The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia
Abstract
1. Introduction
2. Cardiovascular Disease, L-arginine, and NO
3. L-arginine in Therapeutic Applications
4. Hypoxic Conditions and L-arginine
5. Supplementation of L-arginine
6. L-arginine Paradox
7. L-arginine and Sport
8. Individual Physiological Reactivity and NO
9. Resistance to Hypoxia, Mitochondrial Energy Support, and L-arginine
10. L-arginine, Hypoxia, and Methodological Challenge
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef] [PubMed]
- Marchionni, S.; Sell, C.; Lorenzini, A. Development and Longevity: Cellular and Molecular Determinants—A Mini-Review. Gerontology 2020, 66, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Taormina, G.; Ferrante, F.; Vieni, S.; Grassi, N.; Russo, A.; Mirisola, M.G. Longevity: Lesson from Model Organisms. Genes 2019, 10, 518. [Google Scholar] [CrossRef]
- Farmaki, A.E.; Rayner, N.W.; Matchan, A.; Spiliopoulou, P.; Gilly, A.; Kariakli, V.; Kiagiadaki, C.; Tsafantakis, E.; Zeggini, E.; Dedoussis, G. The mountainous Cretan dietary patterns and their relationship with cardiovascular risk factors: The Hellenic Isolated Cohorts MANOLIS study. Public. Health Nutr. 2017, 20, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.C.; Dumitrescu, L.; Goodloe, R.; Brown-Gentry, K.; Boston, J.; McClellan, B., Jr.; Sutcliffe, C.; Wiseman, R.; Baker, P.; Pericak-Vance, M.A.; et al. Rare variant APOC3 R19X is associated with cardio-protective profiles in a diverse population-based survey as part of the Epidemiologic Architecture for Genes Linked to Environment Study. Circ. Cardiovasc. Genet. 2014, 7, 848–853. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Sztalryd, C.; Horenstein, R.B.; Holleran, S.; Matveyenko, A.; Thomas, T.; Nandakumar, R.; Ngai, C.; Karmally, W.; Ginsberg, H.N.; et al. Effects of APOC3 Heterozygous Deficiency on Plasma Lipid and Lipoprotein Metabolism. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 63–72. [Google Scholar] [CrossRef]
- Burtscher, J.; Millet, G.P.; Burtscher, M. Does living at moderate altitudes in Austria affect mortality rates of various causes? An ecological study. BMJ Open 2021, 11, e048520. [Google Scholar] [CrossRef]
- Burtscher, J.; Millet, G.P.; Renner-Sattler, K.; Klimont, J.; Hackl, M.; Burtscher, M. Moderate Altitude Residence Reduces Male Colorectal and Female Breast Cancer Mortality More Than Incidence: Therapeutic Implications? Cancers 2021, 13, 4420. [Google Scholar] [CrossRef]
- Hirschler, V. Cardiometabolic risk factors in native populations living at high altitudes. Int. J. Clin. Pract. 2016, 70, 113–118. [Google Scholar] [CrossRef]
- Burtscher, M.; Millet, G.P.; Klimont, J.; Burtscher, J. Differences in the prevalence of physical activity and cardiovascular risk factors between people living at low (<1001 m) compared to moderate (1001–2000 m) altitude. AIMS Public. Health 2021, 8, 624–635. [Google Scholar] [CrossRef]
- Moncada, S. Nitric oxide. J. Hypertens. Suppl. 1994, 12, S35-9. [Google Scholar]
- McNeal, C.J.; Meininger, C.J.; Reddy, D.; Wilborn, C.D.; Wu, G. Safety and Effectiveness of Arginine in Adults. J. Nutr. 2016, 146, 2587S–2593S. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hou, Y.; Hu, S.; Bazer, F.W.; Meininger, C.J.; McNeal, C.J.; Wu, G. Catabolism and safety of supplemental L-arginine in animals. Amino Acids 2016, 48, 1541–1552. [Google Scholar] [CrossRef]
- Zembowicz, A. Biological role of metabolic pathways from L-arginine to nitric oxide. Folia Med. Cracov. 1992, 33, 103–116. (In Polish) [Google Scholar] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Viribay, A.; Burgos, J.; Fernández-Landa, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Weckman, A.M.; McDonald, C.R.; Baxter, J.B.; Fawzi, W.W.; Conroy, A.L.; Kain, K.C. Perspective: L-arginine and L-citrulline Supplementation in Pregnancy: A Potential Strategy to Improve Birth Outcomes in Low-Resource Settings. Adv. Nutr. 2019, 10, 765–777. [Google Scholar] [CrossRef]
- Arikawe, A.P.; Udenze, I.C.; Olusanya, A.W.; Akinnibosun, O.A.; Dike, I.; Duru, B.N. L-arginine supplementation lowers blood pressure, protein excretion and plasma lipid profile in experimental salt-induced hypertension in pregnancy: Relevance to preeclampsia. Pathophysiology 2019, 26, 191–197. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Tkachenko, H.; Lukash, O. Photoperiod-induced alterations in biomarkers of oxidative stress and biochemical pathways in rats of different ages: Focus on individual physiological reactivity. Chronobiol. Int. 2021, 38, 1673–1691. [Google Scholar] [CrossRef]
- Friebe, A.; Sandner, P.; Schmidtko, A. cGMP: A unique 2nd messenger molecule—Recent developments in cGMP research and development. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 287–302. [Google Scholar] [CrossRef]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 2019, 88, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Dzhalilova, D.; Makarova, O. Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics. Biomedicines 2020, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Lukyanova, L.D.; Kirova, Y.I. Mitochondria-controlled signaling mechanisms of brain protection in hypoxia. Front. Neurosci. 2015, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Redaelli, S.; Magliocca, A.; Malhotra, R.; Ristagno, G.; Citerio, G.; Bellani, G.; Berra, L.; Rezoagli, E. Nitric oxide: Clinical applications in critically ill patients. Nitric Oxide 2022, 121, 20–33. [Google Scholar] [CrossRef]
- Rashid, J.; Kumar, S.S.; Job, K.M.; Liu, X.; Fike, C.D.; Sherwin, C.M.T. Therapeutic Potential of Citrulline as an Arginine Supplement: A Clinical Pharmacology Review. Paediatr. Drugs 2020, 22, 279–293. [Google Scholar] [CrossRef]
