Arachidonic Acid Pathways and Male Fertility: A Systematic Review
Abstract
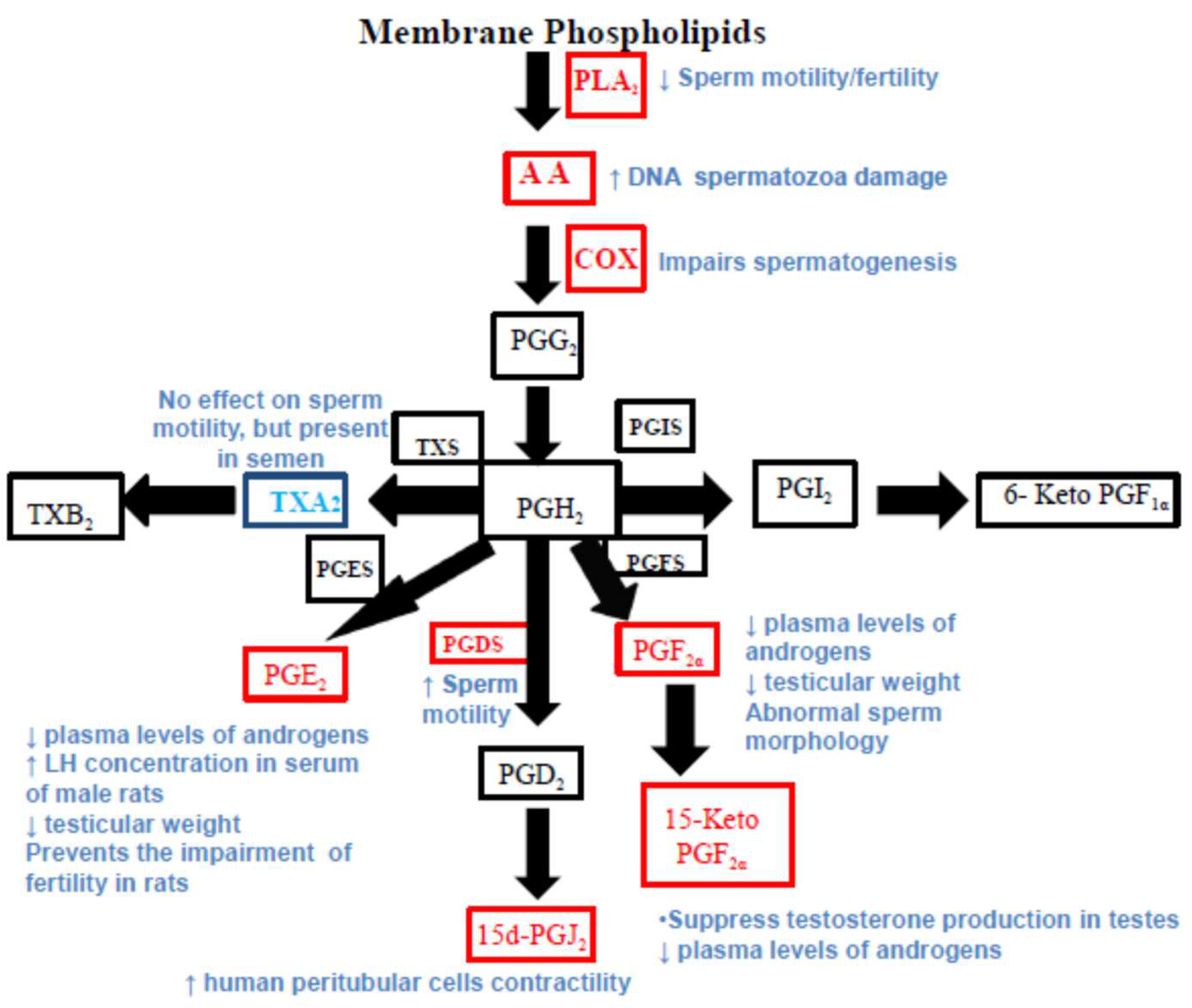
1. Introduction
2. Methods
2.1. Research Methods and Reporting
2.2. Study Design
2.3. Eligibility Criteria
2.4. Literature Search, Information Sources and Selection of Articles
2.5. Data Synthesis
3. Results
3.1. Findings from Animal Studies
| No. | Study | Type of Animal | AA Pathway Mediator/Enzyme Assessed | Results |
|---|---|---|---|---|
| 1 | Castracane et al., 1974 [33] | Male Rats | PGE1, PGE2 | A single PGE2 (sub-q injection) increases gradually the LH concentration in serum of river male rats of known fertility. |
| 2 | Rej et al., 1980 [35] | Male Rats | PGE1, PGE2, PGF2α | The incapacity of PGs (1 mg/kg of PGE(1 and 2) and PGF2α) to affect the fertilizing capacity of spermatozoa in both injected and contralateral sides suggest that androgen that has been available following the treatment schedule was enough to keep the fertilizing capacity despite of the PGs site of injection. |
| 3 | Ericsson et al., 1973 [38] | Male Rats | PGE1, PGE2 | Testosterone added concurrently with PGE1 and PGE2 maintained both the fertilizing capacity of epididymal spermatozoa, and the weight of accessory sex glands. |
| 4 | Bartke et al., 1973 [36] | Mice | PGF2α | PGs can suppress steroidogenesis in the testis and this can bring to a decrease in plasma testosterone levels in intact adult male mice. |
| 5 | Kimball et al., 1978 [40] | Male Rats | 15-Me-PGF2α | 15-Me-PGF2a can suppress testosterone production in testes. |
| 6 | Memon, 1973 [34] | Male Rats | PGE2, PGF2α | The testicular weight and plasma testosterone is lower in rats treated with PGE2, and PGF2α.. LH increased significantly in male rats with bilateral injection of PGs. |
| 7 | Free et al., 1972 [41] | Rats | PGE1, PGE2, PGF2α | PGE1, PGE2, PGF2α can lower the blood flow through the testes. No further data were reported on fertility. |
| 8 | Lubice-Nawrocki et al., 1973 [37] | Hamster | PGE1, PGF2α | The treatment of hamsters for a short period of time with PGF2α, or PGE1 had no effect on fertilizing ability. |
| 9 | Gerozissis et al., 1981 [32] | Rats | PGE2 | PGE2 was found in the seminal fluid of rats, but no further data were reported on sperm fertility (motility, morphology, etc.). |
| 10 | Waheed et al., 2015 [26] | DromedaryCamels | sPLA2, PGDS | sPLA2 is a fertility-associated biomarker in seminal plasma and serum of dromedary camels. Prostaglandin D synthase (lipocalcin-type) is negatively related to camels’ fertility. |
| 11 | Roqueta-Rivera et al., 2010 [30] | Male Mice | AA | AA was not as effective as DHA in restoring fertility, sperm count, and spermiogenesis in male mice. |
| 12 | Hale et al., 2019 [28] | Male mice | AA | Higher AA levels were found in Acsl6 −/− testes. |
| 13 | Stoffel et al., 2020 [29] | Mice | AA | AA is one of the main components of testicular membranes. Only the AA/DHA (1:1 M) diet fully restored male spermatogenesis in the ω6/ω3- fatty acid desaturase (fads2−/−) cohorts. |
| 14 | Bao et al., 2004 [25] | Male Mice | PLA2 | Group VIA Phospholipase A2 (iPLA2β) reduces the motility of spermatozoa. |
| 15 | Khatibjoo, 2018 [31] | BroilerBreeder | AA | Higher absolute amount of n-3 and n-6 PUFA are more important for the reproductive and performance traits of breeders than the n-6/n-3 FA ratios. |
| 16 | Hafiez, 1974 [39] | Rats | PGE2 | PGE2 prevented the impairment of fertility in rats. |
| 17 | Fouchécourt et al., 2002 [27] | Ram/Bull | PGDS | PGDS levels were increased in animals with normal or high fertility, and showed a high capacity to bind to testosterone. |
3.2. Summary of the Results Reported by Human Clinical Trials
| No. | Study | Sample Size and Characteristic/Age Range or Mean Age | AA Mediators Studied | Results |
|---|---|---|---|---|
| 1 | Skakkeback et al., 1976 [51] | Semen samples of 2 hypogonadal men/26 and 36 years old respectively | PGE1, PGE2, 19-OH-PGEs | The seminal 19-OH PGEs levels change based on blood testosterone levels, and may be involved in reproductive process. |
| 2 | Cosentino et al., 1984 [13] | 163 semen samples from 145 men/24 to 50 years old | PGF2α, PGE | PGs levels in the semen are essential for assessing the human male fertility and are related to certain male fertility parameters. Patients with low seminal PGE levels also had reduced sperm motility. High levels of seminal PGF2α may be related to impaired testicular function. Seminal PGF2α is positively correlated to abnormal sperm morphology. |
| 3 | Lewy et al., 1979 [49] | Semen of 10 infertile males and 7 fertile males/N.S. | 19-OH-PGE, PGE, 6-keto-PGF1α | Lower levels of PGE and 19-OH-PGE were found in the infertile group in respect to the fertile men. 6-keto F1α levels in human semen did not change, and PGI2 is not important for male fertility. |
| 4 | Bygdeman et al., 1970 [52] | Single semen samples from 150 men, classified in 3 classes: (Group A, fertile men (n = 29); Group B, men in noninvestigated infertile marriages (n = 100); and Group C, men in infertile marriages with no abnormal clinical or laboratory findings (n = 21)/N.S. | PGE1, PGE2,PGE3, PGA1, PGA2,PGB1,PGB2, 19-hydroxy PGA1, 19-hydroxy PGA2, 19-hydroxy PGB1, 19-hydroxy PGB2 | The PGE levels in the seminal plasma have an essential role in male fertility. Seminal PGE, increase the human female reproductive tract uterine contraction. |
