leads to impaired resolution of UPR [77]. | Park et al. [53] Winnay et al. [77] |
| ERα | Unidentified | ERα interaction with p85α activates AKT and eNOS, which provides a cardioprotective effect. | Simoncini et al. [80] Hirsch et al. [4] |
6. The Role of PIK3R1 in Hormonal Regulation of Metabolic Homeostasis

7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asano, T.; Fujishiro, M.; Kushiyama, A.; Nakatsu, Y.; Yoneda, M.; Kamata, H.; Sakoda, H. Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol. Pharm. Bull. 2007, 30, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Majerus, P.W. Phosphatidylinositol signalling reactions. Semin. Cell Dev. Biol. 1998, 9, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bankaitis, V.A.; Grabon, A. Phosphatidylinositol synthase and diacylglycerol platforms bust a move. Dev. Cell 2011, 21, 810–812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirsch, E.; Costa, C.; Ciraolo, E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J. Endocrinol. 2007, 194, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.; Kiger, A.A. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014, 127, 923–928. [Google Scholar] [CrossRef]
- Fox, M.; Mott, H.R.; Owen, D. Class IA PI3K regulatory subunits: p110-independent roles and structures. Biochem. Soc. Trans. 2020, 48, 1397–1417. [Google Scholar] [CrossRef]
- Rathinaswamy, M.K.; Burke, J.E. Class I phosphoinositide 3-kinase (PI3K) regulatory subunits and their roles in signaling and disease. Adv. Biol. Regul. 2020, 75, 100657. [Google Scholar] [CrossRef]
- Vogt, P.K.; Hart, J.R.; Gymnopoulos, M.; Jiang, H.; Kang, S.; Bader, A.G.; Zhao, L.; Denley, A. Phosphatidylinositol 3-kinase: The oncoprotein. Curr. Top. Microbiol. Immunol. 2010, 347, 79–104. [Google Scholar] [CrossRef]
- Luo, J.; Field, S.J.; Lee, J.Y.; Engelman, J.A.; Cantley, L.C. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signaling via the formation of a sequestration complex. J. Cell Biol. 2005, 170, 455–464. [Google Scholar] [CrossRef]
- Songyang, Z.; Shoelson, S.E.; Chaudhuri, M.; Gish, G.; Pawson, T.; Haser, W.G.; King, F.; Roberts, T.; Ratnofsky, S.; Lechleider, R.J.; et al. SH2 domains recognize specific phosphopeptide sequences. Cell 1993, 72, 767–778. [Google Scholar] [CrossRef]
- Yu, J.; Wjasow, C.; Backer, J.M. Regulation of the p85/p110alpha phosphatidylinositol 3′-kinase. Distinct roles for the n-terminal and c-terminal SH2 domains. J. Biol. Chem. 1998, 273, 30199–30203. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, B.D.; Lu, Y.; Mao, M.; Zhang, J.; LaPushin, R.; Siminovitch, K.; Mills, G.B. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J. Biol. Chem. 2001, 276, 27455–27461. [Google Scholar] [CrossRef]
- Holt, K.H.; Olson, L.; Moye-Rowley, W.S.; Pessin, J.E. Phosphatidylinositol 3-kinase activation is mediated by high-affinity interactions between distinct domains within the p110 and p85 subunits. Mol. Cell Biol. 1994, 14, 42–49. [Google Scholar] [CrossRef]
- Fruman, D.A.; Cantley, L.C.; Carpenter, C.L. Structural organization and alternative splicing of the murine phosphoinositide 3-kinase p85 alpha gene. Genomics 1996, 37, 113–121. [Google Scholar] [CrossRef]
- Inukai, K.; Anai, M.; Van Breda, E.; Hosaka, T.; Katagiri, H.; Funaki, M.; Fukushima, Y.; Ogihara, T.; Yazaki, Y.; Kikuchi; et al. A novel 55-kDa regulatory subunit for phosphatidylinositol 3-kinase structurally similar to p55PIK Is generated by alternative splicing of the p85alpha gene. J. Biol. Chem. 1996, 271, 5317–5320. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mauvais-Jarvis, F.; Bluher, M.; Fisher, S.J.; Jozsi, A.; Goodyear, L.J.; Ueki, K.; Kahn, C.R. p50alpha/p55alpha phosphoinositide 3-kinase knockout mice exhibit enhanced insulin sensitivity. Mol. Cell Biol. 2004, 24, 320–329. [Google Scholar] [CrossRef]
