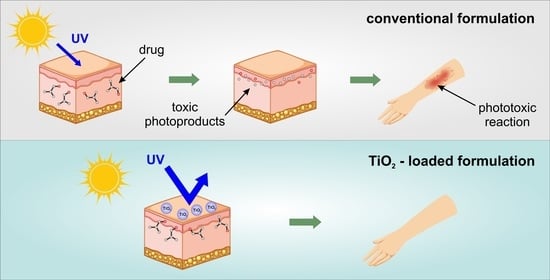
Phototoxic or Photoprotective?—Advances and Limitations of Titanium (IV) Oxide in Dermal Formulations—A Review
Abstract
1. Introduction
2. Photolability of Drugs Applied to the Skin
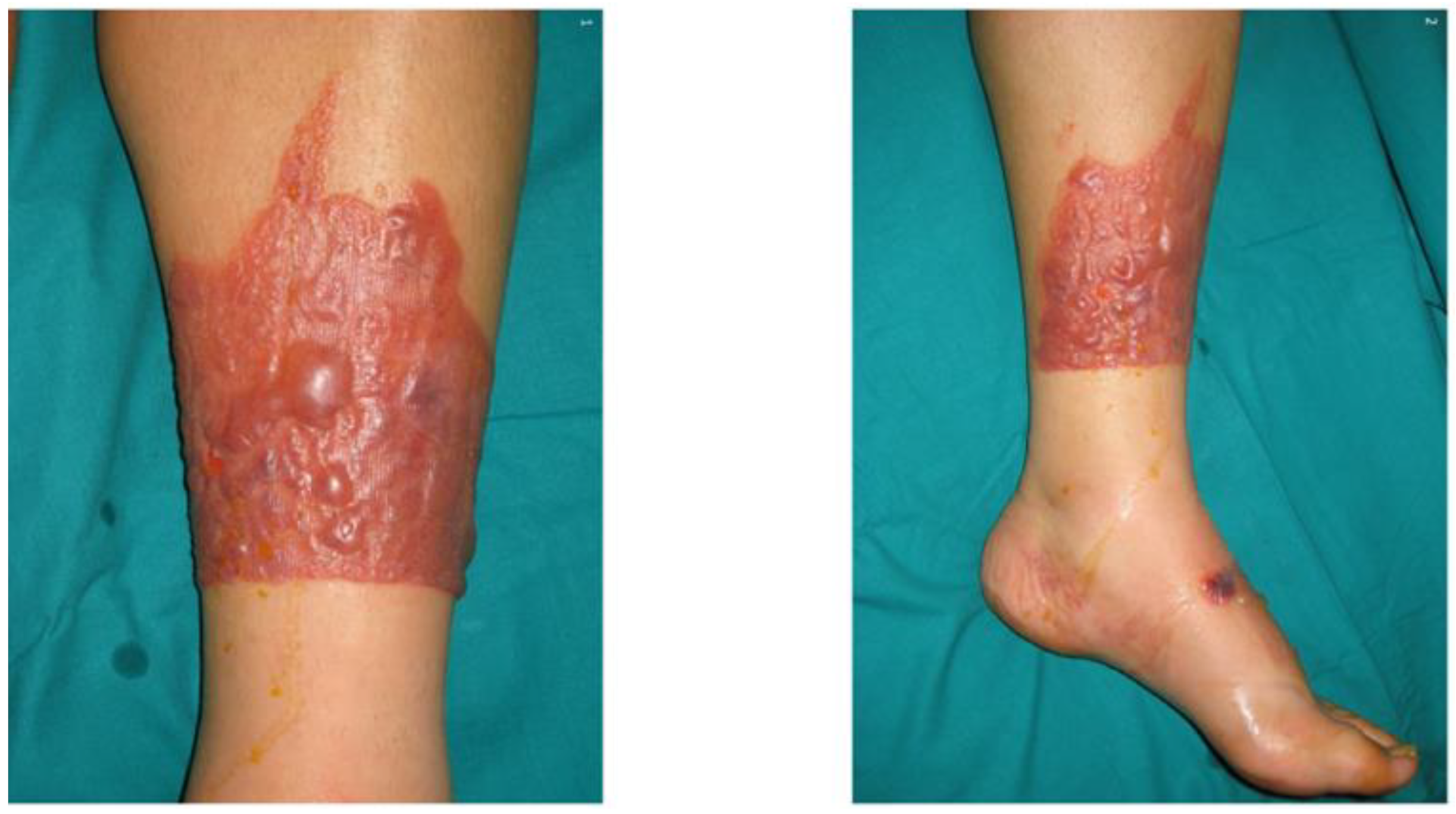
| Phototoxicity | Photoallergy | References | |
|---|---|---|---|
| Clinical symptoms | erythema, vesicles, and bullae, burning, stinging, hyperpigmentation | eczematous, pruritic rash | [3] |
| Histological effects | necrotic keratinocytes, epidermal degeneration, sparse dermal infiltrate of lymphocytes, macrophages, and neutrophils | spongiotic dermatitis, dermal lymphohistiocytic infiltrate | [3,17] |
| Pathophysiology | direct tissue injury | type iv delayed hypersensitivity response | [2] |
| Occurrence after first exposure | yes | no | [2,3] |
| Onset after exposure | minutes to hours | 24 to 48 h | [2,14] |
| Dose of agent needed for the reaction | large | small | [14] |
| Cross-reactivity with other agents | none | common | [2,17] |
| Diagnosis | clinical | photopatch tests | [3,19] |
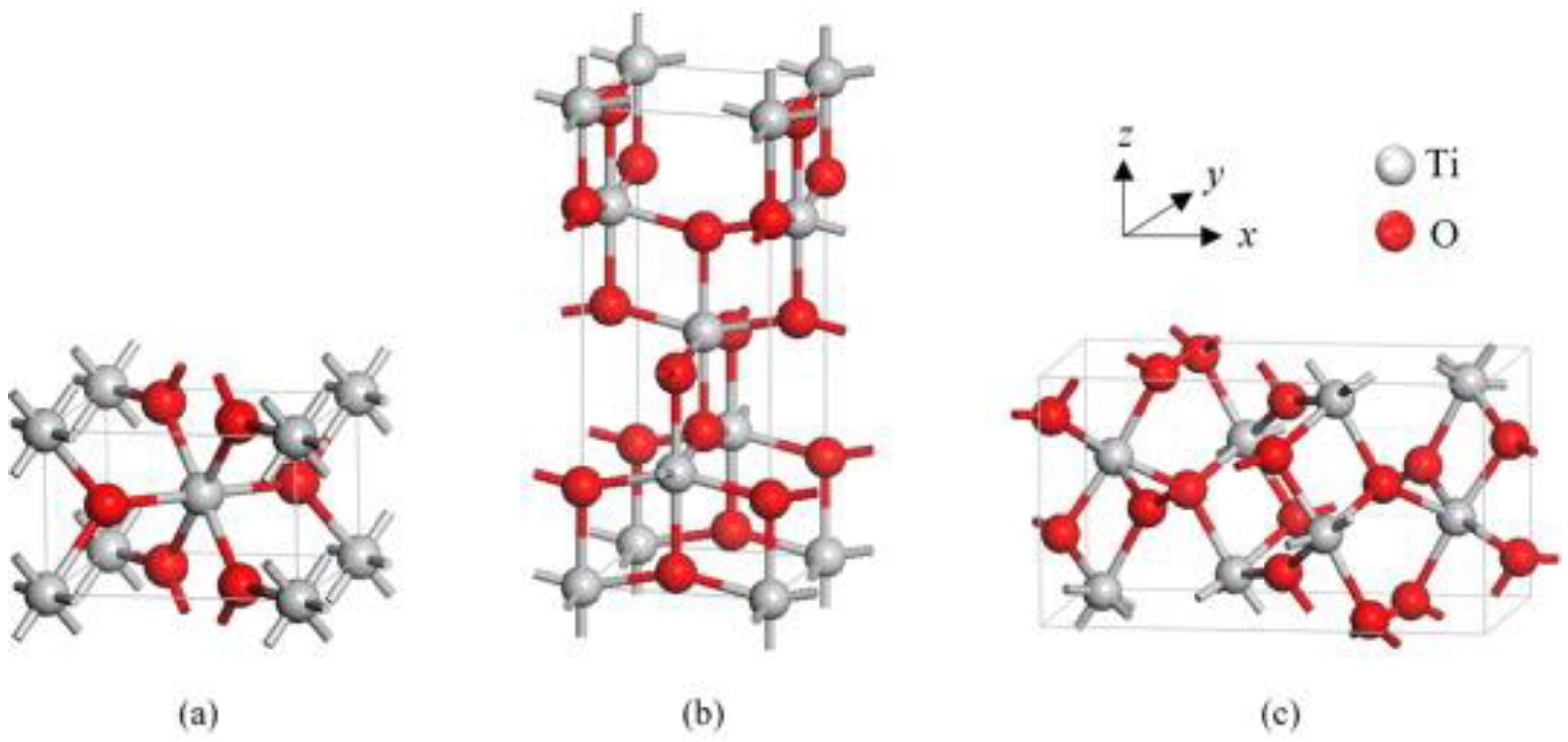
3. Titanium Dioxide
3.1. General Information
3.2. Titanium Dioxide Nanoparticles (NPs) Photoprotection vs. Phototoxicity
3.3. Illuminated TiO2-NPs Generate Reactive Oxygen Species (ROS)
3.4. Factors Influencing the Phototoxic Potential of TiO2-NPs
3.4.1. Polymorphic Form
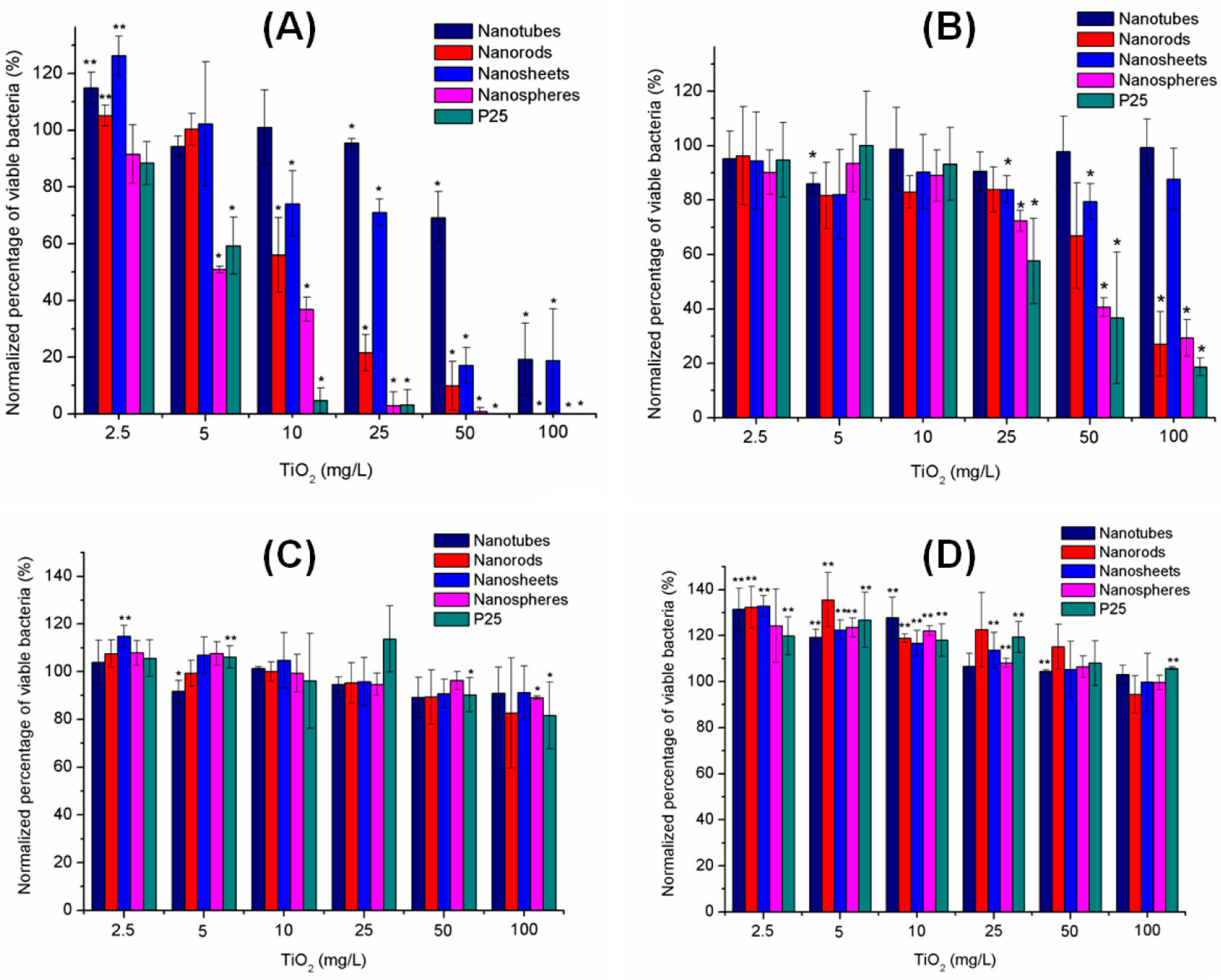
3.4.2. Material Size, Surface, and Morphology