| 5 | Collier et al., 1975 [53] | 12 men in infertile marriages with no abnormal findings/N.S. | PGE | PGE levels in the semen of infertile men is lower than semen of fertile men, but no abnormality on sperm motility had been detected by semen analysis. |
| 6 | Kelly et al., 1979 [65] | 57 semen samples/20–40 years old | PGE1, PGE2, 19-OH PGE1, 19-OH PGE2 | In the semen samples of polyzoospermic group the PGs levels decreased significantly. Reduced PGs levels in polyzoospermia may suggest that the function of seminal PGs is to modify sperm metabolism at the time of ejaculation. |
| 7 | Asplund, 1947 [64] | 155 specimens of sperm/N.S. | PGs | No correlation between seminal PGs concentration and sperm morphology, motility and concentration exists. |
| 8 | Hawkins et al., 1961 [48] | n = 4 normal human testicular samples, n = 13 pathological samples, n = 6 additional samples/26 to 43 years old | PGs | The higher the spermatozoa percentage the lower the number of PGs. However, in a specific group of infertile patients, higher concentration of PGs were related to abnormal sperm motility. 15dPGJ2 is potentially involved through ROS, in enhancing hypertrophy and deprivation on the contractility of peritubular cells from human testes and is potentially involved in development of human male sub-/infertility. |
| 9 | Horton et al., 1964 [50] | 14 semen samples/N.S. | PGE1 | PGs concentration in human semen varied from 24 to 783μg/mL. No data were reported on male fertility. |
| 10 | Brummer et al., 1972 [54] | 104 samples divided in 4 groups/N.S. | PGE, PGA | Lower levels of PGE were observed in the samples of seminal fluid of infertile men. High PGE levels may increase fertility, through the increase of sperm count and motility. |
| 11 | Huleihel et al., 1999 [55] | 17 samples divided in 2 groups Fertile men (n = 7), versus patients with oligoteratoasthenoazoospermia (OTA) (n = 10)/N.S. | PGE2 | Higher levels of PGE2 were observed in the seminal plasma of fertile men in respect to seminal plasma of patient with oligoteratoasthenoazoospermia (OTA), but no differences in sperm cells functions and parameters were observed. |
| 12 | Isidori et al., 1980 [42] | 15 normal volunteers, and 30 patients with PGE seminal levels inferior to normal minimal values; 8 patients with seminal PGE levels greater than normal maximal values; 22 patients with seminal 19-OH PGE levels inferior to normal minimal values; 16 patients with seminal 19-OH PGE levels greater than normal maximal values/N.S. | PGE, 19-OH PGE | The reduced adenylcyclase and testicular androgen activity may be responsible of the negative impact of low seminal PGs levels in sperm concentration and motility. Indeed, reduced sensitivity of receptors to increased titers of PGs, and DNA synthesis inhibition in testes may be responsible of the negative impact of high seminal PGs levels. |
| 13 | Sturde, 1968 [44] | Semen samples from volunteers/N.S. | 19-OH PGF, 19-OH PGF2α, 19-OH PGE2, PGE1, PGE2, PGF2α | 19-hydroxy PGF and 19-hydroxy PGE have a significant role in sperm motility, potentially through the ATP content in spermatozoa. |
| 14 | Schell et al., 2007 [21] | Normal patients with obstructive azoospermia (n = 6), and impaired spermatogenesis (n = 8)/N.S. | PGD2 synthetases | PGD2 synthetases are found in interstitial cells of men with impaired spermatogenesis. |
| 15 | Gerozissis et al., 1981 [34] | Semen of fertile men/30–50 years with a proven fertility (children 3–5 years of age). | 19-OH-PGE, 19-OH-PGFαPGD2, PGE2, PGE1, PGF2α, PGF-1α, 6-keto-PGF1α 13,14-dihydro-15-keto-PGFα | 19-OH-PGE, 19-OH-PGFα,PGE2, PGE1, PGF2α, PGF-1α, 6-keto-PGF1α, PGD2, 13,14-dihydro-15-keto-PGFα were found in human semen of fertile men. |
| 16 | Templeton et al., 1978 [4] | Semen of 23 fertile men/20–40 years old | PGE, 19-OH PGE, PGF, 19-PGF | No significant difference was showed in the PGE levels in the semen of fertile and non-fertile men. The sperm count and motility were normal. |
| 17 | Tusell et al., 1980 [43] | Semen of 7 volunteers/25–30 years old | 19-OH PGE, 19-OH PGF, PGE | PGE, PGF, and 19-hydroxylated E and F were detected through gas and liquid chromatography in semen of fertile men. |
| 18 | Bendvold et al., 1987 [5] | Semen of 31 men/N.S. | PGE, PGF, 19-hydroxy-PGE, 19-hydroxy-PGF | A positive correlation exists between PG content and sperm density in fertile men. PGE and 19-hydroxy-PGE were the main PGs in human semen. |
| 19 | Schlegel et al., 1981 [67] | Semen of 10 fertile and 55 infertile men/N.S. | PGE2, PGF2α | PGF2α can act on sperm motility, but not through its receptors. High levels of PGE were found in patients with persisting varicocele and in patients with very poor motility and low sperm counts. |
| 20 | Signorini et al., 2022 [63] | Infertile Italian patients (n = 67) versus fertile men (n = 18)/29 to 40 years old | Resolvin D1; F2-IsoPs | Resolvin D1 levels increase in patients with idiopathic infertility, leukocytospermia, varicocele in respect to fertile men. Resolvin D1 and F2-IsoPs reduce the sperm quality. Resolvin D1 levels correlated negatively with sperm progressive motility, vitality, fertility index; but positively with sperm necrosis and immaturity. |
| 21 | Longini et al., 2020 [59] | Semen samples of 61 Italian men/27–42 years old | F2-dihomo-IsoPs, F2-IsoPs, F4-NeuroPs | F2-IsoPs are related to male infertility by affecting the sperm quality. F2-IsoPs showed a negative correlation with sperm motility and a positive one with sperm immaturity. |