- Marshall, J.D.S.; Whitecross, D.E.; Mellor, P.; Anderson, D.H. Impact of p85α Alterations in Cancer. Biomolecules 2019, 9, 29. [Google Scholar] [CrossRef]
- Cheung, L.W.; Mills, G.B. Targeting therapeutic liabilities engendered by PIK3R1 mutations for cancer treatment. Pharmacogenomics 2016, 17, 297–307. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Díaz, J.; Chagoyen, M.; Olazabal-Morán, M.; González-García, A.; Carrera, A.C. The Opposing Roles of PIK3R1/p85α and PIK3R2/p85β in Cancer. Trends Cancer 2019, 5, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chiu, Y.H.; Asara, J.; Cantley, L.C. Inhibition of PI3K binding to activators by serine phosphorylation of PI3K regulatory subunit p85alpha Src homology-2 domains. Proc. Natl. Acad. Sci. USA 2011, 108, 14157–14162. [Google Scholar] [CrossRef] [PubMed]
- Foukas, L.C.; Beeton, C.A.; Jensen, J.; Phillips, W.A.; Shepherd, P.R. Regulation of phosphoinositide 3-kinase by its intrinsic serine kinase activity in vivo. Mol. Cell Biol. 2004, 24, 966–975. [Google Scholar] [CrossRef][Green Version]
- Dhand, R.; Hiles, I.; Panayotou, G.; Roche, S.; Fry, M.J.; Gout, I.; Totty, N.F.; Truong, O.; Vicendo, P.; Yonezawa, K.; et al. PI 3-kinase is a dual specificity enzyme: Autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994, 13, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Han, M.W.; Ryu, I.S.; Lee, J.C.; Kim, S.H.; Chang, H.W.; Lee, Y.S.; Lee, S.; Kim, S.W.; Kim, S.Y. Phosphorylation of PI3K regulatory subunit p85 contributes to resistance against PI3K inhibitors in radioresistant head and neck cancer. Oral. Oncol. 2018, 78, 56–63. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, Q.; Liu, J.H.; Zhang, J.; Wang, H.; Koul, D.; McMurray, J.S.; Fang, X.; Yung, W.K.; Siminovitch, K.A.; et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J. Biol. Chem. 2003, 278, 40057–40066. [Google Scholar] [CrossRef]
- von Willebrand, M.; Williams, S.; Saxena, M.; Gilman, J.; Tailor, P.; Jascur, T.; Amarante-Mendes, G.P.; Green, D.R.; Mustelin, T. Modification of phosphatidylinositol 3-kinase SH2 domain binding properties by Abl- or Lck-mediated tyrosine phosphorylation at Tyr-688. J. Biol. Chem. 1998, 273, 3994–4000. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, B.; Lu, Y.; Watt, S.; Kumar, R.; Zhang, J.; Siminovitch, K.A.; Mills, G.B. SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J. Biol. Chem. 1999, 274, 27583–27589. [Google Scholar] [CrossRef]
- Cosentino, C.; Di Domenico, M.; Porcellini, A.; Cuozzo, C.; De Gregorio, G.; Santillo, M.R.; Agnese, S.; Di Stasio, R.; Feliciello, A.; Migliaccio, A.; et al. p85 regulatory subunit of PI3K mediates cAMP-PKA and estrogens biological effects on growth and survival. Oncogene 2007, 26, 2095–2103. [Google Scholar] [CrossRef]
- Donini, C.F.; Di Zazzo, E.; Zuchegna, C.; Di Domenico, M.; D’Inzeo, S.; Nicolussi, A.; Avvedimento, E.V.; Coppa, A.; Porcellini, A. The p85α regulatory subunit of PI3K mediates cAMP-PKA and retinoic acid biological effects on MCF7 cell growth and migration. Int. J. Oncol. 2012, 40, 1627–1635. [Google Scholar] [CrossRef]
- de la Cruz-Herrera, C.F.; Baz-Martínez, M.; Lang, V.; El Motiam, A.; Barbazán, J.; Couceiro, R.; Abal, M.; Vidal, A.; Esteban, M.; Muñoz-Fontela, C.; et al. Conjugation of SUMO to p85 leads to a novel mechanism of PI3K regulation. Oncogene 2016, 35, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef] [PubMed]