3.5. Cytotoxicity as a Response to Photocatalytic Properties of TiO2-NPs
3.6. Might Modifications of TiO2-NPs Surface Decrease Phototoxicity?
4. Titanium Dioxide Nanoparticles as Photostabilizing Agents in Topical Drug Formulations
5. The Use of TiO2 in Sunscreens and Cosmetics
6. Conclusions
- The phototoxic potential of TiO2-NPs must always be taken into account when designing cosmetic and drug formulations;
- Optimization of physical characteristics, such as polymorphic form, size, shape, active surface area, and hydrophilic/hydrophobic profile of the particles makes it possible to reduce the toxicity of the material and to increase the UV/visible light-scattering capacity depending on the intended use of the product;
- Coating NPs with inert inorganic or organic shells, as well as incorporating them into organic structures, can effectively reduce their toxicity and increase their protective properties;
- TiO2 can be effectively used not only as a sunscreen, but also as a substance enhancing the stability of formulations containing photolabile ingredients;
- Given the inorganic sunscreen materials available on the market, TiO2 appears to be invaluable for effective protection against harmful UV radiations as it has no other equivalent, showing high protection against UVB radiation and moderately high protection against UVA radiation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, A.F. Phototherapy: From Ancient Egypt to the New Millennium. J. Perinatol. 