| 22 | Safarinejad et al., 2010 [47] | 82 males/34.2 ± 4.1 years old | AA | Higher levels of AA were detected in infertile men compared to fertile men. A strong negative correlation was found between the AA:DHA and AA:EPA ratios and total sperm count, sperm motility, and sperm morphology. |
| 23 | Shrivastav et al., 1989 [45] | 18 randomly selected IDDM male diabetic patients/mean age 31 years, (age range 21–39 years) | PGE2, PGF2α, PGl2,TXA2 | In diabetic patients, higher levels of PGE2, PGF2α, PGl2, TXA2 were observed in seminal plasma. Despite increased seminal plasma PG concentrations are associated with oligospermia and reduced sperm motility the current data did not showed these sperm defects in the diabetic males. |
| 24 | Collodel et al., 2021 [60] | 49 infertile couples/29–37 years | F2-IsoPs | F2-IsoPs can be a marker of sperm immaturity, for the evaluation of semen and follicular fluid quality. |
| 25 | Andersen et al., 2016 [46] | 144 samples/≥18 years old | AA | A positive correlation between AA levels and spermatozoa was observed. |
| 26 | Collodel et al., 2018 [61] | 38 patients/26–40 years | 8-Iso PGF2α | A significant positive correlation between F2-IsoP levels and sperm immaturity was observed in infertile patients with varicocele. |
| 27 | Chen et al., 2007 [17] | 90 semen samples/N.S. | L-PGDS | L-PGDS in seminal plasma is positively correlated with sperm motility and density. |
| 28 | Moretti et al., 2019 [56] | 1 patient/44 years old | PLA2 | Reduced levels of PLA2 were observed in sperm of globozoospermic patient respect to those of fertile men. |
| 29 | Moretti et al., 2022 [62] | 147 patients with infertility/26–43 years | F2-IsoPs | F2-IsoP levels above 29.96 ng/mL are potentially related to idiopathic infertility and other pathological conditions and is an index of altered sperm quality in infertile patients. |
| 30 | Gerozissis et al., 1982 [7] | Men with proven fertility; Vasectomized men; Men with an obstructive sterility/N.S. | PGE1, PGE2, PGF1α,PGF2α,19-OH-PGE (1 + 2), 19-OH-PGFα | The major part of PGs in human semen derive from seminal vesicles. High levels of seminal PGF 2α may be related to impaired testicular function. |
| 31 | Dorp et al., 1968 [66] | Semen samples/N.K. | PGs | No correlation was found between seminal PGs and spermatozoa morphology and concentration. |
3.3. Potential Effects of NSAIDs in Seminal Fluid PGs
| No. | Study | Type of Study/Sample Characteristics | Compound Analyzed, Dosage and Route of Administration | AA Mediator Assessed | Results |
|---|---|---|---|---|---|
| 1 | Bendvold et al., 1985 [68] | Human study/n = 6 before, during and after treatment with naproxen | Naproxen 250 mg 3 times daily for 2 weeks; Oral administration | PGE, PGF, 19-hydroxy-PGE, and 19-hydroxy-PGF, 8α -1 9-hydroxy-PGF2α, 8ß-19-hydroxy-PGF1α | In human seminal fluid, naproxen reduced the concentration of all PGs. However, no statistically significant role of naproxen was observed on sperm density, motility, or morphology. The data suggest that the reduction of PGs is not secondary to the effect of naproxen on sperm characteristics. |
| 2 | Collier et al., 1971 [69] | Human study/n = 4 (22–29 years old) | Aspirin 600 mg; Oral Administration | PGE2 and PGF2α | PGE2 and PGF2α levels in human seminal fluid were reduced during treatment with aspirin. The mechanisms responsible for controlling the concentrations of PGE2 and PGF2α in semen may be different. No further data were reported on the role of aspirin in sperm motility, density. |
| 3 | Horton et al., 1973 [70] | Human study/n = 2 (25 and 52 years old respectively) | Aspirin 3.6–7.2 g/day; Oral administration | PGE | The seminal plasma levels of PGE were reduced by 80% in patients taking 7.2 g/day of Aspirin. Sperm density was not assessed. |
| 4 | Barbosa 2020 [81] | Animal Study/Male rats (23 days old) | Ibuprofen 0; 2.4; 7.2 or 14.3 mg/kg/day; Oral administration | PGs | The pre-pubertal treatment with ibuprofen had a negative effect in sperm quality and quantity affecting the reproduction. The male offspring had an accelerated sperm transit time in the epididymis, while the fertility potential reduced in the female offspring. |
| 5 | Löscher et al., 1988 [84] | Animal Study/Male rabbits | Phenylbutazone 100 mg/kg; Subcutaneous injection | PGE2, PGF2α | The prolonged treatment with NSAIDs did not affect rabbit male fertility. However, chronic treatment with phenylbutazone may improve sperm quality, increase ejaculate volume, and improve sperm fertility. |
| 6 | Löscher et al., 1986 [85] | Animal Study/Male rats | Acetylsalicylic acid 50 or 150 mg; Naproxen 10 mg; Indomethacin 2 mg; Phenylbutazone 20 mg; Intraperitoneal injection | PGE2 | The reduction of prostaglandin synthesis in male rats does not have any affect in fertility. This can be explained by the very low seminal prostaglandin levels in rats in confront to other animals. |
| 7 | Conte et al., 1985 [86] | Human study/n = 15 fertile men (20–30 years); n = 20 infertile oligozoospermic men (20–40 years); n = 10 infertile oligozoospermic patients (20–40 years) | Indomethacin (100 mg/die) for 30 days; Oral administration | PGE, 19-OH PGE | Indomethacin improved significantly the sperm count and motility in infertile oligozoospermic patients with high levels of PGs |
