Simultaneously Targeting Two Coupled Signalling Molecules in the Mesenchymal Stem Cell Support Efficiently Sensitises the Multiple Myeloma Cell Line H929 to Bortezomib
Abstract
1. Introduction
2. Results
2.1. BM-MSC Favour Slightly the Growth of H929 Cells Greatly the Resistance to BTZ
2.2. BM-MSC Can Even Restore the Viability of H929 Cells Previously Treated with BTZ
2.3. The BM-MSC Supportive Capacity and Resistance to BTZ Involves PKC Activity
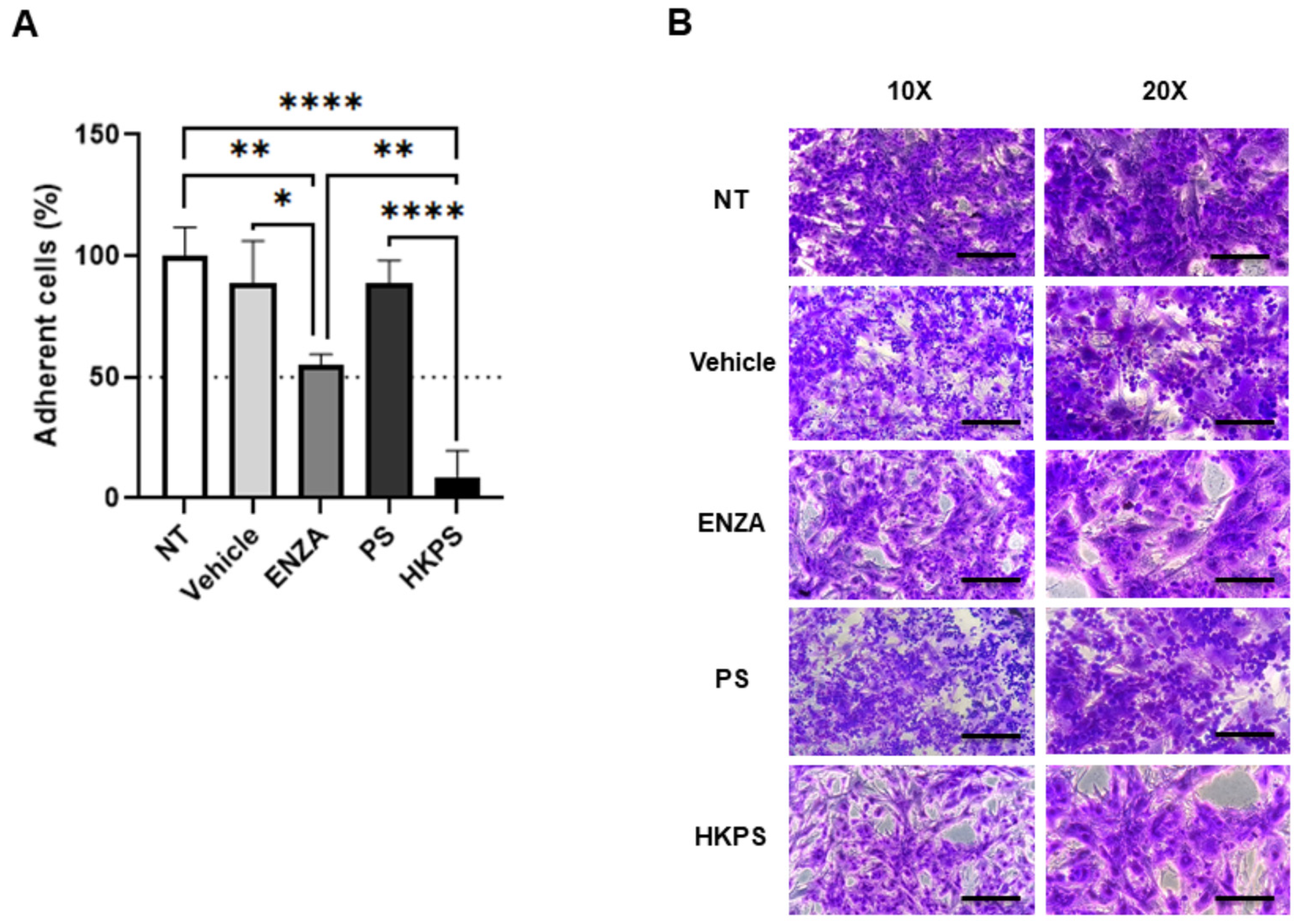
2.4. BM-MSC PKC Mediates Cell-to-Cell Adhesion and BTZ Resistance of H929 Cells
2.5. Pre-Treatment of BM-MSC with BAY11-7082 also Inhibits Cell Adhesion and Increases BTZ Susceptibility of H929 Cells
2.6. Pre-Treatments of BM-MSC with ENZA or HKPS Differentially Affect the Activation of NF-κB Signalling Pathway Induced in BM-MSC by Co-Cultivation with H929 Cells
2.7. Targeting of BM-MSC Support Alone or the Co-Cultures with HKPS and BAY11-7082, Is More Effective Than Individual Treatments in Inducing H929 Susceptibility to BTZ
3. Discussion
4. Materials and Methods
4.1. BM-MSC Isolation and Characterization
4.2. Multiple Myeloma Cell Line (H929)
4.3. Establishment of the Co-Cultures of BM-MSC with H929 Cells
4.4. Peptide Synthesis
4.5. BM-MSC Pre-Treatment with PKC and NF-κB Inhibitors
4.6. Population Doubling Time of H929 Cells
4.7. Cytotoxicity Assays
4.8. H929 Cell Adhesion Assay
4.9. Assessment of NF-κB Pathway Activation in BM-MSC
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| BM | Bone marrow |
| MSC | Mesenchymal stem cells |
| ME | Microenvironment |
| SDF-1 | Stem-derived factor 1 |
| CXCR4 | Chemokine receptor 4 |
| MIF | Macrophage migration inhibitory factor |
| VLA | Very late antigen |
| IL | Interleukin |
| CAM-DR | Cell adhesion-mediated drug resistance |
| STAT | Signal transducer and activator of transcription |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphoinositol-3-kinase |
| NF- κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| (c)PKC | (Classical) protein kinase C |
| RACK1 | Receptor for activated C kinase 1 |
| BTZ | Bortezomib |
| DEXA | Dexamethasone |
| FBS | Foetal bovine serum |
| ENZA | Enzastaurin |
| DMSO | Dimethyl sulfoxide |
| NT | Non-treated |
| TNFR(SF6) | Tumour necrosis factor receptor (superfamily member 6) |
| LTBR | Lymphotoxin beta receptor |
| SOCS6 | Suppressor of cytokine signalling 6 |
| STING | Simulator of interferon genes |
| TRAIL(R) | TNF-related apoptosis-inducing ligand (receptor) |
| IRAK | Interleukin receptor-associated kinase |
| IκB | Inhibitor of kappa B |
| JNK | Janus kinase |
| APRIL | A proliferation-inducing ligand |
| BAFF | B-cell activating factor |
| MNC | Mononuclear cells |
| BCA | Bicinchoninic acid |