2001, 21, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Rok, J.; Rzepka, Z.; Wrześniok, D. Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms. Pharmaceuticals 2021, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.; Worswick, S. Photosensitizing Drug Reactions. Clin. Dermatol. 2022, 40, 57–63. [Google Scholar] [CrossRef]
- Hofmann, G.A.; Weber, B. Drug-Induced Photosensitivity: Culprit Drugs, Potential Mechanisms and Clinical Consequences. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2021, 19, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lugović-Mihić, L.; Duvančić, T.; Ferček, I.; Vuković, P.; Japundzic, I.; Ćesić, D. Drug-Induced Photosensitivity—A Continuing Diagnostic Challenge. Acta Clin. Croat. 2017, 56, 277–283. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). EFSA Statement on the Review of the Risks Related to the Exposure to the Food Additive Titanium Dioxide (E 171) Performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17, e05714. [Google Scholar] [CrossRef]
- New Questions and Answers from the EMA Regarding Titanium Dioxide in Medicines—ECA Academy. Available online: https://www.gmp-compliance.org/gmp-news/new-questions-and-answers-from-the-ema-regarding-titanium-dioxide-in-medicines (accessed on 27 March 2023).
- EUR-Lex—32022R0063—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2022/63/oj (accessed on 15 March 2023).