| 8 | Yegnanarayan et al., 1978 [87] | Animal Study/Male rats | Acetyl salicylic acid, indomethacin, oxyphenbutazone 4 mg/kg, 100 mg/kg, 400 mg/kg for short term experiments; and one fifth of the above-mentioned doses for long-term experiments; Oral administration | PGE2, PGF2α | The number of fertile coatings or pregnancies was significantly less in the groups mated with indomethacin and oxyphenbutazone treated males. |
| 9 | Freixa et al., 1984 [83] | Human study/n = 6 (20–25 years old) | Flurbiprofen 100 mg; Oral administration | PGE, 19-OH PGEs | Flurbiprofen reduced PGE values, and produces a small alteration in sperm head with a larger and spherical head. |
| 10 | Fouchécourt et al., 2002 [27] | Animal Study/Rats | Flurbiprofen 3.3 mg/kg, Indomethacin 1.7 mg/kg; Intraperitoneal injection | PGE2, PGF2 | Flurbiprofen and Indomethacin did not affect male reproduction in rats. |
| 11 | Cenedella et al., 1973 [79] | Animal Study/Mice | Aspirin (50 mg/kg twice daily); Oral administration | PGs | Interestingly the fertility increased in male mice that were classified initially as sub-fertile under treatment with aspirin (50 mg/kg twice daily for a total of 12 days). |
| 12 | Vyas et al., 2016 [71] | Animal Study/Male rats | Aspirin 12.5 mg/kg for 30 days and 60 days; Oral administration | PGs | The subchronic dose of aspirin (12.5 mg/kg for 30 days and 60 days) given to male rats changed the reproductive profile of male rats, and reduced sperm mobility and density. |
| 13 | Stutz et al., 2004 [77] | Human study/n = 277 | Aspirin 1–8 g/mo; Oral administration | PGs | Aspirin can have a deleterious effect on seminal parameters. In moderate aspirin users the percentages of motile, progressive and rapid progressive gametes decreased. |
| 14 | Kennedy et al., 2003 [72] | Animal Study/White Turkeys | Diclofenac, Indomethacin, Aspirin, Tolmetin 0–15 mM; In-vitro | PGE1, PGE2, PGF2α | The NSAIDs studied decreased the avian mobility of sperm. |
| 15 | Martini et al., 2003 [78] | Human study/20–60 years old | Aspirin 2600 mg/day for 3 days; Oral administration | PGs | The chronic use of Aspirin has a negative impact on male fertility, specifically on sperm motility, morphology, and seminal volume. |
| 16 | Tanyildizi et al., 2003 [73] | Animal Study/Rams | Aspirin 75 mg kg−1 body weight; Oral administration/Metamizol t 50 mg kg−1 body weight; Intramuscular injection | PGs | The concomitant use of two NSAIDs, respectively Aspirin and Metamizol decreased both the semen volumes, and sperm concentrations. |
| 17 | Stutz et al., 2000 [82]. | Animal Study/Mice | Aspirin 14.3 mg/kg day−1 Intramuscular injection/Ibuprofen 5.6 mg/kg day−1, Intraperitoneal injection /Piroxicam 0.28 mg/kg day−1, Intraperitoneal injection | PGs | Ibuprofen reduced testosterone levels, but did not modify the sperm functional activity. Aspirin reduced the percentage of gametes that were swelled, and irreversibly alters structural and/or functional properties of sperm plasma membrane. |
| 18 | Didolkar et al., 1980 [74] | Animal Study/Rats | Aspirin 5 mg/100 g body weight/day; Oral administration | PGs | Aspirin used for a long period of time can impair spermatogenesis and brings to an increase of plasma LH levels. |
| 19 | Didolkar et al., 1980 [75] | Animal Study/Rats | Aspirin 5 mg/100 g body weight/day for 30 days; Oral administration | PGs | Aspirin can have a deleterious effect on seminal parameters, bringing to impairment of spermatogenesis. |
| 20 | Biswas et al., 1978 [76] | Animal Study/Rats | Aspirin 5 mg/100 g body weight/day; Intraperitoneal injection | PGE2 | The spermatid count was decreased following treatment with Aspirin. |
| 21 | Scott et al., 1978 [80] | Animal Study/Rats | Aspirin 300 mg/kg body weight, 150 mg/kg body weight) for 12 days and 6 days; Oral administration | PGs | Data did not conclude on the role of Aspirin on spermatogenesis. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capra, V.; Rovati, G.E.; Mangano, P.; Buccellati, C.; Murphy, R.C.; Sala, A. Transcellular biosynthesis of eicosanoid lipid mediators. Biochim. Biophys. Acta 2015, 1851, 377–382. [Google Scholar] [CrossRef]
- Von Euler, U.S. On the specific vaso-dilating and plain muscle stimulating substances from accessory genital glands in man and certain animals. J. Physiol. 1936, 88, 213. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.W. Prostaglandin synthesis in the male and female reproductive tract. J. Reprod. Fertil. 1981, 62, 293–304. [Google Scholar] [CrossRef]
- Templeton, A.A.; Cooper, I.; Kelly, R.W. Prostaglandin concentrations in the semen of fertile men. J. Reprod. Fertil. 1978, 52, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bendvold, E.; Gottlieb, C.; Svanborg, K.; Bygdeman, M.; Eneroth, P. Concentration of prostaglandins in seminal fluid of fertile men. Int. J. Androl. 1987, 10, 463–469. [Google Scholar] [CrossRef]
- Gerozissis, K.; Jouannet, P.; Soufir, J.C.; Dray, F. Origin of prostaglandins in human semen. J. Reprod. Fertil. 1982, 65, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.J.; Hastings, N.E.; Ellis, L.C. Prostaglandins and cyclic nucleotides in the ram reproductive tract. J. Androl. (Abstr.) 1982, 3, 39. [Google Scholar]
- Ollero, M.; Powers, R.D.; Alvarez, J.G. Variation of docosahexaenoic acid content in subsets of human spermatozoa at different stages of maturation: Implications for sperm lipoperoxidative damage. Mol. Reprod. Dev. 2000, 55, 326–334. [Google Scholar] [CrossRef]