- King, M.J.; Sale, G.J. Dephosphorylation of insulin-receptor autophosphorylation sites by particulate and soluble phosphotyrosyl-protein phosphatases. Biochem. J. 1990, 266, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Eck, M.J.; Dhe-Paganon, S.; Trüb, T.; Nolte, R.T.; Shoelson, S.E. Structure of the IRS-1 PTB domain bound to the juxtamembrane region of the insulin receptor. Cell 1996, 85, 695–705. [Google Scholar] [CrossRef]
- Carracedo, A.; Pandolfi, P.P. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene 2008, 27, 5527–5541. [Google Scholar] [CrossRef]
- Stokoe, D.; Stephens, L.R.; Copeland, T.; Gaffney, P.R.; Reese, C.B.; Painter, G.F.; Holmes, A.B.; McCormick, F.; Hawkins, P.T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 1997, 277, 567–570. [Google Scholar] [CrossRef]
- Mîinea, C.P.; Sano, H.; Kane, S.; Sano, E.; Fukuda, M.; Peränen, J.; Lane, W.S.; Lienhard, G.E. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 2005, 391, 87–93. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Spangle, J.M.; Ohlson, C.E.; Cheng, H.; Roberts, T.M.; Cantley, L.C.; Zhao, J.J. PI3K-p110α mediates the oncogenic activity induced by loss of the novel tumor suppressor PI3K-p85α. Proc. Natl. Acad. Sci. USA 2017, 114, 7095–7100. [Google Scholar] [CrossRef]
- Fruman, D.A.; Mauvais-Jarvis, F.; Pollard, D.A.; Yballe, C.M.; Brazil, D.; Bronson, R.T.; Kahn, C.R.; Cantley, L.C. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat. Genet. 2000, 26, 379–382. [Google Scholar] [CrossRef]
- Terauchi, Y.; Tsuji, Y.; Satoh, S.; Minoura, H.; Murakami, K.; Okuno, A.; Inukai, K.; Asano, T.; Kaburagi, Y.; Ueki, K.; et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat. Genet. 1999, 21, 230–235. [Google Scholar] [CrossRef]
- Terauchi, Y.; Matsui, J.; Kamon, J.; Yamauchi, T.; Kubota, N.; Komeda, K.; Aizawa, S.; Akanuma, Y.; Tomita, M.; Kadowaki, T. Increased serum leptin protects from adiposity despite the increased glucose uptake in white adipose tissue in mice lacking p85alpha phosphoinositide 3-kinase. Diabetes 2004, 53, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Szanto, I.; Kahn, C.R. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc. Natl. Acad. Sci. USA 2000, 97, 2355–2360. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Tran, T.T.; Kondo, T.; Luo, J.; Ueki, K.; Cantley, L.C.; Kahn, C.R. Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses insulin action via positive regulation of PTEN. Proc. Natl. Acad. Sci. USA 2006, 103, 12093–12097. [Google Scholar] [CrossRef] [PubMed]
- Chagpar, R.B.; Links, P.H.; Pastor, M.C.; Furber, L.A.; Hawrysh, A.D.; Chamberlain, M.D.; Anderson, D.H. Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 2010, 107, 5471–5476. [Google Scholar] [CrossRef]
- Cheung, L.W.; Walkiewicz, K.W.; Besong, T.M.; Guo, H.; Hawke, D.H.; Arold, S.T.; Mills, G.B. Regulation of the PI3K pathway through a p85α monomer-homodimer equilibrium. eLife 2015, 4, e06866. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Aleman, J.O.; Ueki, K.; Luo, J.; Asano, T.; Kaneto, H.; Stephanopoulos, G.; Cantley, L.C.; Kahn, C.R. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Mol. Cell Biol. 2007, 27, 2830–2840. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sobkiw, C.L.; Hirshman, M.F.; Logsdon, M.N.; Li, T.Q.; Goodyear, L.J.; Cantley, L.C. Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cell Metab. 2006, 3, 355–366. [Google Scholar] [CrossRef]