- Araujo, M.A.R. Ketoprofen Hypersensibility and Idiosyncratic Response—A Case Report. Immunopharmacol. Immunotoxicol. 2020, 42, 174–177. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, Y.; Yu, H.; Lu, C.; Han, K. Investigation on Thermal Degradation Properties of Oleic Acid and Its Methyl and Ethyl Esters through TG-FTIR. Energy Convers. Manag. 2017, 149, 495–504. [Google Scholar] [CrossRef]
- Ambrogi, V.; Nocchetti, M.; Latterini, L. Promethazine-Montmorillonite Inclusion Complex to Enhance Drug Photostability. Langmuir ACS J. Surf. Colloids 2014, 30, 14612–14620. [Google Scholar] [CrossRef]
- Kryczyk-Poprawa, A.; Zupkó, I.; Bérdi, P.; Żmudzki, P.; Popiół, J.; Muszyńska, B.; Opoka, W. Photostability Testing of a Third-Generation Retinoid-Tazarotene in the Presence of UV Absorbers. Pharmaceutics 2020, 12, 899. [Google Scholar] [CrossRef]
- Choi, Y.-G.; Lee, J.H.; Bae, I.-H.; Ah, Y.-C.; Ki, H.-M.; Bae, J.-H.; Park, Y.-H.; Lee, K.C.; Lim, K.-M. Titanium Dioxide Inclusion in Backing Reduce the Photoallergenicity of Ketoprofen Transdermal Patch. Arch. Toxicol. 2011, 85, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lankerani, L.; Baron, E.D. Photosensitivity to Exogenous Agents. J. Cutan. Med. Surg. 2004, 8, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Dawe, R.S.; Ibbotson, S.H. Drug-Induced Photosensitivity. Dermatol. Clin. 2014, 32, 363–368. [Google Scholar] [CrossRef]
- Epstein, J.H.; Wintroub, B.U. Photosensitivity Due to Drugs. Drugs 1985, 30, 42–57. [Google Scholar] [CrossRef]
- Glatz, M.; Hofbauer, G.F.L. Phototoxic and Photoallergic Cutaneous Drug Reactions. Adverse Cutan. Drug Erupt. 2012, 97, 167–179. [Google Scholar] [CrossRef]
- Gómez Moyano, E.; Martín Carmona, J.; Gómez Huelgas, R.; Martínez Pilar, L. Reacción fototóxica causada por contacto con ketoprofeno tópico. Med. Clin. 2021, 156, 153. [Google Scholar] [CrossRef]
- Zuba, E.B.; Koronowska, S.; Osmola-Mańkowska, A.; Jenerowicz, D. Drug-Induced Photosensitivity. Acta Dermatovenerol. Croat. 2016, 24, 55. [Google Scholar]
- Gumieniczek, A.; Berecka-Rycerz, A.; Hubicka, U.; Żmudzki, P.; Lejwoda, K.; Kozyra, P. Photodegradation of the H1 Antihistaminic Topical Drugs Emedastine, Epinastine, and Ketotifen and ROS Tests for Estimations of Their Potent Phototoxicity. Pharmaceutics 2020, 12, 560. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Zhou, Y.; Lu, J.; Chovelon, J.-M.; Ji, Y. Aquatic Photolysis of Ketoprofen Generates Products with Photosensitizing Activity and Toxicity. Water Res. 2022, 210, 117982. [Google Scholar] [CrossRef]
- Ioele, G.; Tavano, L.; De Luca, M.; Ragno, G.; Picci, N.; Muzzalupo, R. Photostability and Ex-Vivo Permeation Studies on Diclofenac in Topical Niosomal Formulations. Int. J. Pharm. 2015, 494, 490–497. [Google Scholar] [CrossRef]
- Sammartino, M.P.; Castrucci, M.; Ruiu, D.; Visco, G.; Campanella, L. Photostability and Toxicity of Finasteride, Diclofenac and Naproxen under Simulating Sunlight Exposure: Evaluation of the Toxicity Trend and of the Packaging Photoprotection. Chem. Cent. J. 2013, 7, 181. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Improving the Isotretinoin Photostability by Incorporating in Microemulsion Matrix. ISRN Pharm. 2011, 2011, 838016. [Google Scholar] [CrossRef] [PubMed]
- Kryczyk, A.; Żmudzki, P.; Koczurkiewicz, P.; Piotrowska, J.; Pękala, E.; Hubicka, U. The Impact of ZnO and TiO2 on the Stability of Clotrimazole under UVA Irradiation: Identification of Photocatalytic Degradation Products and in vitro Cytotoxicity Assessment. J. Pharm. Biomed. Anal. 2017, 145, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, A.; Abdouss, M.; Afshar Taromi, F. Fabrication of Biocompatible Antibacterial Nanowafers Based on HNT/PVA Nanocomposites Loaded with Minocycline for Burn Wound Dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110685. [Google Scholar] [CrossRef]
- Niu, X.-Z.; Glady-Croué, J.; Croué, J.-P. Photodegradation of Sulfathiazole under Simulated Sunlight: Kinetics, Photo-Induced Structural Rearrangement, and Antimicrobial Activities of Photoproducts. Water Res. 2017, 124, 576–583. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Samat, M.H.; Ali, A.M.M.; Taib, M.F.M.; Hassan, O.H.; Yahya, M.Z.A. Hubbard U Calculations on Optical Properties of 3d Transition Metal Oxide TiO2. Results Phys. 2016, 6, 891–896. [Google Scholar] [CrossRef]
- Hiroi, Z. Inorganic Structural Chemistry of Titanium Dioxide Polymorphs. Inorg. Chem. 2022, 61, 8393–8401. [Google Scholar] [CrossRef]
- Revision of the Opinion on Titanium Dioxide (Nano Form). Available online: https://health.ec.europa.eu/publications/revision-opinion-titanium-dioxide-nano-form_en (accessed on 27 March 2023).