- Arishe, O.; McKenzie, J.; Priviero, F.; Ebeigbe, A.B.; Webb, R.C. L-arginase induces vascular dysfunction in old spontaneously hypertensive rats. J. Afr. Assoc. Physiol. Sci. 2019, 7, 119–127. [Google Scholar] [CrossRef]
- Lee, C.W.; Li, D.; Channon, K.M.; Paterson, D.J. L-arginine supplementation reduces cardiac noradrenergic neurotransmission in spontaneously hypertensive rats. J. Mol. Cell Cardiol. 2009, 47, 149–155. [Google Scholar] [CrossRef]
- Jurisic, A.; Jurisic, Z.; Lefkou, E.; Girardi, G. Pravastatin plus L-arginine prevents adverse pregnancy outcomes in women with uteroplacental vascular dysfunction. Vasc. Pharmacol. 2021, 137, 106824. [Google Scholar] [CrossRef]
- Sandqvist, A.; Schneede, J.; Kylhammar, D.; Henrohn, D.; Lundgren, J.; Hedeland, M.; Bondesson, U.; Rådegran, G.; Wikström, G. Plasma L-arginine levels distinguish pulmonary arterial hypertension from left ventricular systolic dysfunction. Heart Vessel. 2018, 33, 255–263. [Google Scholar] [CrossRef]
- Sztormowska-Achranowicz, K.; Jankowski, Z.; Kocić, I. Protective effect of nicotinamide and L-arginine against monocrotaline-induced pulmonary hypertension in rats: Gender dependence. Pharmacol. Rep. 2020, 72, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.S.; Lakin, R.O.; Campos, P.; Allemang, M.; Kim, A.; Sarac, T.P.; Hausladen, A.; Stamler, J.S. The LargPAD Trial: Phase IIA evaluation of l-arginine infusion in patients with peripheral arterial disease. J. Vasc. Surg. 2017, 66, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lakin, R.O.; Zhu, W.; Feiten, L.; Kashyap, V.S. Techniques to harvest diseased human peripheral arteries and measure endothelial function in an ex vivo model. J. Vasc. Surg. 2013, 58, 470–477. [Google Scholar] [CrossRef]
- Curtiss, P.; Schwager, Z.; Lo Sicco, K.; Franks, A.G., Jr. The clinical effects of l-arginine and asymmetric dimethylarginine: Implications for treatment in secondary Raynaud’s phenomenon. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 497–503. [Google Scholar] [CrossRef]
- Agostoni, A.; Marasini, B.; Biondi, M.L.; Bassani, C.; Cazzaniga, A.; Bottasso, B.; Cugno, M. L-arginine therapy in Raynaud’s phenomenon? Int. J. Clin. Lab. Res. 1991, 21, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Sudar-Milovanovic, E.; Obradovic, M.; Jovanovic, A.; Zaric, B.; Zafirovic, S.; Panic, A.; Radak, D.; Isenovic, E.R. Benefits of L-Arginine on Cardiovascular System. Mini Rev. Med. Chem. 2016, 16, 94–103. [Google Scholar] [CrossRef]
- Javrushyan, H.; Nadiryan, E.; Grigoryan, A.; Avtandilyan, N.; Maloyan, A. Antihyperglycemic activity of L-norvaline and L-arginine in high-fat diet and streptozotocin-treated male rats. Exp. Mol. Pathol. 2022, 126, 104763. [Google Scholar] [CrossRef]
- Shved, M.I.; Yastremska, I.O.; Martynyuk, L.P.; Yastremska, S.O.; Dobrianskyi, T.O. Management of central hemodynamic and endothelial function disturbances in patients with myocardial infarction combined with metabolic syndrome. Pol. Merkur. Lek. 2021, 49, 325–328. [Google Scholar]
- Tripolt, N.J.; Aberer, F.; Riedl, R.; Url, J.; Dimsity, G.; Meinitzer, A.; Stojakovic, T.; Aziz, F.; Hödl, R.; Brachtl, G.; et al. Effects of linagliptin on endothelial function and postprandial lipids in coronary artery disease patients with early diabetes: A randomized, placebo-controlled, double-blind trial. Cardiovasc. Diabetol. 2018, 17, 71. [Google Scholar] [CrossRef]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Salmani, M.; Alipoor, E.; Navid, H.; Farahbakhsh, P.; Yaseri, M.; Imani, H. Effect of l-arginine on cardiac reverse remodeling and quality of life in patients with heart failure. Clin. Nutr. 2021, 40, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Shushanof, M.; Bouskela, E.; Bottino, D. Oral L-Arginine (5 g/day) for 14 Days Improves Microcirculatory Function in Healthy Young Women and Healthy and Type 2 Diabetes Mellitus Elderly Women. J. Vasc. Res. 2022, 59, 24–33. [Google Scholar] [CrossRef]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Does l-citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Song, M.; Li, L.; Niu, L.; Chen, Y.; Han, B.; Sun, X.; Yang, Z.; Lei, Y.; Chen, X. Endogenous dual stimuli-activated NO generation in the conventional outflow pathway for precision glaucoma therapy. Biomaterials 2021, 277, 121074. [Google Scholar] [CrossRef]
- Barcelos, G.T.; Rossato, D.D.; Perini, J.L.; Pinheiro, L.P.; Carvalho, C.; Jaenisch, R.B.; Rhoden, C.R.; Lago, P.D.; Nunes, R.B. Effects of l-arginine supplementation associated with continuous or interval aerobic training on chronic heart failure rats. Metabolism 2017, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nesher, N.; Frolkis, I.; Schwartz, D.; Chernichovski, T.; Levi, S.; Pri-Paz, Y.; Chernin, G.; Shtabsky, A.; Ben-Gal, Y.; Paz, Y. L-arginine improves endothelial function, independently of arginine uptake, in aortas from chronic renal failure female rats. Am. J. Physiol. Renal Physiol. 2014, 306, F449–F456. [Google Scholar] [CrossRef]
- Saha, B.K.; Burns, S.L. The Story of Nitric Oxide, Sepsis and Methylene Blue: A Comprehensive Pathophysiologic Review. Am. J. Med. Sci. 2020, 360, 329–337. [Google Scholar] [CrossRef]
- Russell, J.A.; Rush, B.; Boyd, J. Pathophysiology of Septic Shock. Crit. Care Clin. 2018, 34, 43–61. [Google Scholar] [CrossRef]
- Infante, T.; Costa, D.; Napoli, C. Novel Insights Regarding Nitric Oxide and Cardiovascular Diseases. Angiology 2021, 72, 411–425. [Google Scholar] [CrossRef]
