Polystyrene Microplastics Exacerbate Candida albicans Infection Ability In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
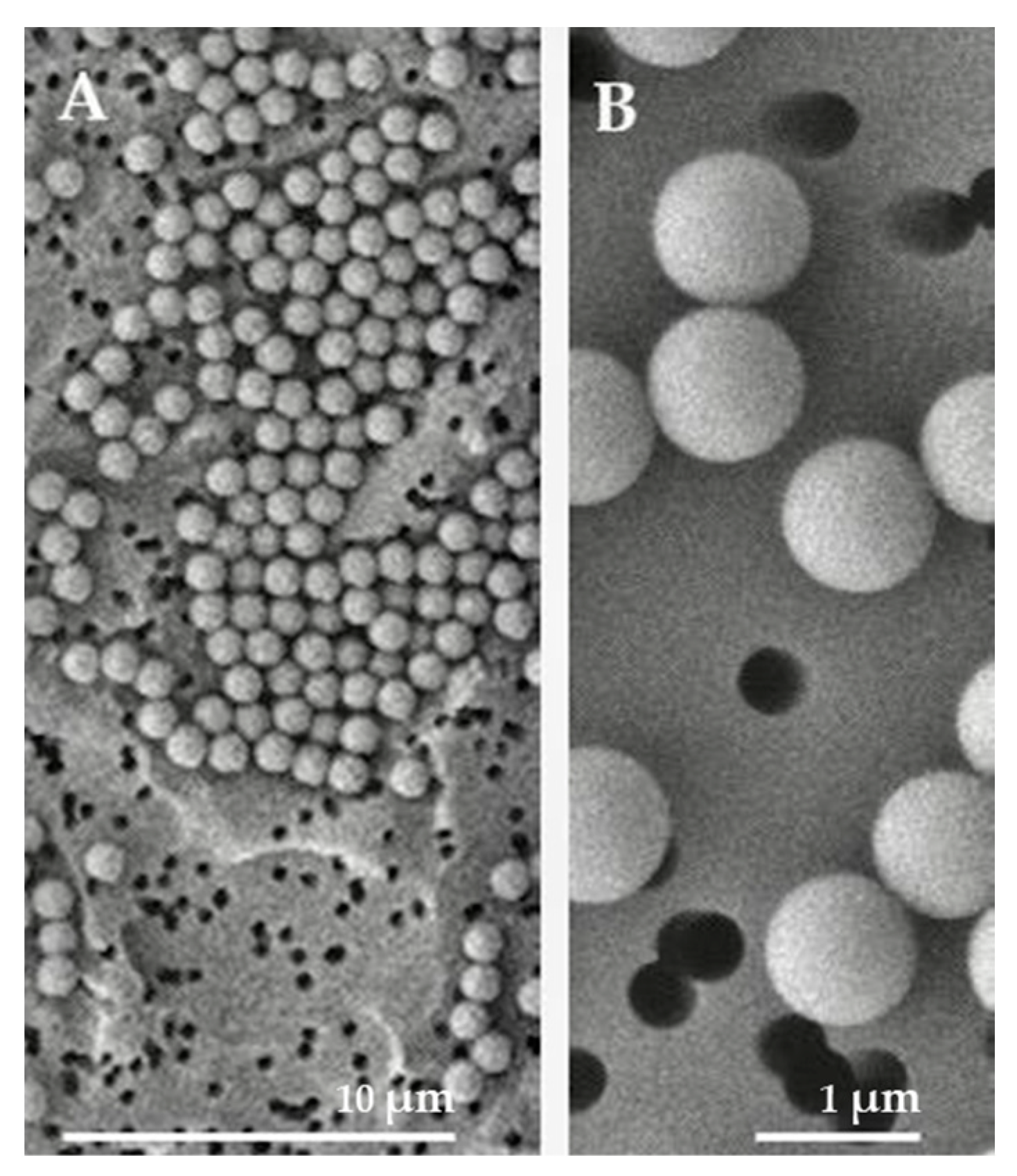
2.1. Characteristics of PSMPs
2.2. Toxic Effect of PSMPs on HT29 Cells
2.3. FACScan Results
2.4. C. albicans Adheres to PSMPs to Form a Biofilm
2.5. Evaluation of C. albicans Invasion Capability
2.6. Effect of PSMPs on the Modulation of Inflammatory Cytokine Activity in HT29 Cells
2.7. In Vivo Survival Analysis
2.8. Effects of PSMPs on the Expression of the Gene Encoding Gallerimycin and Galiomicin
2.9. Effects of PSMPs on the Gene Expression of HWP1 and ALS3
3. Discussion
4. Materials and Methods
4.1. Polystyrene Microplastics Characterization
4.2. Cell and Fungal Culture
4.3. PSMPs Cytotoxicity Assessment
4.4. Citofluorimetric Assay
4.5. Biofilm Formation Ability in Presence of PSMPs
4.6. Candida Internalization and PSMPs Exposure
4.7. Bio-Plex Assay for Cytokine Measurement
4.8. Monitoring G. mellonella Larvae
4.9. Analysis of Gallerimycin and Galiomicin Gene Expression
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Kumar, A.; Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment—Biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019, 191, 668. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Choi, D.; Bang, J.; Kim, T.; Oh, Y.; Hwang, Y.; Hong, J. In vitro chemical and physical toxicities of polystyrene microfragments in human-derived cells. J. Hazard. Mater. 2020, 400, 123308. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Ma, Y.; Koh, J.Y.C.; Lim, H.K.; Shi, P.; Tay, C.Y. Elucidating the Size-Dependency of In Vitro Digested Polystyrene Microplastics on Human Intestinal Cells Health and Function. Macromol. Chem. Phys. 2022, 223, 2100454. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. Proc. Natl. Acad. Sci. USA 2019, 116, 723–731. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, J.; Liu, Y.; Zhang, Y.; Tang, C.; Wang, X.; Wang, P.; Chen, W.; Wei, B.; Liu, M. Oral immunization of BALB/c mice with recombinant Helicobacter pylori antigens and double mutant heat-labile toxin (dmLT) induces prophylactic protective immunity against H. pylori infection. Microb. Pathog. 2020, 145, 104229. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Liu, L.; Chen, L.; Teng, J.; Zhu, X.; Zhao, J.; Wang, Q. Spatial and seasonal variations in biofilm formation on microplastics in coastal waters. Sci. Total Environ. 2021, 770, 145303. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Eo, S.; Han, G.M.; Shim, W.J. Rapid Production of Micro- and Nanoplastics by Fragmentation of Expanded Polystyrene Exposed to Sunlight. Environ. Sci. Technol. 