- Gupta, S.; Tripathi, M. A Review on the Synthesis of TiO2 Nanoparticles by Solution Route. Open Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Baldassari, S.; Komarneni, S.; Mariani, E.; Villa, C. Microwave-Hydrothermal Process for the Synthesis of Rutile. Mater. Res. Bull. 2005, 40, 2014–2020. [Google Scholar] [CrossRef]
- Hu, Y.; Tsai, H.-L.; Huang, C.-L. Phase Transformation of Precipitated TiO2 Nanoparticles. Mater. Sci. Eng. A 2003, 344, 209–214. [Google Scholar] [CrossRef]
- Dubey, R.S. Temperature-Dependent Phase Transformation of TiO2 Nanoparticles Synthesized by Sol-Gel Method. Mater. Lett. 2018, 215, 312–317. [Google Scholar] [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. [Google Scholar] [CrossRef]
- Egerton, T. UV-Absorption—The Primary Process in Photocatalysis and Some Practical Consequences. Molecules 2014, 19, 18192–18214. [Google Scholar] [CrossRef]
- Egerton, T.A.; Tooley, I.R. UV Absorption and Scattering Properties of Inorganic-Based Sunscreens: Absorption and Scattering by TiO2. Int. J. Cosmet. Sci. 2012, 34, 117–122. [Google Scholar] [CrossRef]
- Meo, S.D.; Venditti, P.; Victor, V.M.; Napolitano, G. Harmful and Beneficial Role of ROS 2020. Oxid. Med. Cell. Longev. 2022, 2022, 9873652. [Google Scholar] [CrossRef]
- Martínez de Toda, I.; Vida, C.; Sanz San Miguel, L.; De la Fuente, M. Function, Oxidative, and Inflammatory Stress Parameters in Immune Cells as Predictive Markers of Lifespan throughout Aging. Oxid. Med. Cell. Longev. 2019, 2019, e4574276. [Google Scholar] [CrossRef]
- Gojznikar, J.; Zdravković, B.; Vidak, M.; Leskošek, B.; Ferk, P. TiO2 Nanoparticles and Their Effects on Eukaryotic Cells: A Double-Edged Sword. Int. J. Mol. Sci. 2022, 23, 12353. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, M.; Sagar, B.; Doshi, S.; Chandrasekaran, N.; Mukherjee, A. Comparative Study on Toxicity of ZnO and TiO2 Nanoparticles on Artemia Salina: Effect of Pre-UV-A and Visible Light Irradiation. Environ. Sci. Pollut. Res. 2017, 24, 5633–5646. [Google Scholar] [CrossRef]
- Fenoglio, I.; Ponti, J.; Alloa, E.; Ghiazza, M.; Corazzari, I.; Capomaccio, R.; Rembges, D.; Oliaro-Bosso, S.; Rossi, F. Singlet Oxygen Plays a Key Role in the Toxicity and DNA Damage Caused by Nanometric TiO2 in Human Keratinocytes. Nanoscale 2013, 5, 6567–6576. [Google Scholar] [CrossRef]
- Coral, J.A.; Kitchens, C.L.; Brumaghim, J.L.; Klaine, S.J. Correlating Quantitative Measurements of Radical Production by Photocatalytic TiO2 with Daphnia Magna Toxicity. Environ. Toxicol. Chem. 2021, 40, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively Generated Damage to the Guanine Moiety of DNA: Mechanistic Aspects and Formation in Cells. Acc. Chem. Res. 2008, 41, 1075–1083. [Google Scholar] [CrossRef]
- Vequizo, J.J.M.; Matsunaga, H.; Ishiku, T.; Kamimura, S.; Ohno, T.; Yamakata, A. Trapping-Induced Enhancement of Photocatalytic Activity on Brookite TiO2 Powders: Comparison with Anatase and Rutile TiO2 Powders. ACS Catal. 2017, 7, 2644–2651. [Google Scholar] [CrossRef]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, M.; Wojtyła, S.; Macyk, W. On Oxygen Activation at Rutile- and Anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Kirkland, D.; Aardema, M.J.; Battersby, R.V.; Beevers, C.; Burnett, K.; Burzlaff, A.; Czich, A.; Donner, E.M.; Fowler, P.; Johnston, H.J.; et al. A Weight of Evidence Review of the Genotoxicity of Titanium Dioxide (TiO2). Regul. Toxicol. Pharmacol. 2022, 136, 105263. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Sugino, S.; Kato, H.; Tabei, Y.; Nakamura, A.; Yoshida, Y. Does Photocatalytic Activity of TiO2 Nanoparticles Correspond to Photo-Cytotoxicity? Cellular Uptake of TiO2 Nanoparticles Is Important in Their Photo-Cytotoxicity. Toxicol. Mech. Methods 2016, 26, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Hering, H.; Zoschke, C.; König, F.; Kühn, M.; Luch, A.; Schreiver, I. Phototoxic versus Photoprotective Effects of Tattoo Pigments in Reconstructed Human Skin Models: In vitro Phototoxicity Testing of Tattoo Pigments: 3D versus 2D. Toxicology 2021, 460, 152872. [Google Scholar] [CrossRef]
- Yin, J.-J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of Nano Titanium Dioxides in HaCaT Keratinocytes—Generation of Reactive Oxygen Species and Cell Damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef]
- Coleman, H.M.; Marquis, C.P.; Scott, J.A.; Chin, S.-S.; Amal, R. Bactericidal Effects of Titanium Dioxide-Based Photocatalysts. Chem. Eng. J. 2005, 113, 55–63. [Google Scholar] [CrossRef]
- Uchino, T.; Tokunaga, H.; Ando, M.; Utsumi, H. Quantitative Determination of OH Radical Generation and Its Cytotoxicity Induced by TiO2-UVA Treatment. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2002, 16, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size Matters: Why Nanomaterials Are Different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Y.; Whalen, J.K.; McShane, H.; Driscoll, B.T.; Sunahara, G.I. Evidence That Nano-TiO2 Induces Acute Cytotoxicity to the Agronomically Beneficial Nitrogen-Fixing Bacteria Sinorhizobium meliloti. Can. J. Microbiol. 2021, 68, 67–72. [Google Scholar] [CrossRef]
- Sanders, K.; Degn, L.L.; Mundy, W.R.; Zucker, R.M.; Dreher, K.; Zhao, B.; Roberts, J.E.; Boyes, W.K. In vitro Phototoxicity and Hazard Identification of Nano-Scale Titanium Dioxide. Toxicol. Appl. Pharmacol. 2012, 258, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Wyrwoll, A.J.; Lautenschläger, P.; Bach, A.; Hellack, B.; Dybowska, A.; Kuhlbusch, T.A.J.; Hollert, H.; Schäffer, A.; Maes, H.M. Size Matters—The Phototoxicity of TiO2 Nanomaterials. Environ. Pollut. 2016, 208, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Almquist, C.B.; Biswas, P. Role of Synthesis Method and Particle Size of Nanostructured TiO2 on Its Photoactivity. J. Catal. 2002, 212, 145–156. [Google Scholar] [CrossRef]
- Specific Surface Area of Titanium Dioxide (TiO2) Particles Influences Cyto- and Photo-Toxicity—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23295712/ (accessed on 11 February 2023).