- Li, Y.; Yoon, B.; Dey, A.; Nguyen, V.Q.; Park, J.H. Recent progress in nitric oxide-generating nanomedicine for cancer therapy. J. Control. Release 2022, 352, 179–198. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Grabanska, K.; Musialik, K.; Cieslewicz, A.; Skoluda, A.; Jablecka, A. Effect of 3-month L-arginine supplementation on insulin resistance and tumor necrosis factor activity in patients with visceral obesity. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 816–823. [Google Scholar]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P.; Parveen, A.; Kim, S.Y. Nitric Oxide as a Target for Phytochemicals in Anti-Neuroinflammatory Prevention Therapy. Int. J. Mol. Sci. 2021, 22, 4771. [Google Scholar] [CrossRef]
- Leo, F.; Suvorava, T.; Heuser, S.K.; Li, J.; LoBue, A.; Barbarino, F.; Piragine, E.; Schneckmann, R.; Hutzler, B.; Good, M.E.; et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 2021, 144, 870–889. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.W.; Giam, B.; Kuruppu, S.; Head, G.A.; Kaye, D.M. Impaired l-arginine-nitric oxide pathway contributes to the pathogenesis of resistant hypertension. Clin. Sci. 2019, 133, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Povalko, N.; Inoue, E.; Nakamura, H.; Ishii, A.; Suzuki, Y.; Yoneda, M.; Kanda, F.; Kubota, M.; Okada, H.; et al. Therapeutic regimen of L-arginine for MELAS: 9-year, prospective, multicenter, clinical research. J. Neurol. 2018, 265, 2861–2874. [Google Scholar] [CrossRef] [PubMed]
- Fago, A.; Jensen, F.B. Hypoxia tolerance, nitric oxide, and nitrite: Lessons from extreme animals. Physiology 2015, 30, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Pérez-Padilla, J.R. Adaptation to Moderate Altitude Hypoxemia: The Example of the Valley of Mexico. Rev. Investig. Clin. 2022, 74, 4–15. [Google Scholar] [CrossRef]
- Friedman, S.M. Lifestyle (Medicine) and Healthy Aging. Clin. Geriatr. Med. 2020, 36, 645–653. [Google Scholar] [CrossRef]
- Tenopoulou, M.; Doulias, P.T. Endothelial nitric oxide synthase-derived nitric oxide in the regulation of metabolism. F1000Research 2020, 9, F1000 Faculty Rev-1190. [Google Scholar] [CrossRef]
- Soodaeva, S.; Klimanov, I.; Kubysheva, N.; Popova, N.; Batyrshin, I. The State of the Nitric Oxide Cycle in Respiratory Tract Diseases. Oxid. Med. Cell Longev. 2020, 2020, 4859260. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Wilkinson, M.L.; Gow, A.J. Nitric oxide regulation of cellular metabolism: Adaptive tuning of cellular energy. Nitric Oxide 2023, 131, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Porrini, C.; Ramarao, N.; Tran, S.L. Dr. NO and Mr. Toxic—The versatile role of nitric oxide. Biol. Chem. 2020, 401, 547–572. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey Man, H.S.; Tsui, A.K.; Marsden, P.A. Nitric oxide and hypoxia signaling. Vitam. Horm. 2014, 96, 161–192. [Google Scholar] [CrossRef] [PubMed]
- Wierońska, J.M.; Cieślik, P.; Kalinowski, L. Nitric Oxide-Dependent Pathways as Critical Factors in the Consequences and Recovery after Brain Ischemic Hypoxia. Biomolecules 2021, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef]
- d’Unienville, N.M.A.; Blake, H.T.; Coates, A.M.; Hill, A.M.; Nelson, M.J.; Buckley, J.D. Effect of food sources of nitrate, polyphenols, L-arginine and L-citrulline on endurance exercise performance: A systematic review and meta-analysis of randomised controlled trials. J. Int. Soc. Sports Nutr. 2021, 18, 76. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Jobgen, W.S.; Kim, S.W.; Lassala, A.; Li, P.; Matis, J.H.; Meininger, C.J.; Spencer, T.E. Pharmacokinetics and safety of arginine supplementation in animals. J. Nutr. 2007, 137 (Suppl. S2), 1673S–1680S. [Google Scholar] [CrossRef]
- Shayo, S.C.; Kawade, S.; Ogiso, K.; Yoshihiko, N. Strategies to ameliorate endothelial dysfunction associated with metabolic syndrome, where are we? Diabetes Metab. Syndr. 2019, 13, 2164–2169. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Z.; Li, W.; Fan, X.; Han, F.; Huang, Y.; Yu, Y.; Qian, L.; Xiong, Y. Arginase: Biological and Therapeutic Implications in Diabetes Mellitus and Its Complications. Oxid. Med. Cell Longev. 2022, 2022, 2419412. [Google Scholar] [CrossRef] [PubMed]
- Kuchanowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tables of Composition and Nutritional Value of Foods; PZWL Medical Publishing House: Warsaw, Poland, 2023. [Google Scholar]
- Oliveira, C.H.; Dias, K.M.M.; Bernardes, R.D.; Diana, T.F.; Rodrigueiro, R.J.B.; Calderano, A.A.; Albino, L.F.T. The effects of arginine supplementation through different ratios of arginine:lysine on performance, skin quality and creatine levels of broiler chickens fed diets reduced in protein content. Poult. Sci. 2022, 101, 102148. [Google Scholar] [CrossRef] [PubMed]
- Ognik, K.; Całyniuk, Z.; Mikulski, D.; Stępniowska, A.; Konieczka, P.; Jankowski, J. The effect of different dietary ratios of lysine, arginine and methionine on biochemical parameters and hormone secretion in turkeys. J. Anim. Physiol. Anim. Nutr. 2021, 105, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Ognik, K.; Konieczka, P.; Mikulski, D. Effects of different levels of arginine and methionine in a high-lysine diet on the immune status, performance, and carcass traits of turkeys. Poult. Sci. 2020, 99, 4730–4740. [Google Scholar] [CrossRef] [PubMed]
- Boguszewski, M.C.S.; Boguszewski, C.L.; Chemaitilly, W.; Cohen, L.E.; Gebauer, J.; Higham, C.; Hoffman, A.R.; Polak, M.; Yuen, K.C.J.; Alos, N.; et al. Safety of growth hormone replacement in survivors of cancer and intracranial and pituitary tumours: A consensus statement. Eur. J. Endocrinol. 2022, 186, P35–P52. [Google Scholar] [CrossRef]
- Kaczka, P.; Michalczyk, M.M.; Jastrząb, R.; Gawelczyk, M.; Kubicka, K. Mechanism of Action and the Effect of Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation on Different Types of Physical Performance—A Systematic Review. J. Hum. Kinet. 2019, 68, 211–222. [Google Scholar] [CrossRef]
- Goli, P.; Yazdi, M.; Heidari-Beni, M.; Kelishadi, R. Growth Hormone Response to L-Arginine Alone and Combined with Different Doses of Growth Hormone-Releasing Hormone: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 8739289. [Google Scholar] [CrossRef]