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.-V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Prim. 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Giannakoulas, N.; Ntanasis-Stathopoulos, I.; Terpos, E. The Role of Marrow Microenvironment in the Growth and Development of Malignant Plasma Cells in Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 4462. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.G.; Stojanov, P.; Carter, S.L.; Cruz-Gordillo, P.; Lawrence, M.S.; Auclair, D.; Sougnez, C.; Knoechel, B.; Gould, J.; Saksena, G.; et al. Widespread Genetic Heterogeneity in Multiple Myeloma: Implications for Targeted Therapy. Cancer Cell 2014, 25, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bolli, N.; Avet-Loiseau, H.; Wedge, D.C.; Van Loo, P.; Alexandrov, L.B.; Martincorena, I.; Dawson, K.J.; Iorio, F.; Nik-Zainal, S.; Bignell, G.R.; et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 2014, 5, 2997. [Google Scholar] [CrossRef]
- Gupta, V.A.; Ackley, J.; Kaufman, J.L.; Boise, L.H. BCL2 Family Inhibitors in the Biology and Treatment of Multiple Myeloma. Blood Lymphat. Cancer 2021, 11, 11–24. [Google Scholar] [CrossRef]
- Damiano, J.S.; Dalton, W.S. Integrin-Mediated Drug Resistance in Multiple Myeloma. Leuk. Lymphoma 2000, 38, 71–81. [Google Scholar] [CrossRef]
- Sanz-rodríguez, F.; Teixidó, J. VLA-4-Dependent Myeloma Cell Adhesion. Leuk. Lymphoma 2001, 41, 239–245. [Google Scholar] [CrossRef]
- Noll, J.E.; Williams, S.A.; Purton, L.E.; Zannettino, A.C.W. Tug of war in the haematopoietic stem cell niche: Do myeloma plasma cells compete for the HSC niche? Blood Cancer J. 2012, 2, e91. [Google Scholar] [CrossRef]
- Raab, M.S.; Podar, K.; Breitkreutz, I.; Richardson, P.G.; Anderson, K.C. Multiple myeloma. Lancet 2009, 374, 324–339. [Google Scholar] [CrossRef]
- Alsayed, Y.; Ngo, H.; Runnels, J.; Leleu, X.; Singha, U.K.; Pitsillides, C.M.; Spencer, J.A.; Kimlinger, T.; Ghobrial, J.M.; Jia, X.; et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)–dependent migration and homing in multiple myeloma. Blood 2007, 109, 2708–2717. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Q.; Li, T.; Qian, J.; Lu, Y.; Li, Y.; Bi, E.; Reu, F.; Qin, Y.; Drazba, J.; et al. Role of Myeloma-Derived MIF in Myeloma Cell Adhesion to Bone Marrow and Chemotherapy Response. J. Natl. Cancer Inst. 2016, 108, djw131. [Google Scholar] [CrossRef]
- Azab, A.K.; Runnels, J.M.; Pitsillides, C.; Moreau, A.-S.; Azab, F.; Leleu, X.; Jia, X.; Wright, R.; Ospina, B.; Carlson, A.L.; et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 2009, 113, 4341–4351. [Google Scholar] [CrossRef]
- Hazlehurst, L.A.; Dalton, W.S. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 2001, 20, 43–50. [Google Scholar] [CrossRef]
- Shain, K.H.; Dalton, W.S. Environmental-mediated drug resistance: A target for multiple myeloma therapy. Expert Rev. Hematol. 2009, 2, 649–662. [Google Scholar] [CrossRef]