2020, 54, 11191–11200. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.E.; Hua, T.; Sang, Q.-X.A. Effects of Polystyrene Microplastics on Human Kidney and Liver Cell Morphology, Cellular Proliferation, and Metabolism. ACS Omega 2022, 7, 34136–34153. [Google Scholar] [CrossRef] [PubMed]
- de Alteriis, E.; Maione, A.; Falanga, A.; Bellavita, R.; Galdiero, S.; Albarano, L.; Salvatore, M.M.; Galdiero, E.; Guida, M. Activity of Free and Liposome-Encapsulated Essential Oil from Lavandula angustifolia against Persister-Derived Biofilm of Candida auris. Antibiotics 2022, 11, 26. [Google Scholar] [CrossRef]
- Beck, P.; Cotton, J.; Platnich, J.; Muruve, D.; Buret, A.; Jijon, H. Interleukin-8 in gastrointestinal inflammation and malignancy: Induction and clinical consequences. Int. J. Interferon Cytokine Mediat. Res. 2016, 8, 13. [Google Scholar] [CrossRef]
- Allen, J.E. IL-4 and IL-13: Regulators and Effectors of Wound Repair. Annu. Rev. Immunol. 2023, 41, 229–254. [Google Scholar] [CrossRef]
- Dougan, M.; Dranoff, G.; Dougan, S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019, 50, 796–811. [Google Scholar] [CrossRef]
- Browne, N.; Heelan, M.; Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.-M.; Bolan, N.S.; Tsang, D.C.W.; Li, Y.; Qin, M.; Hou, D. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. J. Hazard. Mater. 2021, 401, 123415. [Google Scholar] [CrossRef] [PubMed]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; de Britto, M.; Velázquez, A.; Pastor, S.; Hernández, A.; Marcos, R.; Cortés, C. Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Abbasi, S. Routes of human exposure to micro(nano)plastics. Curr. Opin. Toxicol. 2021, 27, 41–46. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- González-Acedo, A.; García-Recio, E.; Illescas-Montes, R.; Ramos-Torrecillas, J.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J. Evidence from in vitro and in vivo studies on the potential health repercussions of micro- and nanoplastics. Chemosphere 2021, 280, 130826. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, W.; Pang, Z.; Liu, J.; Qiu, J.; Luan, T.; Deng, J.; Fang, Z. Polystyrene microplastics significantly facilitate influenza A virus infection of host cells. J. Hazard. Mater. 2023, 446, 130617. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Schneider, H.A.; Nett, J.E.; Sheppard, D.C.; Filler, S.G.; Andes, D.R.; Mitchell, A.P. Complementary adhesin function in C. albicans biofilm formation. Curr. Biol. 2008, 18, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Wong, W.F.; Madhavan, P.; Yong, V.C.; Looi, C.Y. Transcriptomic and Genomic Approaches for Unravelling Candida albicans Biofilm Formation and Drug Resistance—An Update. Genes. 2018, 9, 540. [Google Scholar] [CrossRef]