- Sugiharto; Merawati, D.; Pranoto, A.; Susanto, H. Physiological response of endurance exercise as a growth hormone mediator in adolescent women’s. J. Basic Clin. Physiol. Pharmacol. 2022, 34, 61–67. [Google Scholar] [CrossRef]
- Collier, S.R.; Casey, D.P.; Kanaley, J.A. Growth hormone responses to varying doses of oral arginine. Growth Horm. IGF Res. 2005, 15, 136–139. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Karimzadeh, I.; Sagheb, M.M.; Khalili, H. The Renal Safety of L-Carnitine, L-Arginine, and Glutamine in Athletes and Bodybuilders. J. Ren. Nutr. 2019, 29, 221–234. [Google Scholar] [CrossRef]
- Szefel, J.; Danielak, A.; Kruszewski, W.J. Metabolic pathways of L-arginine and therapeutic consequences in tumors. Adv. Med. Sci. 2019, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cziráki, A.; Lenkey, Z.; Sulyok, E.; Szokodi, I.; Koller, A. L-Arginine-Nitric Oxide-Asymmetric Dimethylarginine Pathway and the Coronary Circulation: Translation of Basic Science Results to Clinical Practice. Front. Pharmacol. 2020, 11, 569914. [Google Scholar] [CrossRef]
- Tsikas, D.; Bollenbach, A.; Hanff, E.; Kayacelebi, A.A. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): The ADMA, SDMA and hArg paradoxes. Cardiovasc. Diabetol. 2018, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020, 91, 1076–1084. [Google Scholar] [CrossRef]
- Koleva, D.I.; Orbetzova, M.M.; Nikolova, J.G.; Deneva, T.I. Pathophysiological Role of Adiponectin, Leptin and Asymmetric Dimethylarginine in the Process of Atherosclerosis. Folia Med. 2016, 58, 234–240. [Google Scholar] [CrossRef]
- Meirelles, C.M.; Matsuura, C. Acute supplementation of L-arginine affects neither strength performance nor nitric oxide production. J. Sports Med. Phys. Fit. 2018, 58, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, S.W.; Seo, J.; Jung, Y.P.; Kim, H.; Kim, A.J.; Kim, S.; Lim, K. Dietary Arginine and Citrulline Supplements for Cardiovascular Health and Athletic Performance: A Narrative Review. Nutrients 2023, 15, 1268. [Google Scholar] [CrossRef]
- Kina-Tanada, M.; Sakanashi, M.; Tanimoto, A.; Kaname, T.; Matsuzaki, T.; Noguchi, K.; Uchida, T.; Nakasone, J.; Kozuka, C.; Ishida, M.; et al. Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice. Diabetologia 2017, 60, 1138–1151. [Google Scholar] [CrossRef]
- Paul, S.; Pan, S.; Mukherjee, A.; De, P. Nitric Oxide Releasing Delivery Platforms: Design, Detection, Biomedical Applications, and Future Possibilities. Mol. Pharm. 2021, 18, 3181–3205. [Google Scholar] [CrossRef]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Gemelli against COVID-19 Post-Acute Care Team. Effects of l-Arginine Plus Vitamin C Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients 2022, 14, 4984. [Google Scholar] [CrossRef]
- Huerta Ojeda, Á.; Domínguez de Hanna, A.; Barahona-Fuentes, G. The effect of supplementation with L-arginine and L-citrulline on physical performance: A systematic review. Nutr. Hosp. 2019, 36, 1389–1402. (In Spanish) [Google Scholar] [CrossRef]
- Mone, P.; Izzo, R.; Marazzi, G.; Manzi, M.V.; Gallo, P.; Campolongo, G.; Cacciotti, L.; Tartaglia, D.; Caminiti, G.; Varzideh, F.; et al. L-Arginine Enhances the Effects of Cardiac Rehabilitation on Physical Performance: New Insights for Managing Cardiovascular Patients During the COVID-19 Pandemic. J. Pharmacol. Exp. Ther. 2022, 381, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ruano, J.; Teixeira, V.H. Prevalence of dietary supplement use by gym members in Portugal and associated factors. J. Int. Soc. Sports Nutr. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Snow, R.J.; Gatta, P.A.D.; Bellofiore, N.; Ellery, S.J. Creatine metabolism in the uterus: Potential implications for reproductive biology. Amino Acids 2020, 52, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Moreno, Y.; Rawson, E.S.; Forero, D.A.; Stout, J.R.; Kerksick, C.M.; Roberts, M.D.; Kreider, R.B. A Convergent Functional Genomics Analysis to Identify Biological Regulators Mediating Effects of Creatine Supplementation. Nutrients 2021, 13, 2521. [Google Scholar] [CrossRef]
- Witham, M.D.; Clarke, C.L.; Hutcheon, A.; Gingles, C.; Gandy, S.; Priba, L.; Nicholas, R.S.; Cavin, I.; Sumukadas, D.; Struthers, A.D.; et al. Effect of allopurinol on phosphocreatine recovery and muscle function in older people with impaired physical function: A randomised controlled trial. Age Ageing 2020, 49, 1003–1010. [Google Scholar] [CrossRef]
- Evans, R.W.; Fernstrom, J.D.; Thompson, J.; Morris, S.M., Jr.; Kuller, L.H. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J. Nutr. Biochem. 2004, 15, 534–539. [Google Scholar] [CrossRef]
- Yousefi Rad, E.; Nazarian, B.; Saboori, S.; Falahi, E.; Hekmatdoost, A. Effects of l-arginine supplementation on glycemic profile: Evidence from a systematic review and meta-analysis of clinical trials. J. Integr. Med. 2020, 18, 284–291. [Google Scholar] [CrossRef]
- Campbell, B.; Roberts, M.; Kerksick, C.; Wilborn, C.; Marcello, B.; Taylor, L.; Nassar, E.; Leutholtz, B.; Bowden, R.; Rasmussen, C.; et al. Pharmacokinetics, safety, and effects on exercise performance of L-arginine alpha-ketoglutarate in trained adult men. Nutrition 2006, 22, 872–881. [Google Scholar] [CrossRef]
- Abel, T.; Knechtle, B.; Perret, C.; Eser, P.; von Arx, P.; Knecht, H. Influence of chronic supplementation of arginine aspartate in endurance athletes on performance and substrate metabolism—A randomized, double-blind, placebo-controlled study. Int. J. Sports Med. 2005, 26, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Gholamalizadeh, M.; Tabrizi, R.; Nowrouzi-Sohrabi, P.; Rastgoo, S.; Doaei, S. The effect of L-arginine supplementation on maximal oxygen uptake: A systematic review and meta-analysis. Physiol. Rep. 2021, 9, e14739. [Google Scholar] [CrossRef] [PubMed]