- Mahtouk, K.; Moreaux, J.; Hose, D.; Rème, T.; Meißner, T.; Jourdan, M.; Rossi, J.F.; Pals, S.T.; Goldschmidt, H.; Klein, B. Growth factors in multiple myeloma: A comprehensive analysis of their expression in tumor cells and bone marrow environment using Affymetrix microarrays. BMC Cancer 2010, 10, 198. [Google Scholar] [CrossRef]
- Slany, A.; Haudek-Prinz, V.; Meshcheryakova, A.; Bileck, A.; Lamm, W.; Zielinski, C.; Gerner, C.; Drach, J. Extracellular Matrix Remodeling by Bone Marrow Fibroblast-like Cells Correlates with Disease Progression in Multiple Myeloma. J. Proteome Res. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Brocke-Heidrich, K.; Kretzschmar, A.K.; Pfeifer, G.; Henze, C.; Löffler, D.; Koczan, D.; Thiesen, H.-J.; Burger, R.; Gramatzki, M.; Horn, F. Interleukin-6–dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family–independent survival pathway closely associated with Stat3 activation. Blood 2004, 103, 242–251. [Google Scholar] [CrossRef]
- Hu, L.; Shi, Y.; Hsu, J.; Gera, J.; Van Ness, B.; Lichtenstein, A. Downstream effectors of oncogenic ras in multiple myeloma cells. Blood 2003, 101, 3126–3135. [Google Scholar] [CrossRef]
- Juliano, R.L. Signal Transduction by Cell Adhesion Receptors and the Cytoskeleton: Functions of Integrins, Cadherins, Selectins, and Immunoglobulin-Superfamily Members. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 283–323. [Google Scholar] [CrossRef]
- Landowski, T.H.; Olashaw, N.E.; Agrawal, D.; Dalton, W.S. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-κB (RelB/p50) in myeloma cells. Oncogene 2003, 22, 2417–2421. [Google Scholar] [CrossRef]
- Novotny-Diermayr, V.; Zhang, T.; Gu, L.; Cao, X. Protein Kinase C δ Associates with the Interleukin-6 Receptor Subunit Glycoprotein (gp) 130 via Stat3 and Enhances Stat3-gp130 Interaction. J. Biol. Chem. 2002, 277, 49134–49142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zong, C.S.; Hermanto, U.; Lopez-Bergami, P.; Ronai, Z.; Wang, L.-H. RACK1 Recruits STAT3 Specifically to Insulin and Insulin-Like Growth Factor 1 Receptors for Activation, Which Is Important for Regulating Anchorage-Independent Growth. Mol. Cell Biol. 2006, 26, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Mahtouk, K.; Attal, M.; Gadelorge, M.; Huynh, A.; Fleury-Cappellesso, S.; Danho, C.; Laharrague, P.; Klein, B.; Rème, T.; et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007, 21, 1079–1088. [Google Scholar] [CrossRef]
- Todoerti, K.; Lisignoli, G.; Storti, P.; Agnelli, L.; Novara, F.; Manferdini, C.; Codeluppi, K.; Colla, S.; Crugnola, M.; Abeltino, M.; et al. Distinct transcriptional profiles characterize bone microenvironment mesenchymal cells rather than osteoblasts in relationship with multiple myeloma bone disease. Exp. Hematol. 2010, 38, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; De Veirman, K.; De Becker, A.; Vanderkerken, K.; Van Riet, I. Mesenchymal stem cells in multiple myeloma: A therapeutical tool or target? Leukemia 2018, 32, 1500–1514. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Kostopoulos, I.V.; Tsopanidou, A.; Orologas-Stavrou, N.; Kastritis, E.; Tsitsilonis, O.E.; Dimopoulos, M.A.; Terpos, E. Ex Vivo Models Simulating the Bone Marrow Environment and Predicting Response to Therapy in Multiple Myeloma. Cancers 2020, 12, 2006. [Google Scholar] [CrossRef]