- Tong, T.; Shereef, A.; Wu, J.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.-F.; Gray, K.A. Effects of Material Morphology on the Phototoxicity of Nano-TiO2 to Bacteria. Environ. Sci. Technol. 2013, 47, 12486–12495. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute Toxicity and Biodistribution of Different Sized Titanium Dioxide Particles in Mice after Oral Administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Pourjafar, M. NLRP3 Inflammasome, Oxidative Stress, and Apoptosis Induced in the Intestine and Liver of Rats Treated with Titanium Dioxide Nanoparticles: In vivo and in vitro Study. Int. J. Nanomed. 2019, 14, 1919–1936. [Google Scholar] [CrossRef]
- Xue, C.; Luo, W.; Yang, X.L. A Mechanism for Nano-Titanium Dioxide-Induced Cytotoxicity in HaCaT Cells under UVA Irradiation. Biosci. Biotechnol. Biochem. 2015, 79, 1384–1390. [Google Scholar] [CrossRef]
- Karch, J.; Molkentin, J.D. Identifying the Components of the Elusive Mitochondrial Permeability Transition Pore. Proc. Natl. Acad. Sci. USA 2014, 111, 10396–10397. [Google Scholar] [CrossRef]
- Geng, R.; Ren, Y.; Rao, R.; Tan, X.; Zhou, H.; Yang, X.; Liu, W.; Lu, Q. Titanium Dioxide Nanoparticles Induced HeLa Cell Necrosis under UVA Radiation through the ROS-MPTP Pathway. Nanomaterials 2020, 10, 2029. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Lee, T.G.; Reipa, V.; Heo, M.B. Titanium Dioxide Induces Apoptosis under UVA Irradiation via the Generation of Lysosomal Membrane Permeabilization-Dependent Reactive Oxygen Species in HaCat Cells. Nanomaterials 2021, 11, 1943. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in Cancer: Mechanisms and Clinical Implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, X.; Geng, R.; Lu, Q.; Rao, R.; Tan, X.; Yang, X.; Liu, W. Increased Level of A2,6-Sialylated Glycans on HaCaT Cells Induced by Titanium Dioxide Nanoparticles under UV Radiation. Nanomaterials 2018, 8, 253. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, J.; Chatterjee, N.; Roca, C.P.; Yoon, D.; Kim, S.; Kim, Y.; Choi, J. JAK/STAT and TGF-ß Activation as Potential Adverse Outcome Pathway of TiO2NPs Phototoxicity in Caenorhabditis Elegans. Sci. Rep. 2017, 7, 17833. [Google Scholar] [CrossRef] [PubMed]
- Hering, H.; Zoschke, C.; Kühn, M.; Gadicherla, A.K.; Weindl, G.; Luch, A.; Schreiver, I. TatS: A Novel in vitro Tattooed Human Skin Model for Improved Pigment Toxicology Research. Arch. Toxicol. 2020, 94, 2423–2434. [Google Scholar] [CrossRef]
- Wani, M.R.; Shadab, G. Titanium Dioxide Nanoparticle Genotoxicity: A Review of Recent in vivo and in vitro Studies. Toxicol. Ind. Health 2020, 36, 514–530. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kim, J.-Y.; Lee, W.; Lee, W.H.; Jang, H.-D.; Lee, S.B. UV Protection Characteristics and Fabrication of Modified TiO2 Nanoparticles. J. Ind. Eng. Chem. 2004, 10, 428–434. [Google Scholar]
- Furusawa, T.; Honda, K.; Ukaji, E.; Sato, M.; Suzuki, N. The Microwave Effect on the Properties of Silica-Coated TiO2 Fine Particles Prepared Using Sol–Gel Method. Mater. Res. Bull. 2008, 43, 946–957. [Google Scholar] [CrossRef]
- Park, O.K.; Kang, Y.S. Preparation and Characterization of Silica-Coated TiO2 Nanoparticle. Colloids Surf. Physicochem. Eng. Asp. 2005, 257–258, 261–265. [Google Scholar] [CrossRef]
- Siddiquey, I.A.; Furusawa, T.; Sato, M.; Honda, K.; Suzuki, N. Control of the Photocatalytic Activity of TiO2 Nanoparticles by Silica Coating with Polydiethoxysiloxane. Dye. Pigment. 2008, 76, 754–759. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Yin, S.; Sato, T.; Ghannam, T.; Al-Hoshan, M.; Al-Salhi, M. Investigation of Photocatalytic Activity and UV-Shielding Properties for Silica Coated Titania Nanoparticles by Solvothermal Coating. J. Alloy. Compd. 2010, 508, L1–L4. [Google Scholar] [CrossRef]
- Martin, N.; Wassmur, B.; Slomberg, D.; Labille, J.; Lammel, T. Influence of TiO2 Nanocomposite UV Filter Surface Chemistry and Their Interactions with Organic UV Filters on Uptake and Toxicity toward Cultured Fish Gill Cells. Ecotoxicol. Environ. Saf. 2022, 243, 113984. [Google Scholar] [CrossRef]
- Camaioni, A.; Massimiani, M.; Lacconi, V.; Magrini, A.; Salustri, A.; Sotiriou, G.A.; Singh, D.; Bitounis, D.; Bocca, B.; Pino, A.; et al. Silica Encapsulation of ZnO Nanoparticles Reduces Their Toxicity for Cumulus Cell-Oocyte-Complex Expansion. Part. Fibre Toxicol. 2021, 18, 33. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, R.; Cao, D.; Kong, X.; Lu, Y. Photocatalytic Production of Hydroxyl Radicals by Commercial TiO2 Nanoparticles and Phototoxic Hazard Identification. Toxicology 2018, 406–407, 1–8. [Google Scholar] [CrossRef]
- Asfour, M.H.; Kassem, A.A.; Salama, A. Topical Nanostructured Lipid Carriers/Inorganic Sunscreen Combination for Alleviation of All-Trans Retinoic Acid-Induced Photosensitivity: Box-Behnken Design Optimization, in vitro and in vivo Evaluation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2019, 134, 219–232. [Google Scholar] [CrossRef]
- Lodén, M.; Akerström, U.; Lindahl, K.; Berne, B. Novel Method for Studying Photolability of Topical Formulations: A Case Study of Titanium Dioxide Stabilization of Ketoprofen. J. Pharm. Sci. 2005, 94, 781–787. [Google Scholar] [CrossRef]
- Wright, F.; Weller, R.B. Risks and Benefits of UV Radiation in Older People: More of a Friend than a Foe? Maturitas 2015, 81, 425–431. [Google Scholar] [CrossRef]
- Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 23 February 2023).