- Esen, O.; Eser, M.C.; Abdioglu, M.; Benesova, D.; Gabrys, T.; Karayigit, R. Eight Days of L-Citrulline or L-Arginine Supplementation Did Not Improve 200-m and 100-m Swimming Time Trials. Int. J. Environ. Res. Public. Health 2022, 19, 4462. [Google Scholar] [CrossRef]
- Esen, O.; Karayigit, R. One-Week L-Arginine Supplementation Had No Effect on 200m Freestyle Swimming Time Trial in Moderately-Trained Male Swimmers. J. Diet. Suppl. 2022, 1–11. [Google Scholar] [CrossRef]
- Esen, O.; Nicholas, C.; Morris, M.; Bailey, S.J. No Effect of Beetroot Juice Supplementation on 100-m and 200-m Swimming Performance in Moderately Trained Swimmers. Int. J. Sports Physiol. Perform. 2019, 14, 706–710. [Google Scholar] [CrossRef]
- Julian, C.G.; Moore, L.G. Human Genetic Adaptation to High Altitude: Evidence from the Andes. Genes 2019, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Du, Q.; Wang, L.; Chen, B. Impacts of oxygen deficiency on embryo life-history traits of migratory locust Locusta migratoria from low and high altitudes. Insect Sci. 2022, 13129. [Google Scholar] [CrossRef]
- Kronsbein, H.; Gerlach, D.A.; Heusser, K.; Hoff, A.; Hoffmann, F.; Diedrich, A.; Ehmke, H.; Jordan, J.; Tank, J. Testing individual baroreflex responses to hypoxia-induced peripheral chemoreflex stimulation. Clin. Auton. Res. 2020, 30, 531–540. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Lukash, O.; Nosar, V.; Portnychenko, A.; Portnichenko, V.; Wszedybyl-Winklewska, M.; Winklewski, P.J. Liver mitochondrial respiratory plasticity and oxygen uptake evoked by cobalt chloride in rats with low and high resistance to extreme hypobaric hypoxia. Can. J. Physiol. Pharmacol. 2019, 97, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Germanova, E.; Khmil, N.; Pavlik, L.; Mikheeva, I.; Mironova, G.; Lukyanova, L. The Role of Mitochondrial Enzymes, Succinate-Coupled Signaling Pathways and Mitochondrial Ultrastructure in the Formation of Urgent Adaptation to Acute Hypoxia in the Myocardium. Int. J. Mol. Sci. 2022, 23, 14248. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N.; Tkachenko, H.; Nosar, V. The effects of intermittent hypoxia training on mitochondrial oxygen consumption in rats exposed to skeletal unloading. Ann. Clin. Lab. Sci. 2013, 43, 54–63. [Google Scholar] [PubMed]
- Huang, Y.J.; Yuan, Y.J.; Liu, Y.X.; Zhang, M.Y.; Zhang, J.G.; Wang, T.C.; Zhang, L.N.; Hu, Y.Y.; Li, L.; Xian, X.H.; et al. Nitric Oxide Participates in the Brain Ischemic Tolerance Induced by Intermittent Hypobaric Hypoxia in the Hippocampal CA1 Subfield in Rats. Neurochem. Res. 2018, 43, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2021, 19, 114–126. [Google Scholar] [CrossRef]
- Lukyanova, L.D.; Kirova, Y.I.; Germanova, E.L. The Role of Succinate in Regulation of Immediate HIF-1α Expression in Hypoxia. Bull. Exp. Biol. Med. 2018, 164, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Knoepp, F.; Wahl, J.; Andersson, A.; Kraut, S.; Sommer, N.; Weissmann, N.; Ramser, K. A Microfluidic System for Simultaneous Raman Spectroscopy, Patch-Clamp Electrophysiology, and Live-Cell Imaging to Study Key Cellular Events of Single Living Cells in Response to Acute Hypoxia. Small Methods 2021, 5, e2100470. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Kim, S.J. Oxygen-dependent regulation of ion channels: Acute responses, post-translational modification, and response to chronic hypoxia. Pflugers Arch. 2021, 473, 1589–1602. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; Del Real, Á.; Sainz-Aja, J.A.; Prieto-Lloret, J.; Olea, E.; Rocher, A.; Rigual, R.J.; Riancho, J.A.; Pérez-Castrillón, J.L. Analysis of Bone Histomorphometry in Rat and Guinea Pig Animal Models Subject to Hypoxia. Int. J. Mol. Sci. 2022, 23, 12742. [Google Scholar] [CrossRef]
- Donald, J.A.; Forgan, L.G.; Cameron, M.S. The evolution of nitric oxide signalling in vertebrate blood vessels. J. Comp. Physiol. B. 2015, 185, 153–171. [Google Scholar] [CrossRef]
- Shepherd, M.; Giordano, D.; Verde, C.; Poole, R.K. The Evolution of Nitric Oxide Function: From Reactivity in the Prebiotic Earth to Examples of Biological Roles and Therapeutic Applications. Antioxidants 2022, 11, 1222. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Joshi, J.C.; Ray, A. Recent advances in stress research: Focus on nitric oxide. Eur. J. Pharmacol. 2015, 765, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, C.C.; Alejandra, T.G.; Guadalupe, D.K.J.; Herminia, V.G.; Lenin, P.; Enrique, B.V.; Evandro, B.M.; Oscar, B.; Iván, G.M. Modulation of the extraneuronal cholinergic system on main innate response leukocytes. J. Neuroimmunol. 2019, 327, 22–35. [Google Scholar] [CrossRef]
- Weigert, A.; von Knethen, A.; Fuhrmann, D.; Dehne, N.; Brüne, B. Redox-signals and macrophage biology. Mol. Asp. Med. 2018, 63, 70–87. [Google Scholar] [CrossRef]
- Xu, L.; Tan, X.; Bai, S.; Wu, H.; Luo, H.; Ye, Y.; Fang, L.; Dai, H.; Huang, L. L-arginine protects cementoblasts against hypoxia-induced apoptosis through Sirt1-enhanced autophagy. J. Periodontol. 2022, 93, 1961–1973. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Tong, L.; Zhang, G.M.; Zhao, X.H.; Sa, Y.P.; Liu, Y.; Lu, D.X.; Ga, Q.; Wu, P. L-Arginine Supplementation Improves Vascular Endothelial Dysfunction Induced by High-Fat Diet in Rats Exposed to Hypoxia. Wilderness Environ. Med. 2020, 31, 400–406. [Google Scholar] [CrossRef]
- Bogdański, P.; Suliburska, J.; Szulińska, M.; Sikora, M.; Walkowiak, J.; Jakubowski, H. L-Arginine and vitamin C attenuate pro-atherogenic effects of high-fat diet on biomarkers of endothelial dysfunction in rats. Biomed. Pharmacother. 2015, 76, 100–106. [Google Scholar] [CrossRef]