- Lemaitre, L.; Do Souto Ferreira, L.; Joubert, M.-V.; Avet-Loiseau, H.; Martinet, L.; Corre, J.; Couderc, B. Imprinting of Mesenchymal Stromal Cell Transcriptome Persists even after Treatment in Patients with Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 3854. [Google Scholar] [CrossRef]
- Beider, K.; Bitner, H.; Leiba, M.; Gutwein, O.; Koren-Michowitz, M.; Ostrovsky, O.; Abraham, M.; Wald, H.; Galun, E.; Peled, A.; et al. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget 2014, 5, 11283–11296. [Google Scholar] [CrossRef]
- André, T.; Najar, M.; Stamatopoulos, B.; Pieters, K.; Pradier, O.; Bron, D.; Meuleman, N.; Lagneaux, L. Immune impairments in multiple myeloma bone marrow mesenchymal stromal cells. Cancer Immunol. Immunother. 2015, 64, 213–224. [Google Scholar] [CrossRef]
- Di Marzo, L.; Desantis, V.; Solimando, A.G.; Ruggieri, S.; Annese, T.; Nico, B.; Fumarulo, R.; Vacca, A.; Frassanito, M.A. Microenvironment drug resistance in multiple myeloma: Emerging new players. Oncotarget 2016, 7, 60698–60711. [Google Scholar] [CrossRef]
- Markovina, S.; Callander, N.S.; O’Connor, S.L.; Xu, G.; Shi, Y.; Leith, C.P.; Kim, K.; Trivedi, P.; Kim, J.; Hematti, P.; et al. Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-κB activity in myeloma cells. Mol. Cancer 2010, 9, 176. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, Y.; Zhang, Y.; Cao, Z.; Jiang, Y. Mesenchymal stem cells derived from multiple myeloma patients protect against chemotherapy through autophagy-dependent activation of NF-κB signaling. Leuk. Res. 2017, 60, 82–88. [Google Scholar] [CrossRef]
- Annunziata, C.M.; Davis, R.E.; Demchenko, Y.; Bellamy, W.; Gabrea, A.; Zhan, F.; Lenz, G.; Hanamura, I.; Wright, G.; Xiao, W.; et al. Frequent Engagement of the Classical and Alternative NF-κB Pathways by Diverse Genetic Abnormalities in Multiple Myeloma. Cancer Cell 2007, 12, 115–130. [Google Scholar] [CrossRef]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.-J.; Van Wier, S.; Tiedemann, R.; Shi, C.-X.; Sebag, M.; et al. Promiscuous Mutations Activate the Noncanonical NF-κB Pathway in Multiple Myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef]
- Xiang, Y.; Remily-Wood, E.R.; Oliveira, V.; Yarde, D.; He, L.; Cheng, J.Q.; Mathews, L.; Boucher, K.; Cubitt, C.; Perez, L.; et al. Monitoring a Nuclear Factor-κB Signature of Drug Resistance in Multiple Myeloma. Mol. Cell. Proteom. 2011, 10, M110.005520. [Google Scholar] [CrossRef]
- Reagan, M.R.; Ghobrial, I.M. Multiple Myeloma Mesenchymal Stem Cells: Characterization, Origin, and Tumor-Promoting Effects. Clin. Cancer Res. 2012, 18, 342–349. [Google Scholar] [CrossRef]