- Lautenschlager, S.; Wulf, H.C.; Pittelkow, M.R. Photoprotection. Lancet 2007, 370, 528–537. [Google Scholar] [CrossRef]
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958. [Google Scholar] [CrossRef]
- Kubáč, L.; Akrman, J.; Kejlová, K.; Bendová, H.; Klánová, K.; Hladíková, Z.; Pikal, P.; Kovaříková, L.; Kašparová, L.; Jírová, D. Characteristics of Titanium Dioxide Microdispersions with Different Photo-Activity Suitable for Sunscreen Formulations. Int. J. Pharm. 2015, 481, 91–96. [Google Scholar] [CrossRef]
- Baumann, L.; Baumann, L. Cosmetic Dermatology and Medicine: Principles and Practice, 2nd ed.; McGraw-Hill: New York, NY, USA, 2009; ISBN 978-0-07-149062-7. [Google Scholar]
- Shedding Light on Sunscreens|Clinical and Experimental Dermatology|Oxford Academic. Available online: https://academic.oup.com/ced/article/23/4/147/6631068 (accessed on 21 February 2023).
- Fajzulin, I.; Zhu, X.; Möller, M. Nanoparticulate Inorganic UV Absorbers: A Review. J. Coat. Technol. Res. 2015, 12, 617–632. [Google Scholar] [CrossRef]
- Gilbert, E.; Pirot, F.; Bertholle, V.; Roussel, L.; Falson, F.; Padois, K. Commonly Used UV Filter Toxicity on Biological Functions: Review of Last Decade Studies. Int. J. Cosmet. Sci. 2013, 35, 208–219. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Dufour, E.K.; Roberts, M.S. Nanotechnology, Cosmetics and the Skin: Is There a Health Risk? Skin Pharmacol. Physiol. 2008, 21, 136–149. [Google Scholar] [CrossRef]
- Kamel, R.; Mostafa, D.M. Rutin Nanostructured Lipid Cosmeceutical Preparation with Sun Protective Potential. J. Photochem. Photobiol. B Biol. 2015, 153, 59–66. [Google Scholar] [CrossRef]
- Diffey, B.L.; Tanner, P.R.; Matts, P.J.; Nash, J.F. In vitro Assessment of the Broad-Spectrum Ultraviolet Protection of Sunscreen Products. J. Am. Acad. Dermatol. 2000, 43, 1024–1035. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Neto, D.M.A.; Freire, R.M.; Rocha, J.S.; Fechine, L.M.U.D.; Denardin, J.C.; Valentini, A.; de Araújo, T.G.; Mazzetto, S.E.; Fechine, P.B.A. Ultrafast Sonochemistry-Based Approach to Coat TiO2 Commercial Particles for Sunscreen Formulation. Ultrason. Sonochem. 2018, 48, 340–348. [Google Scholar] [CrossRef]
- Bartoszewska, M.; Adamska, E.; Kowalska, A.; Grobelna, B. Novelty Cosmetic Filters Based on Nanomaterials Composed of Titanium Dioxide Nanoparticles. Molecules 2023, 28, 645. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Catalano, R.; Bartolomei, V.; Labille, J. Release and Fate of Nanoparticulate TiO2 UV Filters from Sunscreen: Effects of Particle Coating and Formulation Type. Environ. Pollut. 2021, 271, 116263. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Ene, V.L.; Voicu, B.B.; Bucur, M.A.; Neacsu, I.A.; Vasile, B.S.; Iordache, F. Biocompatible Ag/Fe-Enhanced TiO2 Nanoparticles as an Effective Compound in Sunscreens. Nanomaterials 2020, 10, 570. [Google Scholar] [CrossRef]
- Loto, A.M.; Morales, J.M.N.; Cisneros, A.B.; Coria, M.S.; Tulli, F.; Morán Vieyra, F.E.; Borsarelli, C.D. Simple Preparation of Broadband UV Filters Based on TiO2 Coated with Aqueous Extracts of Native Trees from the Chaco Region of Argentina. Photochem. Photobiol. Sci. 2023, 22, 319–331. [Google Scholar] [CrossRef]
- Rabani, I.; Jang, H.-N.; Park, Y.-J.; Tahir, M.S.; Lee, Y.-B.; Moon, E.-Y.; Song, J.W.; Seo, Y.-S. Titanium Dioxide Incorporated in Cellulose Nanofibers with Enhanced UV Blocking Performance by Eliminating ROS Generation. RSC Adv. 2022, 12, 33653–33665. [Google Scholar] [CrossRef]
- Wang, S.Q.; Tooley, I.R. Photoprotection in the Era of Nanotechnology. Semin. Cutan. Med. Surg. 2011, 30, 210–213. [Google Scholar] [CrossRef]


| Active Compound | Photosensitivity or Photostability Assessment | Conclusions | References |
|---|---|---|---|
| H1-receptor antagonists (antihistamines) | |||
| Emedastine | 1. pH-dependent photodegradation in the presence of UV/VIS light 2. HPLC-UV analysis—a quantifying percentage of photodegradation 3. UPLC-MS/MS analysis—identifying photodegradation products, their chemical structure, and possible degradation pathways | photolability in the whole range of pH values | [20] |
| Epinastine | photostability at pH 7.0 and 10.0, decreased at 3.0 | ||
| Ketotifen | moderately photolabile at pH 3.0 and 7.0, completely decreased at 10.0 | ||