- Kurhaliuk, N.M.; Ikkert, O.V.; Vovkanych, L.S.; Horyn, O.V.; Hal’kiv, M.O.; Hordiĭ, S.K. Effect of L-arginine and the nitric oxide synthase blocker L-NNA on calcium capacity in rat liver mitochondria with differing resistance to hypoxia. Ukrains’kyi Biokhimichnyi Zhurnal 2001, 73, 85–89. (In Ukrainian) [Google Scholar]
- Kurhaliuk, N.M.; Serebrovs’ka, T.V.; Nosar, V.I.; Kolesnikova, E.E.; Moĭbenko, O.O. Intermittent hypoxic training and L-arginine as corrective agents for myocardial energy supply under acute hypoxia. Ukrains’kyi Biokhimichnyi Zhurnal 2002, 74, 82–87. (In Ukrainian) [Google Scholar]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; Batiha, G.E. COVID-19 and L-arginine Supplementations: Yet to Find the Missed Key. Curr. Protein Pept. Sci. 2022, 23, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Hirfanoglu, I.; Turkyilmaz, C.; Turkyilmaz, Z.; Onal, E.; Soylemezoglu, F.; Karabulut, R.; Atalay, Y. Neuroprotective effect of L-arginine in a neonatal rat model of hypoxic-ischemia. Int. J. Neurosci. 2019, 129, 1139–1144. [Google Scholar] [CrossRef]
- Atteia, H.H.; Alamri, E.S.; Sirag, N.; Zidan, N.S.; Aljohani, R.H.; Alzahrani, S.; Arafa, M.H.; Mohammad, N.S.; Asker, M.E.; Zaitone, S.A.; et al. Soluble guanylate cyclase agonist, isoliquiritigenin attenuates renal damage and aortic calcification in a rat model of chronic kidney failure. Life Sci. 2023, 317, 121460. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Dasgupta, S.; Anand, G.; Rejish Kumar, V.J.; Sahu, N.P.; Pal, A.K.; Puthiyottil, M. Dietary arginine attenuates hypoxia- induced HIF expression, metabolic responses and oxidative stress in Indian Major Carp, Cirrhinus mrigala. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 259, 110714. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, K. Improved Cardiac Function Following Ischemia Reperfusion Injury Using Exercise Preconditioning and L-Arginine Supplementation via Oxidative Stress Mitigation and Angiogenesis Amelioration. Cardiovasc. Toxicol. 2022, 22, 736–745. [Google Scholar] [CrossRef]
- Zuo, S.; Zhang, Y.; Wang, Z.; Wang, J. Mitochondria-Targeted Mesoporous Titanium Dioxide Nanoplatform for Synergistic Nitric Oxide Gas-Sonodynamic Therapy of Breast Cancer. Int. J. Nanomed. 2022, 17, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Reinero, M.; Beghetti, M.; Tozzi, P.; Segesser, L.K.V.; Samaja, M.; Milano, G. Nitric Oxide-cGMP Pathway Modulation in an Experimental Model of Hypoxic Pulmonary Hypertension. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Potenza, D.M.; Brenna, A.; Ma, Y.; Ren, Z.; Cheng, X.; Ming, X.F.; Yang, Z. Hypoxia Induces Renal Epithelial Injury and Activates Fibrotic Signaling Through Up-Regulation of Arginase-II. Front. Physiol. 2021, 12, 773719. [Google Scholar] [CrossRef]
- López, V.; Uribe, E.; Moraga, F.A. Activation of arginase II by asymmetric dimethylarginine and homocysteine in hypertensive rats induced by hypoxia: A new model of nitric oxide synthesis regulation in hypertensive processes? Hypertens. Res. 2021, 44, 263–275. [Google Scholar] [CrossRef]
- Lüneburg, N.; Siques, P.; Brito, J.; Arriaza, K.; Pena, E.; Klose, H.; Leon-Velarde, F.; Böger, R.H. Long-Term Chronic Intermittent Hypobaric Hypoxia in Rats Causes an Imbalance in the Asymmetric Dimethylarginine/Nitric Oxide Pathway and ROS Activity: A Possible Synergistic Mechanism for Altitude Pulmonary Hypertension? Pulm. Med. 2016, 2016, 6578578. [Google Scholar] [CrossRef]
- La Padula, P.; Costa, L.E.; Karadayian, A.; Lores-Arnaiz, S.; Czerniczyniec, A. Differences in mitochondrial function between brain and heart of senile rats exposed to acute hypobaric hypoxia. Role of nitric oxide. Exp. Gerontol. 2023, 173, 112100. [Google Scholar] [CrossRef] [PubMed]
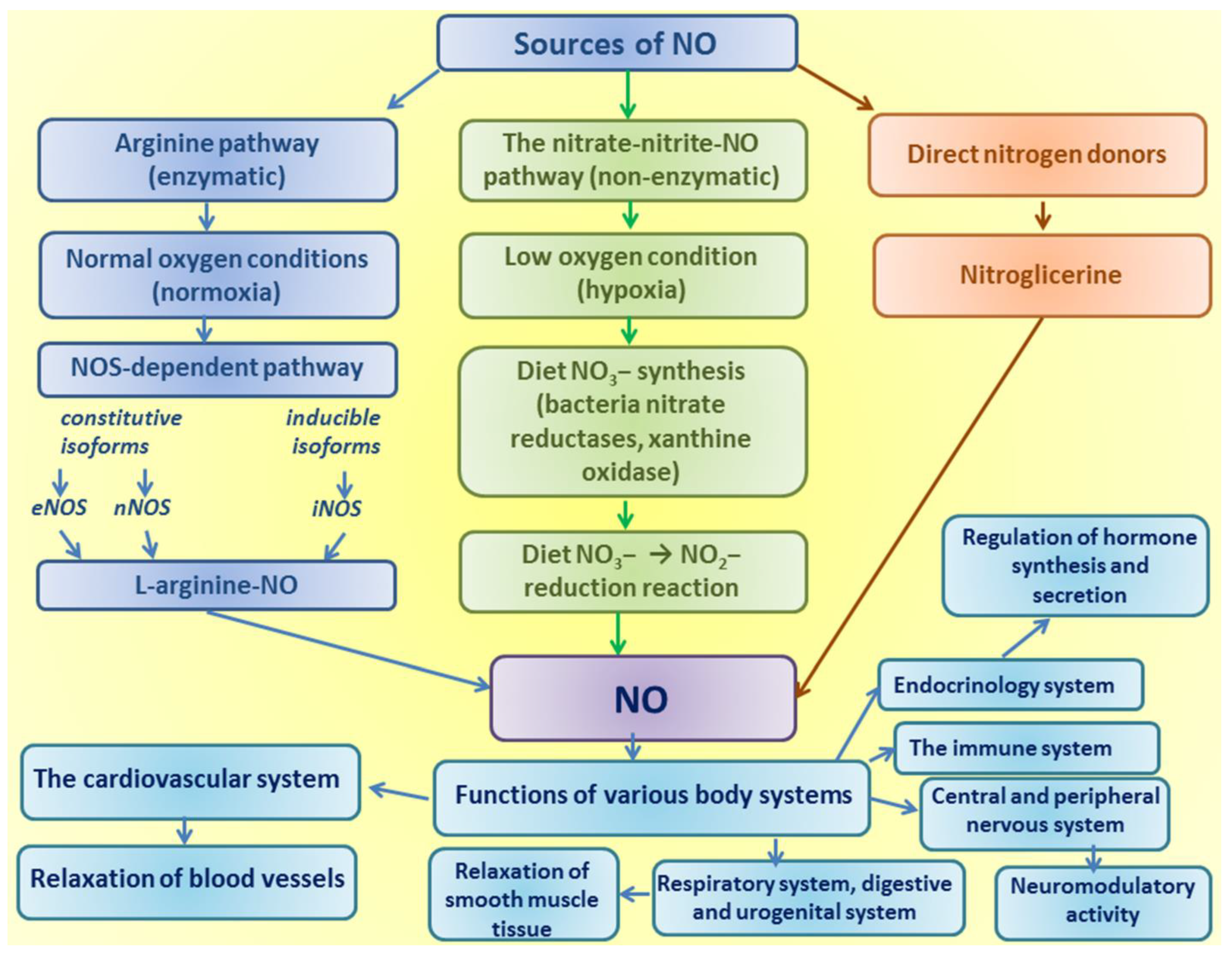

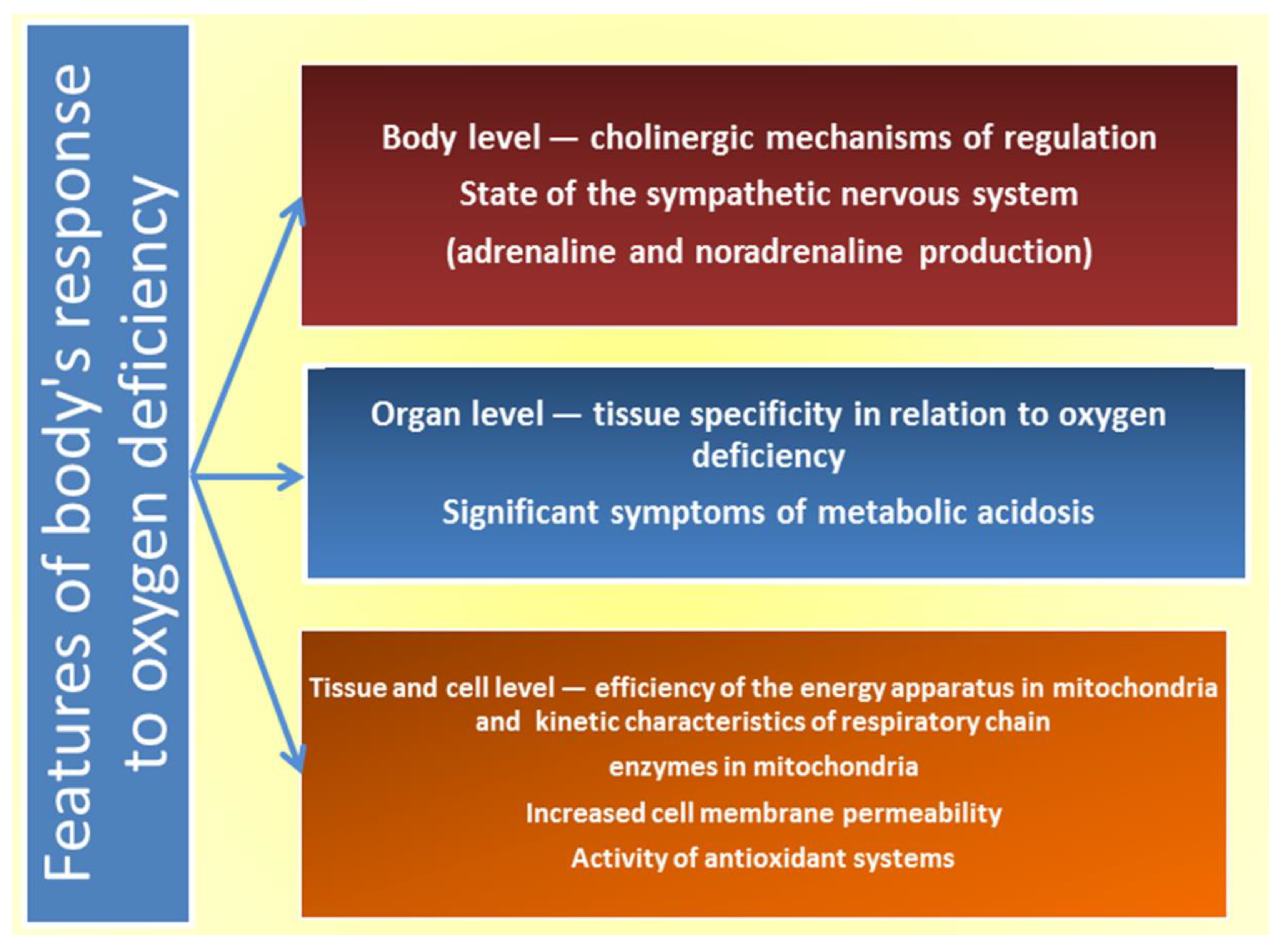
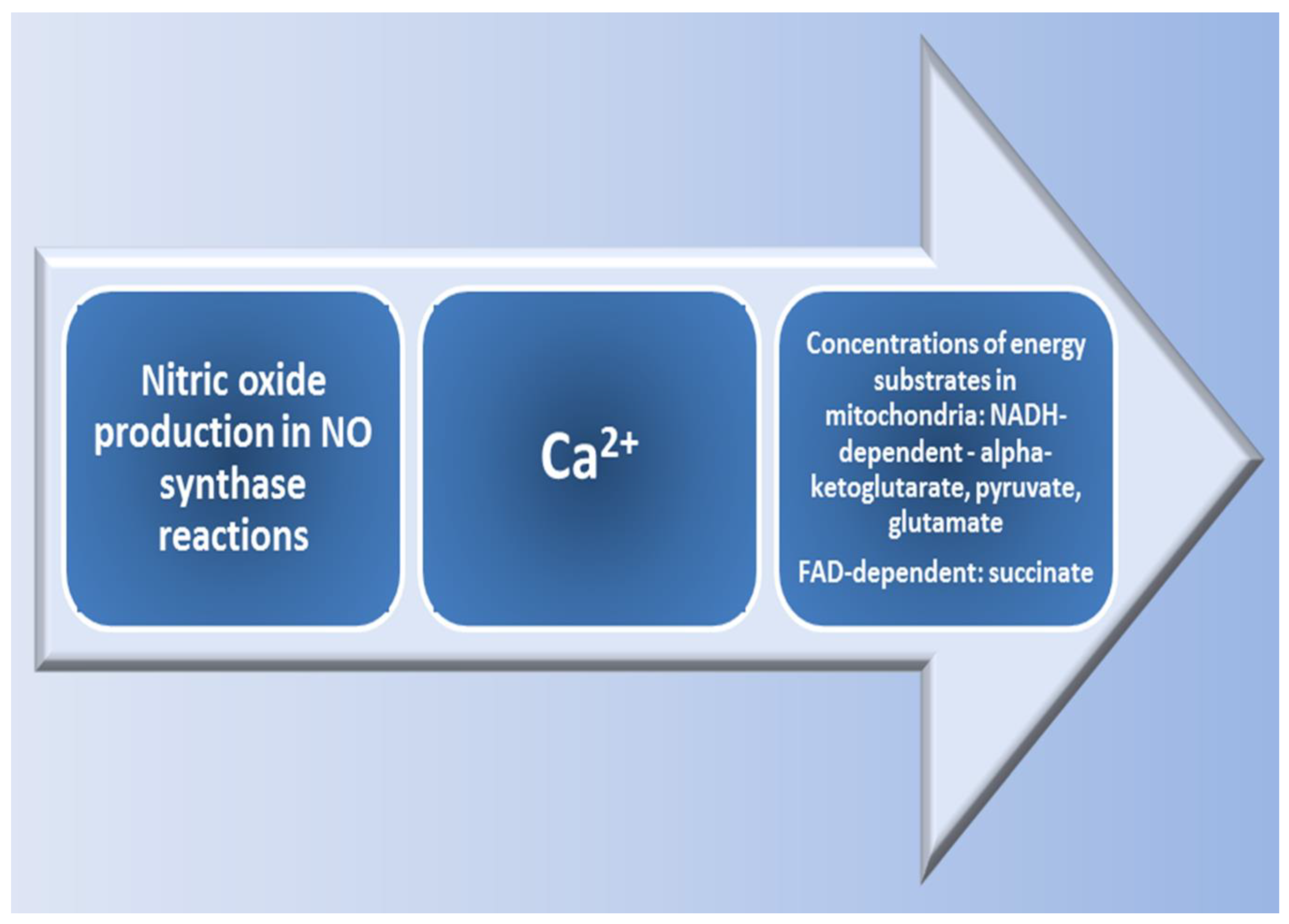


| N | Model | Effects of L-arginine | Mechanisms of L-arginine Action | References |
|---|---|---|---|---|
| 1 | Spontaneously hypertensive male rats SHR, Wistar Kyoto rats, L-arginine (10 g/L in drinking water), 1 week | Via local cardiac noradrenergic hyperactivity | Increased pre-synaptic substrate availability of the NOS-sGC-cGMP pathway reduced tyrosine hydroxylase levels | [28] |
| 2 | Spontaneously hypertensive young (12–14 weeks) and old (11–12 months) male Wistar rats | Aging effects similar to those seen in hypertension; age-dependent vascular dysfunction in SHRs is mediated by arginase | L-arginase reduces L-arginine availability for the formation of nitric oxide | [27] |
| 3 | Depletion of endogenous L-arginine due to maternal malaria infections by L-arginine or L-citrulline supplementation | Strategies for arginine supplementation in pregnancy | Implementation in resource-constrained settings, enhanced placental vascular development, and improved birth outcomes | [17] |
| 4 | Pregnant Female Sprague-Dawley rats, oral L-arginine supplementation from the 16th to 20th week with salt diets | Ameliorated deleterious effects in salt-induced hypertensive pregnant rats | NO vasodilatory effect | [18] |
| 5 | Pregnant patients with uteroplacental vascular dysfunction, high risk of adverse maternal and foetal outcomes | Pravastatin + L-arginine | Improved uteroplacental haemodynamics, increased foetal growth, and prevented early onset preeclampsia | [29] |
| 6 | Summarised data of preclinical and clinical studies of arginine/citrulline supplementation in adults and children | Improved endogenous NO regulation in cardiovascular diseases via endothelial function correction | Synthesis of NO from arginine/citrulline | [26] |
| 7 | Patients with pulmonary arterial hypertension | L-arginine and the L-arginine/ADMA ratio were lower in pulmonary arterial hypertension patients | L-arginine provided useful information in differentiating patients with pulmonary arterial hypertension diseases | [30] |