- De Raeve, H.R.; Vanderkerken, K. The role of the bone marrow microenvironment in multiple myeloma. Histol. Histopathol. 2005, 20, 1227–1250. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.-T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM mesenchymal stromal cell–derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Moschetta, M.; Manier, S.; Glavey, S.; Görgün, G.T.; Roccaro, A.M.; Anderson, K.C.; Ghobrial, I.M. Targeting the bone marrow microenvironment in multiple myeloma. Immunol. Rev. 2015, 263, 160–172. [Google Scholar] [CrossRef]
- Ruiz-Aparicio, P.F.; Vanegas, N.-D.P.; Uribe, G.I.; Ortiz-Montero, P.; Cadavid-Cortés, C.; Lagos, J.; Flechas-Afanador, J.; Linares-Ballesteros, A.; Vernot, J.P. Dual Targeting of Stromal Cell Support and Leukemic Cell Growth by a Peptidic PKC Inhibitor Shows Effectiveness against B-ALL. Int. J. Mol. Sci. 2020, 21, 3705. [Google Scholar] [CrossRef]
- Park, E.; Chen, J.; Moore, A.; Mangolini, M.; Santoro, A.; Boyd, J.R.; Schjerven, H.; Ecker, V.; Buchner, M.; Williamson, J.C.; et al. Stromal cell protein kinase C-β inhibition enhances chemosensitivity in B cell malignancies and overcomes drug resistance. Sci. Transl. Med. 2020, 12, eaax9340. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-Meco, M.T.; Rennert, P. NF-kappaB activation by protein kinase C isoforms and B-cell function. EMBO Rep. 2003, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Shabaneh, T.B.; Downey, S.L.; Goddard, A.L.; Screen, M.; Lucas, M.M.; Eastman, A.; Kisselev, A.F. Molecular Basis of Differential Sensitivity of Myeloma Cells to Clinically Relevant Bolus Treatment with Bortezomib. PLoS ONE 2013, 8, e56132. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Mager, D.E. Network-Based Analysis of Bortezomib Pharmacodynamic Heterogeneity in Multiple Myeloma Cells. J. Pharmacol. Exp. Ther. 2018, 365, 734–751. [Google Scholar] [CrossRef]
- Graff, J.R.; McNulty, A.M.; Hanna, K.R.; Konicek, B.W.; Lynch, R.L.; Bailey, S.N.; Banks, C.; Capen, A.; Goode, R.; Lewis, J.E.; et al. The Protein Kinase Cβ–Selective Inhibitor, Enzastaurin (LY317615.HCl), Suppresses Signaling through the AKT Pathway, Induces Apoptosis, and Suppresses Growth of Human Colon Cancer and Glioblastoma Xenografts. Cancer Res. 2005, 65, 7462–7469. [Google Scholar] [CrossRef]
- Perdomo-Arciniegas, A.M.; Patarroyo, M.E.; Vernot, J.P. Novel Chimeric Peptide Inhibits Protein Kinase C and Induces Apoptosis in Human Immune Cells. Int. J. Pept. Res. Ther. 2008, 14, 64–74. [Google Scholar] [CrossRef]
- Panwalkar, A.; Verstovsek, S.; Giles, F. Nuclear factor-KappaB modulation as a therapeutic approach in hematologic malignancies. Cancer 2004, 100, 1578–1589. [Google Scholar] [CrossRef]
- Vrábel, D.; Pour, L.; Ševčíková, S. The impact of NF-κB signaling on pathogenesis and current treatment strategies in multiple myeloma. Blood Rev. 2019, 34, 56–66. [Google Scholar] [CrossRef]