- McCurdy, C.E.; Schenk, S.; Holliday, M.J.; Philp, A.; Houck, J.A.; Patsouris, D.; MacLean, P.S.; Majka, S.M.; Klemm, D.J.; Friedman, J.E. Attenuated Pik3r1 expression prevents insulin resistance and adipose tissue macrophage accumulation in diet-induced obese mice. Diabetes 2012, 61, 2495–2505. [Google Scholar] [CrossRef]
- Gomez-Hernandez, A.; Lopez-Pastor, A.R.; Rubio-Longas, C.; Majewski, P.; Beneit, N.; Viana-Huete, V.; García-Gómez, G.; Fernandez, S.; Hribal, M.L.; Sesti, G.; et al. Specific knockout of p85α in brown adipose tissue induces resistance to high-fat diet-induced obesity and its metabolic complications in male mice. Mol. Metab. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Kaneko, K.; Ueki, K.; Takahashi, N.; Hashimoto, S.; Okamoto, M.; Awazawa, M.; Okazaki, Y.; Ohsugi, M.; Inabe, K.; Umehara, T.; et al. Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metab. 2010, 12, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Winnay, J.N.; Dirice, E.; Liew, C.W.; Kulkarni, R.N.; Kahn, C.R. p85α deficiency protects β-cells from endoplasmic reticulum stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 1192–1197. [Google Scholar] [CrossRef]
- Park, S.W.; Zhou, Y.; Lee, J.; Lu, A.; Sun, C.; Chung, J.; Ueki, K.; Ozcan, U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 2010, 16, 429–437. [Google Scholar] [CrossRef]
- Chen, T.C.; Kuo, T.; Dandan, M.; Lee, R.A.; Chang, M.; Villivalam, S.D.; Liao, S.C.; Costello, D.; Shankaran, M.; Mohammed, H.; et al. The role of striated muscle Pik3r1 in glucose and protein metabolism following chronic glucocorticoid exposure. J. Biol. Chem. 2021, 296, 100395. [Google Scholar] [CrossRef] [PubMed]
- Winnay, J.N.; Solheim, M.H.; Dirice, E.; Sakaguchi, M.; Noh, H.L.; Kang, H.J.; Takahashi, H.; Chudasama, K.K.; Kim, J.K.; Molven, A.; et al. PI3-kinase mutation linked to insulin and growth factor resistance in vivo. J. Clin. Investig. 2016, 126, 1401–1412. [Google Scholar] [CrossRef]
- Solheim, M.H.; Winnay, J.N.; Batista, T.M.; Molven, A.; Njølstad, P.R.; Kahn, C.R. Mice Carrying a Dominant-Negative Human PI3K Mutation Are Protected From Obesity and Hepatic Steatosis but Not Diabetes. Diabetes 2018, 67, 1297–1309. [Google Scholar] [CrossRef]
- Kuo, T.; Chen, T.C.; Lee, R.A.; Nguyen, N.H.T.; Broughton, A.E.; Zhang, D.; Wang, J.C. Pik3r1 Is Required for Glucocorticoid-Induced Perilipin 1 Phosphorylation in Lipid Droplet for Adipocyte Lipolysis. Diabetes 2017, 66, 1601–1610. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, B.; Li, J.; Li, Y.; Zhang, M.; Ban, B. SHORT syndrome in two Chinese girls: A case report and review of the literature. Mol. Genet. Genom. Med. 2020, 8, e1385. [Google Scholar] [CrossRef]
- Dyment, D.A.; Smith, A.C.; Alcantara, D.; Schwartzentruber, J.A.; Basel-Vanagaite, L.; Curry, C.J.; Temple, I.K.; Reardon, W.; Mansour, S.; Haq, M.R.; et al. Mutations in PIK3R1 cause SHORT syndrome. Am. J. Hum. Genet. 2013, 93, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, C.; Quesada, V.; De Sandre-Giovannoli, A.; Puente, D.A.; Fernández-Toral, J.; Sigaudy, S.; Baban, A.; Lévy, N.; Velasco, G.; López-Otín, C. Exome sequencing identifies a novel mutation in PIK3R1 as the cause of SHORT syndrome. BMC Med. Genet. 2014, 15, 51. [Google Scholar] [CrossRef][Green Version]
- Chudasama, K.K.; Winnay, J.; Johansson, S.; Claudi, T.; König, R.; Haldorsen, I.; Johansson, B.; Woo, J.R.; Aarskog, D.; Sagen, J.V.; et al. SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling. Am. J. Hum. Genet. 2013, 93, 150–157. [Google Scholar] [CrossRef]