| 8 | Male and female rats with pulmonary arterial hypertension (monocrotaline sc, 60/kg b.b.) during nicotinamide (500 mg/kg, 7th day) and L-arginine supplementation (2.5% solution, drinking water, 7th day) | Protective effects on myocardial function and prevention of histopathological changes in pulmonary arteries | Clinical efficacy of supplementation via prevention of pulmonary arterial hypertension in a gender-dependent way | [31] |
| 9 | Patients with peripheral arterial disease and catheter-directed L-arginine delivery | Maximal effects for limb volumetric flow at 100 mg L-arginine supplementation | Correction of endothelial function in patients with peripheral arterial disease | [32] |
| 10 | Human lower extremity anterior tibial artery segments, histology and immunohistochemistry methods, amputation specimens in an ex vivo model | Improved endothelial dysfunction by L-arginine supplementation | Increase in the local levels of nitric oxide in humans and responsiveness to L-arginine as a nitric oxide precursor | [33] |
| 11 | Patients with fibrosis and severe secondary Raynaud’s phenomenon phenotypes at L-arginine-based therapies 1–2 g/day–10 g/day | NO metabolism in fibrosis and severe secondary Raynaud’s phenomenon phenotypes | eNOS, iNOS | [34] |
| 12 | Patients with primary or secondary Raynaud’s phenomenon at L-arginine-based therapy | Endothelial-derived mediators | Increased nitric oxide synthesis | [35] |
| 13 | Review of data from animal and clinical studies | Analysis of the states of hypertension, diabetes, hypercholesterolaemia, and vascular inflammation | L-arginine improved treatment of cardiovascular disorders via the NO pathway | [36] |
| 14 | Rats with high-fat diet and streptozotocin-induced hyperglycaemia, arginase inhibition (via L-norvaline) and supplementation (via L-arginine) | L-arginine acted as a potent antihyperglycaemic agent | Inhibition of arginase provided an antihyperglycaemic effect, NO protected against oxidative stress and hypercholesterolaemia | [37] |
| 15 | Patients with acute coronary syndrome, myocardial infarction and metabolic syndrome, L-arginine (4.2 g)/L-carnitine (2.0 g) in infusions for 28 days | Gradual recovery of myocardial contractility, reduction in diastolic dysfunction | Improved myocardial infarction protocol treatment in therapy of cardiovascular diseases | [38] |
| 16 | Subjects with early diabetes forms; arginine, ornithine, and citrulline level | Dipeptidylpeptidase-4 inhibitor linagliptin effects in subjects with coronary artery disease | No significant improvements in the arginine bioavailability ratios | [39] |
| 17 | Review of clinical and preclinical data in hypertension, ischaemic heart diseases, aging, peripheral artery disease, and diabetes mellitus; L-arginine supplementation | Nitric oxide synthase and arginase action, which are fundamental for the generation of NO | Supplementation of L-arginine prevented the evolution of hypertension and atherosclerosis | [40] |
| 18 | Patients with ischaemic heart failure, 3 g/d L-arginine supplementation, 10 weeks, cardiac reverse remodelling | Cardiac reverse remodelling after L-arginine supplementation | Improvement of cardiac recovery and function and quality of life in patients with ischaemic heart failure | [41] |
| 19 | Patients with type 2 diabetes mellitus different ages, oral supplementation with L-arginine 5 g/day for 14 days | No changes in glycaemia and lipidogram levels, decreased systolic, diastolic, and mean arterial pressure in elderly women, improved vasoreactivity | L-arginine as a precursor of NO synthesis improved endothelial-dependent vasodilatation and vascular/microvascular health in elderly women with or without type 2 diabetes mellitus | [42] |
| 20 | Older adult patients, short-term supplementation with l-citrulline (6 g day−1 for 14 days) | Modest improvement of muscle blood flow during submaximal exercise in older men | Possibility of L-citrulline to increase the L-arginine level | [43] |
| 21 | Glaucoma mouse models, precision glaucoma therapy, hydrophilic L-arginine | Intraocular pressure reduction | NO decreased high intraocular pressure | [44] |
| 22 | Wistar rats with myocardial infarction, L-arginine supplementation (1 g/kg, oral 7×/week), chronic heart failure, aerobic interval training | Interval training associated with L-arginine supplementation improved the hemodynamic parameters, reduction in pulmonary congestion | NO pathway improved the inflammatory profile and antioxidant status during training. | [45] |
| 23 | Wistar rats, chronic renal failure, L-arginine-NO system | Endothelial cell dysfunction at defective nitric oxide generation in chronic renal failure | Profound beneficial effects in chronic renal failure | [46] |
| N | Model | Effects of L-arginine | Mechanisms of L-arginine Action | References |
|---|---|---|---|---|
| 1 | Murine cementoblast apoptosis and root resorption model, hypoxia-induced apoptosis, L-arginine application | Reduced cementoblast apoptosis and root resorption in hypoxia | Via improved Sirt1 activator resveratrol, activated autophagy in the root resorption model | [127] |
| 2 | Male Sprague-Dawley rats exposed to hypoxia, and hypoxia (altitude of 5000 m) plus a high-fat diet model, L-arginine supplementation for 1 week | Increased plasma nitrates and nitrites, endothelial nitric oxide synthase mRNA | Prevention of aortic ultrastructural changes via aortic endothelium effects and endothelium-dependent vasodilator response | [128] |