- Maione, A.; Imparato, M.; Buonanno, A.; Carraturo, F.; Schettino, A.; Schettino, M.T.; Galdiero, M.; de Alteriis, E.; Guida, M.; Galdiero, E. Anti-Biofilm Activity of Phenyllactic Acid against Clinical Isolates of Fluconazole-Resistant Candida albicans. J. Fungi 2023, 9, 355. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Allert, S.; Förster, T.M.; Svensson, C.M.; Richardson, J.P.; Pawlik, T.; Hebecker, B.; Rudolphi, S.; Juraschitz, M.; Schaller, M.; Blagojevic, M.; et al. Candida albicans-Induced Epithelial Damage Mediates Translocation through Intestinal Barriers. mBio 2018, 9. [Google Scholar] [CrossRef]
- Verrillo, M.; Cuomo, P.; Montone, A.M.I.; Savy, D.; Spaccini, R.; Capparelli, R.; Piccolo, A. Humic substances from composted fennel residues control the inflammation induced by Helicobacter pylori infection in AGS cells. PLoS ONE 2023, 18, e0281631. [Google Scholar] [CrossRef]
- Maione, A.; de Alteriis, E.; Carraturo, F.; Galdiero, S.; Falanga, A.; Guida, M.; Di Cosmo, A.; Maselli, V.; Galdiero, E. The Membranotropic Peptide gH625 to Combat Mixed Candida albicans/Klebsiella pneumoniae Biofilm: Correlation between In Vitro Anti-Biofilm Activity and In Vivo Antimicrobial Protection. J. Fungi 2021, 7, 26. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maione, A.; Norcia, M.; Sinoca, M.; Galdiero, M.; Maselli, V.; Feola, A.; Carotenuto, R.; Cuomo, P.; Capparelli, R.; Guida, M.; et al. Polystyrene Microplastics Exacerbate Candida albicans Infection Ability In Vitro and In Vivo. Int. J. Mol. Sci. 2024, 25, 12. https://doi.org/10.3390/ijms25010012
Maione A, Norcia M, Sinoca M, Galdiero M, Maselli V, Feola A, Carotenuto R, Cuomo P, Capparelli R, Guida M, et al. Polystyrene Microplastics Exacerbate Candida albicans Infection Ability In Vitro and In Vivo. International Journal of Molecular Sciences. 2024; 25(1):12. https://doi.org/10.3390/ijms25010012
Chicago/Turabian StyleMaione, Angela, Mariangela Norcia, Marica Sinoca, Marilena Galdiero, Valeria Maselli, Antonia Feola, Rosa Carotenuto, Paola Cuomo, Rosanna Capparelli, Marco Guida, and et al. 2024. "Polystyrene Microplastics Exacerbate Candida albicans Infection Ability In Vitro and In Vivo" International Journal of Molecular Sciences 25, no. 1: 12. https://doi.org/10.3390/ijms25010012