- Avila, M.; Dyment, D.A.; Sagen, J.V.; St-Onge, J.; Moog, U.; Chung, B.H.Y.; Mo, S.; Mansour, S.; Albanese, A.; Garcia, S.; et al. Clinical reappraisal of SHORT syndrome with PIK3R1 mutations: Toward recommendation for molecular testing and management. Clin. Genet. 2016, 89, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Semple, R.K.; Sleigh, A.; Murgatroyd, P.R.; Adams, C.A.; Bluck, L.; Jackson, S.; Vottero, A.; Kanabar, D.; Charlton-Menys, V.; Durrington, P.; et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J. Clin. Investig. 2009, 119, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Huang-Doran, I.; Tomlinson, P.; Payne, F.; Gast, A.; Sleigh, A.; Bottomley, W.; Harris, J.; Daly, A.; Rocha, N.; Rudge, S.; et al. Insulin resistance uncoupled from dyslipidemia due to C-terminal PIK3R1 mutations. JCI Insight 2016, 1, e88766. [Google Scholar] [CrossRef] [PubMed]
- Bulut, G.B.; Sulahian, R.; Yao, H.; Huang, L.J. Cbl ubiquitination of p85 is essential for Epo-induced EpoR endocytosis. Blood 2013, 122, 3964–3972. [Google Scholar] [CrossRef]
- Fang, D.; Wang, H.Y.; Fang, N.; Altman, Y.; Elly, C.; Liu, Y.C. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J. Biol. Chem. 2001, 276, 4872–4878. [Google Scholar] [CrossRef]
- Ko, H.R.; Kim, C.K.; Lee, S.B.; Song, J.; Lee, K.H.; Kim, K.K.; Park, K.W.; Cho, S.W.; Ahn, J.Y. P42 Ebp1 regulates the proteasomal degradation of the p85 regulatory subunit of PI3K by recruiting a chaperone-E3 ligase complex HSP70/CHIP. Cell Death Dis. 2014, 5, e1131. [Google Scholar] [CrossRef]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.H.; Landström, M. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci. Signal 2017, 10, eaal4186. [Google Scholar] [CrossRef]
- Zhang, Z.; Turer, E.; Li, X.; Zhan, X.; Choi, M.; Tang, M.; Press, A.; Smith, S.R.; Divoux, A.; Moresco, E.M.; et al. Insulin resistance and diabetes caused by genetic or diet-induced KBTBD2 deficiency in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E6418–E6426. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Lee, J.Y.; Cantley, L.C. BRD7, a tumor suppressor, interacts with p85α and regulates PI3K activity. Mol. Cell 2014, 54, 193–202. [Google Scholar] [CrossRef]
- Park, S.W.; Herrema, H.; Salazar, M.; Cakir, I.; Cabi, S.; Basibuyuk Sahin, F.; Chiu, Y.H.; Cantley, L.C.; Ozcan, U. BRD7 regulates XBP1s’ activity and glucose homeostasis through its interaction with the regulatory subunits of PI3K. Cell Metab. 2014, 20, 73–84. [Google Scholar] [CrossRef]
- Lee, J.M.; Liu, R.; Park, S.W. The regulatory subunits of PI3K, p85α and p85β, differentially affect BRD7-mediated regulation of insulin signaling. J. Mol. Cell Biol. 2022, 13, 889–901. [Google Scholar] [CrossRef]
- King, H.; Thillai, K.; Whale, A.; Arumugam, P.; Eldaly, H.; Kocher, H.M.; Wells, C.M. PAK4 interacts with p85 alpha: Implications for pancreatic cancer cell migration. Sci. Rep. 2017, 7, 42575. [Google Scholar] [CrossRef] [PubMed]
- Pirola, L.; Johnston, A.M.; Van Obberghen, E. Modulation of insulin action. Diabetologia 2004, 47, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bagrodia, S.; Cerione, R.A. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J. Biol. Chem. 1994, 269, 18727–18730. [Google Scholar] [CrossRef]
- Jiménez, C.; Portela, R.A.; Mellado, M.; Rodríguez-Frade, J.M.; Collard, J.; Serrano, A.; Martínez, A.C.; Avila, J.; Carrera, A.C. Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J. Cell Biol. 2000, 151, 249–262. [Google Scholar] [CrossRef]