- Ollero, M.; Gil-Guzman, E.; Lopez, M.C.; Sharma, R.K.; Agarwal, A.; Larson, K.; Evenson, D.; Thomas, A.J.; Alvarez, J.G. Characterization of subsets of human spermatozoa at different stages of maturation: Implications in the diagnosis and treatment of male in-fertility. Hum. Reprod. 2001, 16, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Wingate, J.K.; De Iuliis, G.N.; Koppers, A.J.; McLaughlin, E.A. Cis-Unsaturated Fatty Acids Stimulate Reactive Oxygen Species Generation and Lipid Peroxidation in Human Spermatozoa. J. Clin. Endocrinol. Metab. 2006, 91, 4154–4163. [Google Scholar] [CrossRef]
- Hedqvist, P.; Gustafsson, L.; Hjemdahl, P.; Svanborg, K. Aspects of prostaglandin action on autonomic neuroeffector transmis-sion. In Advances in Prostaglandin and Thromboxane Research; Samuelsson, B., Ramwell, P., Paoletti, R., Eds.; Raven Press: New York, NY, USA, 1980; Volume 8, p. 125. [Google Scholar]
- Peterson, R.N.; Seyler, B.; Bundman, D.; Freund, M. The effect oftheophylline and dibutyryl cyclic AMP on the uptake of radio-active calcium and phosphate ions by boar and human spermatozoa. J. Reprod. Fertil. 1980, 55, 385. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.J.; Emilson, L.B.; Cockett, A.T. Prostaglandins in semen and their relationship to male fertility: A study of 145 men. Fertil. Steril. 1984, 41, 88–94. [Google Scholar] [CrossRef]
- Cheuk, B.L.Y.; Chew, S.C.; Fiscus, R.R.; Wong, P.Y.D. Cyclooxygenase-2 regulates apoptosis in rat epididymis through prostaglandin D2. Biol. Reprod. 2002, 66, 374–380. [Google Scholar] [CrossRef]
- Olsson, J.E. Correlation between the concentration of β-trace protein and the number of spermatozoa in human semen. J. Reprod. Fertil. 1975, 42, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.G.; Haq, H.A.; Saso, L. Lipocalin type prostaglandin D-synthase: Which role in male fertility? Contraception 2002, 65, 293–295. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Zhu, M.-Y.; Cui, Y.-D.; Huang, T.-H. Relationship between contents of lipocalin-type prostaglandin D synthase on the surface of infertility sperm and in seminal plasma. Biochemistry 2007, 72, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Calandra, R.S.; Mayerhofer, A.; Matzkin, M.E. Cyclooxygenase and prostaglandins in somatic cell populations of the testes. Reproduction 2015, 149, R169–R180. [Google Scholar] [CrossRef]
- Rudolph, L.M.; Bentley, G.E.; Calandra, R.S.; Paredes, A.H.; Tesone, M.; Wu, T.-Y.; Micevych, P.E. Peripheral and Central Mechanisms Involved in the Hormonal Control of Male and Female Reproduction. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Hase, T.; Yoshimura, R.; Matsuyama, M.; Kawahito, Y.; Wada, S.; Tsuchida, K.; Sano, H.; Nakatani, T. Cyclooxygenase-1 and -2 in human testicular tumours. Eur. J. Cancer. 2003, 39, 2043–2049. [Google Scholar] [CrossRef]
- Schell, C.; Frungieri, M.B.; Albrecht, M.; Gonzalez-Calvar, S.I.; Ko¨hn, F.M.; Calandra, R.S.; Mayerhofer, A. A prostaglandin D2 system in the human testis. Fertil. Steril. 2007, 88, 233–236. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The Role of Arachidonic and Linoleic Acid De-rivatives in Pathological Pregnancies and the Human Reproduction Process. Int. J. Mol. Sci. 2020, 21, 9628. [Google Scholar] [CrossRef]
- Lax, Y.; Grossman, S.; Rubinstein, S.; Magid, N.; Breitbart, H. Role of lipoxygenase in the mechanism of acrosome reaction in mammalian spermatozoa. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1990, 1043, 12–18. [Google Scholar] [CrossRef]
- Olive, E.H.; Fabiani, R.; Johansson, L.; Ronquist, G. Arachidonic acid 15-lipoxygenase and traces of E prostaglandins in pu-rified human prostasomes. J. Reprod. Fertil. 1993, 99, 195–199. [Google Scholar]
- Bao, S.; Miller, D.J.; Ma, Z.; Wohltmann, M.; Eng, G.; Ramanadham, S.; Moley, K.; Turk, J. Male Mice that Do Not Express Group VIA Phospholipase A2 Produce Spermatozoa with Impaired Motility and Have Greatly Reduced Fertility. J. Biol. Chem. 2004, 279, 38194–38200. [Google Scholar] [CrossRef] [PubMed]
- Waheed, M.; Ghoneim, I.; Alhaider, A. Seminal plasma and serum fertility biomarkers in dromedary camels (Camelus dromedarius). Theriogenology 2014, 83, 650–654. [Google Scholar] [CrossRef]
- Fouchécourt, S.; Charpigny, G.; Reinaud, P.; Dumont, P.; Dacheux, J.-L. Mammalian Lipocalin-Type Prostaglandin D2 Synthase in the Fluids of the Male Genital Tract: Putative Biochemical and Physiological Functions. Biol. Reprod. 2002, 66, 458–467. [Google Scholar] [CrossRef]
- Hale, B.J.; Fernandez, R.F.; Kim, S.Q.; Diaz, V.D.; Jackson, S.N.; Liu, L.; Brenna, J.T.; Hermann, B.P.; Geyer, C.B.; Ellis, J.M. Acyl-CoA syn-thetase 6 enriches seminiferous tubules with the ω-3 fatty acid docosahexaenoic acid and is required for male fertility in the mouse. J. Biol. Chem. 2019, 294, 14394–14405. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Schmidt-Soltau, I.; Binczek, E.; Thomas, A.; Thevis, M.; Wegner, I. Dietary ω3- and ω6-Polyunsaturated fatty acids reconstitute fertility of Juvenile and adult Fads2-Deficient mice. Mol. Metab. 2020, 36, 100974. [Google Scholar] [CrossRef]
- Roqueta-Rivera, M.; Stroud, C.K.; Haschek, W.M.; Akare, S.J.; Segre, M.; Brush, R.S.; Agbaga, M.-P.; Anderson, R.E.; Hess, R.A.; Nakamura, M.T. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J. Lipid Res. 2010, 51, 360–367. [Google Scholar] [CrossRef]