| 3 | Female and male Wistar rats high-fat diet model, L-arginine supplementation in a dose of 20 g/kg diet, vitamin C supplementation | Increased total antioxidant status, decreased insulin resistance, lowered LDL, reduced level of protein carbonyls | Via homocysteine levels, tumour necrosis factor alpha (TNF-α), oxidative stress biomarkers | [129] |
| 4 | Wistar rats with different resistance to hypoxia model, L-arginine single injection (600 mg/kg), blocker of nitric oxide synthase L-NNA (35 mg/kg) in single injection, liver tissue | ADP-dependent processes of oxidative phosphorylation with the use of different substrates of mitochondrial oxidation processes, calcium mitochondrial capacity, different mechanisms of the L-arginine impact depending on the individual resistance to hypoxia | Via activation of aminotransferase mechanism, ATP-dependent processes of oxidative phosphorylation, increases in mitochondrial calcium capacity in low resistant rats | [130] |
| 5 | Wistar rats intermittent hypoxic training model (11% O2, 15-min sessions with 15 min rest intervals, 5 times daily), acute hypoxia model (inhalation of 7% O2, 30 min), myocardium mitochondria, L-arginine impact, NO blocker, L-NNA impact | ADP-dependent processes of oxidative phosphorylation with the use of different substrates of mitochondrial oxidation processes, increase in the tolerance to episodes of acute hypoxia | Stimulation of oxidative phosphorylation with primary activation of NAD-dependent mitochondrial pathway, a marked increase in the ADP/O ratio | [131] |
| 6 | Coronavirus disease (COVID-19) epidemiological situation, L-arginine supplementation, stress conditions, pulmonary diseases | May attenuate SARS-CoV-2 infection | Via restoration of NO by L-arginine antiviral and immunomodulatory effects, reduction of binding of SARS-CoV-2 to angiotensin-converting enzyme 2, inhibition of transmembrane protease serine-type 2, inhibition of proliferation and replication of SARS-CoV-2 | [132] |
| 7 | Seven-day-old rat hypoxia-ischemia model, L-arginine impact before hypoxia-ischemia, analysis of neuronal apoptosis biomarkers with the dUDP-biotin nick end-labelling (TUNEL) method, apoptosis indexes of the hippocampus and striatum | No significant difference in the right apoptosis indexes of the cortex | Via NO production induced by L-arginine post-treatment | [133] |
| 8 | Hypoxia induced by sodium nitrite (75 mg/kg s.c) neurotoxicity rat model, L-arginine, and carnosine impact | Combination of L-arginine and carnosine protects against hypoxia-induced neurotoxicity | Via inflammatory mediators, including nuclear factor kappa B, angiogenic, anti-inflammatory, and anti-apoptotic properties via tumour necrosis factor-alpha, caspase-3, GABA, noradrenaline, serotonin | [134] |
| 9 | Fish aquaculture system model, fish Cirrhinus mrigala exposed to hypoxia animal model, dietary arginine effects (0.7 and 1.4%) during 60 days | Effective supplement for aquaculture to reduce metabolic changes caused by hypoxia through increased antioxidant protection | Via hypoxia inducible factor (HIF)-1α mRNA | [135] |
| 10 | Myocardial infarction-induced damage rat model, L-arginine treatment in drinking water (4 g/L), high-intensity interval training alone and synergistically with L-arginine effects model, ischemia-reperfusion injury model | Synergic effects in improved left ventricular function at ischemia-reperfusion, oxidative stress mitigation, angiogenesis amelioration | Via cardiac function, angiogenesis, oxidative stress, and infarction size effects | [136] |
| 11 | T-mTNPs@L-Arg multifunctional nanoplatform as mitochondrial targeting nitric oxide model, long-term hypoxia breast cancer model, NO gas therapy model | Synergistic strategy in breast cancer | Effective proposal for nitric oxide gas mitochondrial targeting therapy in cancer | [137] |
| 12 | In vivo rat models at 14-day analysis of progression and treatment of pulmonary hypertension, hypoxic chamber (10% O2) method, different NO donor methods, l-arginine (20 mg/mL), molsidomine (15 mg/kg in drinking water) | Similar effects of different NO donors in enhancing the NO-cGMP pathway in pulmonary hypertension processes | Via modulation of the NO-cGMP pathway in vivo | [138] |
| 13 | Mouse renal epithelial cells in hypoxic conditions model, Arg-II−/− mice model; cultured human renal epithelial cell line HK2 model | Effects caused by hypoxia-mediated metabolism of L-arginine into urea and L-ornithine pathway | HIFs-Arg-II-mtROS-TGFβ1-cascade, type-II arginase, hypoxia-inducible factors HIF1α and HIF2α | [139] |
| 14 | Hypoxia-induced hypertensive rat model | Inhibitory effect on eNOS and activating effect on arginase II and nitric oxide bioavailability | Via nitric oxide concentrations, dimethylarginine dimethylaminohydrolase-2, cystathionine β-synthase, homocysteine | [140] |
| 15 | Adult Wistar rats at chronic intermittent hypoxia (2 days of hypoxia/2 days of normoxia) and chronic hypoxia in whole lung tissue models | Decreased L-arginine/ADMA ratio | Via NO bioavailability changes, asymmetric dimethylarginine increase, oxidative stress biomarker malondialdehyde rise | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurhaluk, N. The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia. Int. J. Mol. Sci. 2023, 24, 8205. https://doi.org/10.3390/ijms24098205
Kurhaluk N. The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia. International Journal of Molecular Sciences. 2023; 24(9):8205. https://doi.org/10.3390/ijms24098205
Chicago/Turabian StyleKurhaluk, Natalia. 2023. "The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia" International Journal of Molecular Sciences 24, no. 9: 8205. https://doi.org/10.3390/ijms24098205
APA StyleKurhaluk, N. (2023). The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia. International Journal of Molecular Sciences, 24(9), 8205. https://doi.org/10.3390/ijms24098205