- Winnay, J.N.; Boucher, J.; Mori, M.A.; Ueki, K.; Kahn, C.R. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat. Med. 2010, 16, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Dunys, J.; Duplan, E.; Checler, F. The transcription factor X-box binding protein-1 in neurodegenerative diseases. Mol. Neurodegener. 2014, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Piperi, C.; Adamopoulos, C.; Papavassiliou, A.G. XBP1: A Pivotal Transcriptional Regulator of Glucose and Lipid Metabolism. Trends Endocrinol. Metab. 2016, 27, 119–122. [Google Scholar] [CrossRef]
- Simoncini, T.; Hafezi-Moghadam, A.; Brazil, D.P.; Ley, K.; Chin, W.W.; Liao, J.K. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000, 407, 538–541. [Google Scholar] [CrossRef]
- Mannella, P.; Brinton, R.D. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: A unified mechanism of estrogen action. J. Neurosci. 2006, 26, 9439–9447. [Google Scholar] [CrossRef]
- Kuo, T.; Lew, M.J.; Mayba, O.; Harris, C.A.; Speed, T.P.; Wang, J.C. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 11160–11165. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Harris, C.A.; Wang, J.C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef] [PubMed]
- del Rincon, J.P.; Iida, K.; Gaylinn, B.D.; McCurdy, C.E.; Leitner, J.W.; Barbour, L.A.; Kopchick, J.J.; Friedman, J.E.; Draznin, B.; Thorner, M.O. Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: Mechanism for growth hormone-mediated insulin resistance. Diabetes 2007, 56, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Khalfallah, Y.; Sassolas, G.; Borson-Chazot, F.; Vega, N.; Vidal, H. Expression of insulin target genes in skeletal muscle and adipose tissue in adult patients with growth hormone deficiency: Effect of one year recombinant human growth hormone therapy. J. Endocrinol. 2001, 171, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.A.; Shao, J.; Qiao, L.; Leitner, W.; Anderson, M.; Friedman, J.E.; Draznin, B. Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology 2004, 145, 1144–1150. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, G.; Huo, J.S.; Barney, D.; Wang, Z.; Livshiz, T.; States, D.J.; Qin, Z.S.; Schwartz, J. Computational and functional analysis of growth hormone (GH)-regulated genes identifies the transcriptional repressor B-cell lymphoma 6 (Bc16) as a participant in GH-regulated transcription. Endocrinology 2009, 150, 3645–3654. [Google Scholar] [CrossRef]
- Barbour, L.A.; Mizanoor Rahman, S.; Gurevich, I.; Leitner, J.W.; Fischer, S.J.; Roper, M.D.; Knotts, T.A.; Vo, Y.; McCurdy, C.E.; Yakar, S.; et al. Increased P85alpha is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J. Biol. Chem. 2005, 280, 37489–37494. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsay, A.; Wang, J.-C. The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity. Int. J. Mol. Sci. 2023, 24, 12665. https://doi.org/10.3390/ijms241612665
Tsay A, Wang J-C. The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity. International Journal of Molecular Sciences. 2023; 24(16):12665. https://doi.org/10.3390/ijms241612665
Chicago/Turabian StyleTsay, Ariel, and Jen-Chywan Wang. 2023. "The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity" International Journal of Molecular Sciences 24, no. 16: 12665. https://doi.org/10.3390/ijms241612665
APA StyleTsay, A., & Wang, J.-C. (2023). The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity. International Journal of Molecular Sciences, 24(16), 12665. https://doi.org/10.3390/ijms241612665