- Matula, Z.; Mikala, G.; Lukácsi, S.; Matkó, J.; Kovács, T.; Monostori, É.; Uher, F.; Vályi-Nagy, I. Stromal Cells Serve Drug Resistance for Multiple Myeloma via Mitochondrial Transfer: A Study on Primary Myeloma and Stromal Cells. Cancers 2021, 13, 3461. [Google Scholar] [CrossRef]
- Melzer, C.; von der Ohe, J.; Hass, R. Concise Review: Crosstalk of Mesenchymal Stroma/Stem-Like Cells with Cancer Cells Provides Therapeutic Potential. Stem Cells 2018, 36, 951–968. [Google Scholar] [CrossRef]
- Ruiz-Aparicio, P.F.; Uribe, G.I.; Linares-Ballesteros, A.; Vernot, J.P. Sensitization to Drug Treatment in Precursor B-Cell Acute Lymphoblastic Leukemia Is Not Achieved by Stromal NF-κB Inhibition of Cell Adhesion but by Stromal PKC-Dependent Inhibition of ABC Transporters Activity. Molecules 2021, 26, 5366. [Google Scholar] [CrossRef]
- Dytfeld, D.; Rosebeck, S.; Kandarpa, M.; Mayampurath, A.; Mellacheruvu, D.; Alonge, M.M.; Ngoka, L.; Jasielec, J.; Richardson, P.G.; Volchenboum, S.; et al. Proteomic profiling of naïve multiple myeloma patient plasma cells identifies pathways associated with favourable response to bortezomib-based treatment regimens. Br. J. Haematol. 2015, 170, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H. Protein Kinase C (PKC) Isozymes and Cancer. New J. Sci. 2014, 2014, 1–36. [Google Scholar] [CrossRef]
- Ho, M.; Chen, T.; Liu, J.; Dowling, P.; Hideshima, T.; Zhang, L.; Morelli, E.; Camci-Unal, G.; Wu, X.; Tai, Y.-T.; et al. Targeting histone deacetylase 3 (HDAC3) in the bone marrow microenvironment inhibits multiple myeloma proliferation by modulating exosomes and IL-6 trans-signaling. Leukemia 2020, 34, 196–209. [Google Scholar] [CrossRef]
- Borella, G.; Da Ros, A.; Borile, G.; Porcù, E.; Tregnago, C.; Benetton, M.; Marchetti, A.; Bisio, V.; Montini, B.; Michielotto, B.; et al. Targeting mesenchymal stromal cells plasticity to reroute acute myeloid leukemia course. Blood 2021, 138, 557–570. [Google Scholar] [CrossRef]
- Podar, K.; Raab, M.S.; Zhang, J.; McMillin, D.; Breitkreutz, I.; Tai, Y.-T.; Lin, B.K.; Munshi, N.; Hideshima, T.; Chauhan, D.; et al. Targeting PKC in multiple myeloma: In vitro and in vivo effects of the novel, orally available small-molecule inhibitor enzastaurin (LY317615.HCl). Blood 2007, 109, 1669–1677. [Google Scholar] [CrossRef]
- Raab, M.S.; Breitkreutz, I.; Tonon, G.; Zhang, J.; Hayden, P.J.; Nguyen, T.; Fruehauf, J.H.; Lin, B.K.; Chauhan, D.; Hideshima, T.; et al. Targeting PKC: A novel role for beta-catenin in ER stress and apoptotic signaling. Blood 2009, 113, 1513–1521. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Ghias, K.; Davies, K.M.; Ma, C.; Weinberg, F.; Munshi, H.G.; Krett, N.L.; Rosen, S.T. Enzastaurin (LY317615), a protein kinase Cβ inhibitor, inhibits the AKT pathway and induces apoptosis in multiple myeloma cell lines. Mol. Cancer Ther. 2006, 5, 1783–1789. [Google Scholar] [CrossRef]