- Khatibjoo, A.; Kermanshahi, H.; Golian, A.; Zaghari, M. The effect of n-6/n-3 fatty acid ratios on broiler breeder performance, hatchability, fatty acid profile and reproduction. J. Anim. Physiol. Anim. Nutr. 2018, 102, 986–998. [Google Scholar] [CrossRef]
- Gerozissis, K.; Dray, F. Radioimmunoassay of prostaglandins in the semen of fertile men. J. Reprod. Fertil. 1981, 61, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Castracane, V.D.; Saksena, S.C. Prostaglandins of E series and LH release in fertile male rats. Prostaglandins 1974, 7, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Memon, G.N. Effects of intratesticular injections of prostaglandins on the testes and accessory sex glands of rats. Contraception 1973, 8, 361–370. [Google Scholar] [CrossRef]
- Rej, S.K.; Chatterjee, A. The possible mode of action of prostaglandins: XVI. A study to assess the local effect of prostaglandins E1, E2 or F2α in the regulation of male fertility. Prostaglandins Med. 1980, 4, 465–470. [Google Scholar] [CrossRef]
- Bartke, A.; Iusto, N.; Caldwell, B.V.; Behrman, H.R. Effects of a cholesterol inhibitor and of prostaglandin F2α on testis cholesterol and on plasma testosterone in mice. Prostaglandins 1973, 3, 97. [Google Scholar] [CrossRef] [PubMed]
- Lubice-Nawrocki, C.M.; Saksena, S.K.; Chang, M.C. Effects of prostaglandins El and F2α on the fertilizing ability of hamster spermatozoa. J. Reprod. Fert. 1973, 35, 557. [Google Scholar] [CrossRef]
- Ericsson, R.J. Prostaglandins (E1 and E2) and reproduction in the male rat. Adv. Biosci. 1973, 9, 737–742. [Google Scholar]
- Hafiez, A.A. Prostaglandin E2 prevents impairment of fertility in rats fed a diet deficient in essential fatty acids. J. Reprod. Fertil. 1974, 38, 273–286. [Google Scholar] [CrossRef]
- Kimball, F.A.; Frielink, R.D.; Porteus, S.E. Effects of 15(s)-15-methyl prostaglandin F2A methyl ester-containin silastic disc in male rats. Fertil. Steril. 1978, 29, 103. [Google Scholar] [CrossRef]
- Free, M.J.; Jaffe, R.A. Effect of prostaglandins on blood flow and pressure in the conscious rat. Prostaglandins 1972, 1, 483–498. [Google Scholar] [CrossRef]
- Isidori, A.; Conte, D.; Laguzzi, G.; Giovenco, P.; Dondero, F. Role of seminal prostaglandins in male fertility. I. Relationship of prostaglandin E and 19-OH prostaglandin E with seminal parameters. J. Endocrinol. Investig. 1980, 3, 1–4. [Google Scholar] [CrossRef]
- Tusell, J.M.; Gelpi, E. Prostaglandins E and F, and 19-hydroxylated E and F (series I and II) in semen of fertile men. Gas and liquid chromatographic separation with selected ion detection. J. Chromatogr. 1980, 181, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Sturde, H.G. Experimental investigations into the question of prostaglandins and their relation to male fertility. Parts I, II, III. Arzneim. Forsch. 1968, 18, 895. [Google Scholar]
- Shrivastav, P.; Swann, J.; Jeremy, J.Y.; Thompson, C.; Shaw, R.W.; Dandona, P. Sperm Function and Structure and Seminal Plasma Prostanoid Concentrations in Men with IDDM. Diabetes Care 1989, 12, 742–744. [Google Scholar] [CrossRef]
- Andersen, J.M.; Rønning, P.O.; Herning, H.; Bekken, S.D.; Haugen, T.B.; Witczak, O. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology 2016, 4, 857–865. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Hosseini, Y.; Dadkhah, F.; Asgari, M.A. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: A comparison between fertile and infertile men. Clin. Nutr. 2010, 29, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.F.; Labrum, A.H. Semen prostaglandin levels in fifty patients attending a fertility clinic. J. Reprod. Fertil. 1961, 2, 1–10. [Google Scholar] [CrossRef]
- Lewy, R.I.; Bills, T.K.; Dalton, J.; Smith, J.P.; Silver, M.J. 19-hydroxy-prostaglandin E and infertility in human males. Prostaglandins Med. 1979, 2, 367. [Google Scholar] [CrossRef] [PubMed]
- Horton, E.W.; Thompson, C.J. Thin-layer chromatography and bioassay of prostaglandins in extracts of semen and tissues of the male reproductive tract. Br. J. Pharmacol. Chemother. 1964, 22, 183–188. [Google Scholar] [CrossRef]
- Skakkeback, N.E.; Kely, R.W.; Corter, C.S. Prostaglandin concentrations in the semen of hypogonadal men during treatment with testosterone. J. Reprod. Fert. 1976, 47, 119. [Google Scholar] [CrossRef]
- Bygdeman, M.; Fredricsson, B.; Svanborg, K.; Samuelsson, B. The Relation Between Fertility and Prostaglandin Content of Seminal Fluid in Man. Fertil. Steril. 1970, 21, 622–629. [Google Scholar] [CrossRef]
- Collier, J.G.; Flower, R.J.; Stanton, S.L. Seminal prostaglandins in infertile men. Fertil. Steril. 1975, 26, 868. [Google Scholar] [CrossRef] [PubMed]
- Brummer, H.C.; Gillespie, A. Seminal prostaglandins and fertility. Clin. Endocrinol. 1972, 1, 363–368. [Google Scholar] [CrossRef]
- Huleihel, M.; Lunenfeld, E.; Horowitz, S.; Levy, A.; Potashnik, G.; Mazor, M.; Glezerman, M. Expression of IL-12, IL-10, PGE2, sIL-2R and sIL-6R in seminal plasma of fertile and infertile men. Andrologia 1999, 31, 283–288. [Google Scholar] [CrossRef]