| Promethazine | 1. intercalation promethazine into the montmorillonite (mont) matrix (promethazine salt complex) 2. XRD, DSC, and FT-IR—the behavior of complexes during different light exposure times 3. data analysis—obtaining kinetic of photodegradation and drug photostability information | promethazine-mont salt complex demonstrates a higher value of photostability; this compound can develop topical formulation without photosensitization and adverse reactions in the skin | [11] |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | |||
| Ketoprofen | 1. irradiation aqueous ketoprofen solutions 2. LC-MS/MS, HR-MS analysis—identifying photodegradation products 3. spectroscopic analysis—characterization of photophysical properties of photolysis products | ketoprofen is a strongly photolabile drug; it is necessary to further study and determine the behavior of ketoprofen under the influence of sunlight | [21] |
| Diclofenac | 1. compounding niosomal gels based on diclofenac and ascorbic acid with antioxidant properties 2. irradiation commercial formulations based on diclofenac and niosomal gels 3. spectroscopic analysis—obtaining kinetic of photodegradation and drug photostability information | photodegradation of diclofenac is oxygen-concentration dependent. Niosomal formulations enhanced diclofenac permeation and strongly increased photostability | [22] |
| Naproxen | 1. irradiation aqueous naproxen solutions 2. HPLC-UV analysis—a quantifying percentage of photodegradation 3. toxicity tests—amperometric biosensor based on suspended yeast cell | naproxen and its photodegradation products exhibit toxic properties which lead to yeast cell culture death | [23] |
| Retinoids | |||
| Isotretinoin | 1. compounding microemulsion based on isotretinoin 2. irradiation isotretinoin-methanol solution and isotretinoin microemulsion 3. spectroscopic analysis—obtaining kinetic of photodegradation and drug photostability information | the inclusion of isotretinoin in the microemulsion matrix increases photostability | [24] |
| Tazarotene | 1. irradiation ethanolic solutions containing tazarotene without/in the presence of ZnO, TiO2, and benzophenone-derivative UV-filters 2. UPLC-MS/MS analysis—identifying photodegradation products, their chemical structure, and possible degradation pathways 3. MTT—analyzing cytotoxic properties | UV irradiation favors retinoids photodegradation. Photodegradation is UV-filter-dependent that exhibits photoprotective properties | [25] |
| Antibiotics and antifungals | |||
| Minocycline | 1. development of nanocomposite film based on polyvinyl alcohol and halloysite nanotubes for minocycline delivery 2. XRD, FT-IR, Zeta potential, TG analysis | minocycline is a slightly photolabile drug. polymeric formulations increased photostability | [26] |
| Sulfathiazole | 1. irradiation aqueous sulfathiazole solutions 2. LC-MS/MS analysis—identifying photodegradation products 3. antimicrobial assays | irradiated sulfathiazole indicated less antibacterial potency against Escherichia coli | [27] |
| Clotrimazole | 1. irradiation clotrimazole-methanol solution with ZnO/TiO2 powder-mixture 2. pH-dependent photodegradation in the presence of UV/VIS light 3. UPLC-MS/MS analysis—identifying photodegradation products, their chemical structure, and possible degradation pathways, obtaining kinetic of photodegradation | photodegradation of clotrimazole is strongly pH dependent. Instability is marked at acidic pH | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gackowski, M.; Osmałek, T.; Froelich, A.; Otto, F.; Schneider, R.; Lulek, J. Phototoxic or Photoprotective?—Advances and Limitations of Titanium (IV) Oxide in Dermal Formulations—A Review. Int. J. Mol. Sci. 2023, 24, 8159. https://doi.org/10.3390/ijms24098159
Gackowski M, Osmałek T, Froelich A, Otto F, Schneider R, Lulek J. Phototoxic or Photoprotective?—Advances and Limitations of Titanium (IV) Oxide in Dermal Formulations—A Review. International Journal of Molecular Sciences. 2023; 24(9):8159. https://doi.org/10.3390/ijms24098159
Chicago/Turabian StyleGackowski, Michał, Tomasz Osmałek, Anna Froelich, Filip Otto, Raphaël Schneider, and Janina Lulek. 2023. "Phototoxic or Photoprotective?—Advances and Limitations of Titanium (IV) Oxide in Dermal Formulations—A Review" International Journal of Molecular Sciences 24, no. 9: 8159. https://doi.org/10.3390/ijms24098159
APA StyleGackowski, M., Osmałek, T., Froelich, A., Otto, F., Schneider, R., & Lulek, J. (2023). Phototoxic or Photoprotective?—Advances and Limitations of Titanium (IV) Oxide in Dermal Formulations—A Review. International Journal of Molecular Sciences, 24(9), 8159. https://doi.org/10.3390/ijms24098159