- Matthews, G.M.; de Matos Simoes, R.; Dhimolea, E.; Sheffer, M.; Gandolfi, S.; Dashevsky, O.; Sorrell, J.D.; Mitsiades, C.S. NF-κB dysregulation in multiple myeloma. Semin. Cancer Biol. 2016, 39, 68–76. [Google Scholar] [CrossRef]
- Moreaux, J.; Legouffe, E.; Jourdan, E.; Quittet, P.; Rème, T.; Lugagne, C.; Moine, P.; Rossi, J.-F.; Klein, B.; Tarte, K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004, 103, 3148–3157. [Google Scholar] [CrossRef]
- Novak, A.J.; Darce, J.R.; Arendt, B.K.; Harder, B.; Henderson, K.; Kindsvogel, W.; Gross, J.A.; Greipp, P.R.; Jelinek, D.F. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A mechanism for growth and survival. Blood 2004, 103, 689–694. [Google Scholar] [CrossRef]
- McMillin, D.W.; Delmore, J.; Weisberg, E.; Negri, J.M.; Geer, D.C.; Klippel, S.; Mitsiades, N.; Schlossman, R.L.; Munshi, N.C.; Kung, A.L.; et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat. Med. 2010, 16, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Boise, L.H.; Kaufman, J.L.; Bahlis, N.J.; Lonial, S.; Lee, K.P. The Tao of myeloma. Blood 2014, 124, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.H.-H.; Shin, E.M.; Tergaonkar, V.; Chng, W.-J. Targeting NF-κB Signaling for Multiple Myeloma. Cancers 2020, 12, 2203. [Google Scholar] [CrossRef]
- Amblard, M.; Fehrentz, J.-A.; Martinez, J.; Subra, G. Methods and Protocols of Modern Solid Phase Peptide Synthesis. MB 2006, 33, 239–254. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Zambrano, P.M.; Meyer-Herrera, J.E.; Ruiz-Aparicio, P.F.; Vernot, J.P. Simultaneously Targeting Two Coupled Signalling Molecules in the Mesenchymal Stem Cell Support Efficiently Sensitises the Multiple Myeloma Cell Line H929 to Bortezomib. Int. J. Mol. Sci. 2023, 24, 8157. https://doi.org/10.3390/ijms24098157
Rojas-Zambrano PM, Meyer-Herrera JE, Ruiz-Aparicio PF, Vernot JP. Simultaneously Targeting Two Coupled Signalling Molecules in the Mesenchymal Stem Cell Support Efficiently Sensitises the Multiple Myeloma Cell Line H929 to Bortezomib. International Journal of Molecular Sciences. 2023; 24(9):8157. https://doi.org/10.3390/ijms24098157
Chicago/Turabian StyleRojas-Zambrano, P. M., J. E. Meyer-Herrera, P. F. Ruiz-Aparicio, and J. P. Vernot. 2023. "Simultaneously Targeting Two Coupled Signalling Molecules in the Mesenchymal Stem Cell Support Efficiently Sensitises the Multiple Myeloma Cell Line H929 to Bortezomib" International Journal of Molecular Sciences 24, no. 9: 8157. https://doi.org/10.3390/ijms24098157
APA StyleRojas-Zambrano, P. M., Meyer-Herrera, J. E., Ruiz-Aparicio, P. F., & Vernot, J. P. (2023). Simultaneously Targeting Two Coupled Signalling Molecules in the Mesenchymal Stem Cell Support Efficiently Sensitises the Multiple Myeloma Cell Line H929 to Bortezomib. International Journal of Molecular Sciences, 24(9), 8157. https://doi.org/10.3390/ijms24098157