- Moretti, E.; Collodel, G.; Salvatici, M.C.; Belmonte, G.; Signorini, C. New insights into sperm with total globozoospermia: In-creased fatty acid oxidation and centrin1 alteration. Syst. Biol. Reprod. Med. 2019, 65, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, C.; Svanborg, K.; Eneroth, P.; Bygdeman, M. Effect of prostaglandins on human sperm function in vitro and seminal adenosine triphosphate content. Fertil. Steril. 1988, 49, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Schell, C.; Albrecht, M.; Spillner, S.; Mayer, C.; Kunz, L.; Kohn, F.M.; Schwarzer, U.; Mayerhofer, A. 15-Deoxy-Δ12-14-Prostaglandin-J2 Induces Hypertrophy and Loss of Contractility in Human Testicular Peritubular Cells: Implications for Human Male Fertility. Endocrinology 2010, 151, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Longini, M.; Moretti, E.; Signorini, C.; Noto, D.; Iacoponi, F.; Collodel, G. Relevance of seminal F2-dihomo-IsoPs, F2-IsoPs and F4-NeuroPs in idiopathic infertility and varicocele. Prostaglandins Other Lipid Mediat. 2020, 149, 106448. [Google Scholar] [CrossRef]
- Collodel, G.; Noto, D.; Signorini, C.; Gambera, L.; Stendardi, A.; Mahmutbegovic, A.; Micheli, L.; Menchiari, A.; Moretti, E. Do Seminal Isoprostanes Have a Role in Assisted Reproduction Outcome? Life 2021, 11, 675. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Longini, M.; Pascarelli, N.A.; Signorini, C. Increased F2-Isoprostane Levels in Semen and Immuno-localization of the 8-Iso Prostaglandin F2α in Spermatozoa from Infertile Patients with Varicocele. Oxid. Med. Cell. Longev. 2018, 2018, 7508014. [Google Scholar] [CrossRef]
- Moretti, E.; Signorini, C.; Ferretti, F.; Noto, D.; Collodel, G. A Study to Validate the Relevance of Semen F2-Isoprostanes on Human Male Infertility. Int. J. Environ. Res. Public Health 2022, 19, 1642. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; Moretti, E.; Noto, D.; Micheli, L.; Ponchia, R.; Collodel, G. Fatty Acid Oxidation and Pro-Resolving Lipid Mediators Are Related to Male Infertility. Antioxidants 2022, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Asplund, J. A Quantitative Determination of the Content of Contractive Substances in Human Sperm and their Significance for the Motility and Vitality of the Spermatozoa. Acta Physiol. Scand. 1947, 13, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.W.; Cooper, I.; Templeton, A.A. Reduced prostaglandin levels in the semen of men with very high sperm concen-trations. J. Reprod. Fertil. 1979, 56, 195. [Google Scholar] [CrossRef]
- Dorp, J. Essential fatty acids and prostaglandin. Nat. Sci. 1968, 56, 125. [Google Scholar]
- Schlegel, W.; Rotermund, S.; Färber, G.; Nieschlag, E. The influence of prostaglandins on sperm motility. Prostaglandins 1981, 21, 87–99. [Google Scholar] [CrossRef]
- Bendvold, E.; Gottlieb, C.; Svanborg, K.; Bygdeman, M.; Eneroth, P.; Cai, Q.-H. The effect of naproxen on the concentration of prostaglandins in human seminal fluid. Fertil. Steril. 1985, 43, 922–926. [Google Scholar] [CrossRef]
- Collier, J.G.; Flower, R.J. Effect of aspirin on human seminal prostaglandins. Lancet 1971, 2, 852. [Google Scholar] [CrossRef]
- Horton, E.W.; Jones, R.L.; Marr, C.G. Effects of aspirin on prostaglandin and fructose levels in human semen. J. Reprod. Fertil. 1973, 33, 385. [Google Scholar] [CrossRef]
- Vyas, A.; Ram, H.; Purohit, A.; Jatwa, R. Adverse effects of subchronic dose of aspirin on reproductive profile of male rats. J. Pharm. 2016, 2016, 6585430. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Korn, N.; Thurston, R.J. Prostaglandin levels in seminal plasma and sperm extracts of the domestic turkey, and the effects of cyclooxygenase inhibitors on sperm mobility. Reprod. Biol. Endocrinol. 2003, 1, 74. [Google Scholar] [CrossRef] [PubMed]
- Tanyıldızı, S.; Bozkurt, T. Effects of acetylsalicylic acid and metamizol on hyaluronidase activity and sperm characteristics in rams. Anim. Reprod. Sci. 2003, 76, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Didolkar, A.K.; Gurjar, A.; Joshi, U.M.; Sheth, A.R.; Roychowdhury, D. Effects of aspirin on blood plasma levels of testosterone, LH and FSH in maturing male rats. Int. J. Androl. 1980, 3, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Didolkar, A.K.; Patel, P.B.; Roychowdhury, D. Effect of aspirin on spermatogenesis in mature and immature rats. Int. J. Androl. 1980, 3, 585–593. [Google Scholar] [CrossRef]
- Biswas, N.M.; Sanyal, S.; Patra, P.B. Antispermatogenic Effect of Aspirin and its Prevention by Prostaglandin E2. Andrologia 1978, 10, 137–141. [Google Scholar] [CrossRef]
- Stutz, G.; Zamudio, J.; Santillán, M.E.; Vincenti, L.; De Cuneo, M.F.; Ruiz, R.D. The Effect of Alcohol, Tobacco, and Aspirin Consumption on Seminal Quality among Healthy Young Men. Arch. Environ. Health 2004, 59, 548–552. [Google Scholar] [CrossRef]
- Martini, A.C.; Molina, R.I.; Tissera, A.D.; Ruiz, R.D.; Fiol de Cuneo, M. Analysis of semen from patients chronically treated with low or moderate doses of aspirin-like drugs. Fertil. Steril. 2003, 80, 221–222. [Google Scholar] [CrossRef]
- Cenedella, R.J.; Crouthamel, W.G. Effect of aspirin upon male mouse fertility. Prostaglandins 1973, 4, 285–290. [Google Scholar] [CrossRef]
- Scott, J.E.; Persaud, T.V. A quantitative study of the effects of acetylsalicylic acid on spermatogenesis and organs of the rat. Int. J. Fertil. 1978, 23, 282–287. [Google Scholar]
- Barbosa, M.G.; Jorge, B.C.; Stein, J.; Ferreira, D.A.S.; Barreto, A.C.D.S.; Reis, A.C.C.; Moreira, S.D.S.; Inocencio, L.C.D.L.; Macorini, L.F.B.; Arena, A.C. Pre-pubertal exposure to ibuprofen impairs sperm parameters in male adult rats and compromises the next generation. J. Toxicol. Environ. Health Part A 2020, 83, 559–572. [Google Scholar] [CrossRef]
- Stutz, G.; Martini, A.C.; Ruiz, R.D.; Fiol De Cuneo, M.; Munoz, L.; Lacuara, J.L. Functional activity of mouse sperm was not affected by low doses of aspirin-like drugs. Arch. Androl. 2000, 44, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Freixa, R.; RosellóCatafau, J.; Gelpí, E.; Iglesias Cortit, J.L.; Ballescá, J.L.; de Paz, J.L.; Iglesias Guiu, J.; Gonzalez Merlo, J.; Puig Parellada, P. Comparative study of antiinflammatory drugs and sulphasalazine in relation to prostaglandin E and 19 hydroxylated prostaglandin E levels and human male fertility. Prostaglandins Leukot. Med. 1984, 16, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Lüttgenau, H.; Schlegel, W.; Krüger, S. Pharmacokinetics of non-steroidal anti-inflammatory drugs in male rabbits after acute and chronic administration and effect of chronic treatment on seminal prostaglandins, sperm quality and fertility. J. Reprod. Fertil. 1988, 82, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.; Blazaki, D. Effect of non-steroidal anti-inflammatory drugs on fertility of male rats. J. Reprod. Fertil. 1986, 76, 65–73. [Google Scholar] [CrossRef]
- Conte, D.; Nordio, M.; Ronnanelli, F.; Manganelli, F.; Giovenco, P.; Dondero, F.; Isidori, A. Role of seminal prostaglandins in male fertility. II. Effects of prostaglandin synthesis inhibition on spermatogenesis in man. J. Endocrinol. Investig. 1985, 8, 289–291. [Google Scholar] [CrossRef]
- Yegnanarayan, R.; Joglekar, G. Anti-Fertility Effect of Non-Steroidal Anti-Inflammatory Drugs. Jpn. J. Pharmacol. 1978, 28, 909–917. [Google Scholar] [CrossRef]
- Abayasekara, D.; Wathes, D. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 275–287. [Google Scholar] [CrossRef]
- Didolkar, A.K.; Sunderam, K. Arachidonic acid is involved in the regulation of hCG-induced steroidogenesis in rat Leydig cells. Life Sci. 1987, 41, 471–477. [Google Scholar] [CrossRef]
- Roldan, E.R.; Shi, Q.X. Sperm phospholipases and acrosomal exocytosis. Front. Biosci. 2007, 12, 89–104. [Google Scholar] [CrossRef]
- Mercado, E.; Villalobos, M.; Domínguez, R.; Rosado, A. Differential binding of PGE-1 and PGF-2α to the human spermatozoa membrane. Life Sci. 1978, 22, 429–436. [Google Scholar] [CrossRef]
- Tokugawa, Y.; Kunishige, I.; Kubota, Y.; Shimoya, K.; Nobunaga, T.; Kimura, T.; Saji, F.; Murata, Y.; Eguchi, N.; Oda, H.; et al. Lipocalin-type prostaglandin D synthase in human male reproductive organs and seminal plasma. Biol. Reprod. 1998, 58, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Kampfer, C.; Spillner, S.; Spinnler, K.; Schwarzer, J.U.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Köhn, F.M.; Matzkin, M.E.; et al. Evidence for an adaptation in ROS 647 scavenging systems in human peritubular testicular cells from infertility 648 patients. Int. J. Androl. 2012, 35, 793–801. [Google Scholar] [CrossRef]
- Banihani, S.A. Effect of ibuprofen on semen quality. Andrologia 2019, 51, e13228. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, D.M.; Hass, U.; Lesné, L.; Lottrup, G.; Jacobsen, P.R.; Desdoits-Lethimonier, C.; Boberg, J.; Petersen, J.H.; Toppari, J.; Jensen, T.K.; et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum. Reprod. 2011, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lind, D.V.; Main, K.M.; Kyhl, H.B.; Kristensen, D.M.; Toppari, J.; Andersen, H.R.; Andersen, M.S.; Skakkebæk, N.E.; Jensen, T.K. Maternal use of mild analgesics during pregnancy associated with reduced anogenital distance in sons: A cohort study of 1027 mother-child pairs. Hum. Reprod. 2017, 32, 223–231. [Google Scholar] [CrossRef]
- Barkay, J.; Harpaz-Kerpel, S.; Ben-Ezra, S.; Gordon, S.; Zuckerman, H. The prostaglandin inhibitor effect of antiinflammatory drugs in the therapy of male infertility. Fert. Steril. 1984, 42, 406–411. [Google Scholar] [CrossRef]
- Mazaud-Guittot, S.; Nicolaz, C.N.; Desdoits-Lethimonier, C.; Coiffec, I.; Ben Maamar, M.; Balaguer, P.; Kristensen, D.M.; Chevrier, C.; Lavoué, V.; Poulain, P.; et al. Paracetamol, Aspirin, and Indomethacin Induce Endocrine Disturbances in the Human Fetal Testis Capable of Interfering with Testicular Descent. J. Clin. Endocrinol. Metab. 2013, 98, E1757–E1767. [Google Scholar] [CrossRef]
- Marley, P.B.; Smith, C.C. Proceedings: The source and a possible function in fertility of seminal prostaglandin-like material, in the mouse. Br. J. Pharmacol. 1974, 52, 114. [Google Scholar]
- Hallesy, D.W.; Short, L.D.; Hill, R. Comparative toxicology of naproxen. Scand. J. Rheumatol. 1973, 2 (Suppl. S2), 20–28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoxha, M.; Barbonetti, A.; Zappacosta, B. Arachidonic Acid Pathways and Male Fertility: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 8207. https://doi.org/10.3390/ijms24098207
Hoxha M, Barbonetti A, Zappacosta B. Arachidonic Acid Pathways and Male Fertility: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(9):8207. https://doi.org/10.3390/ijms24098207
Chicago/Turabian StyleHoxha, Malvina, Arcangelo Barbonetti, and Bruno Zappacosta. 2023. "Arachidonic Acid Pathways and Male Fertility: A Systematic Review" International Journal of Molecular Sciences 24, no. 9: 8207. https://doi.org/10.3390/ijms24098207
APA StyleHoxha, M., Barbonetti, A., & Zappacosta, B. (2023). Arachidonic Acid Pathways and Male Fertility: A Systematic Review. International Journal of Molecular Sciences, 24(9), 8207. https://doi.org/10.3390/ijms24098207







