Pan-Genome Analysis of TIFY Gene Family and Functional Analysis of CsTIFY Genes in Cucumber
Abstract
:1. Introduction
2. Results
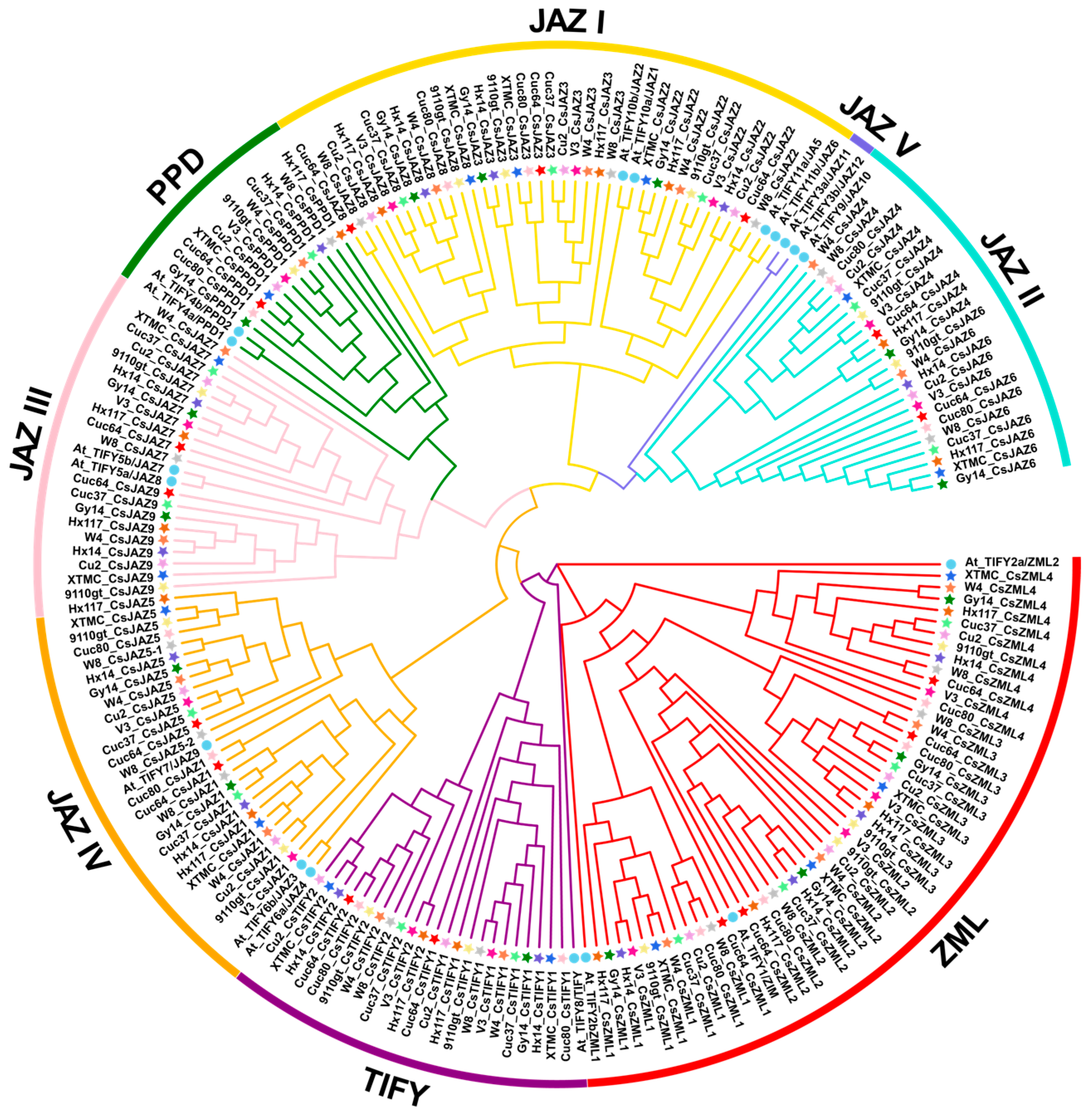
2.1. Identification of TIFY Genes Based on Cucumber Pan-Genome
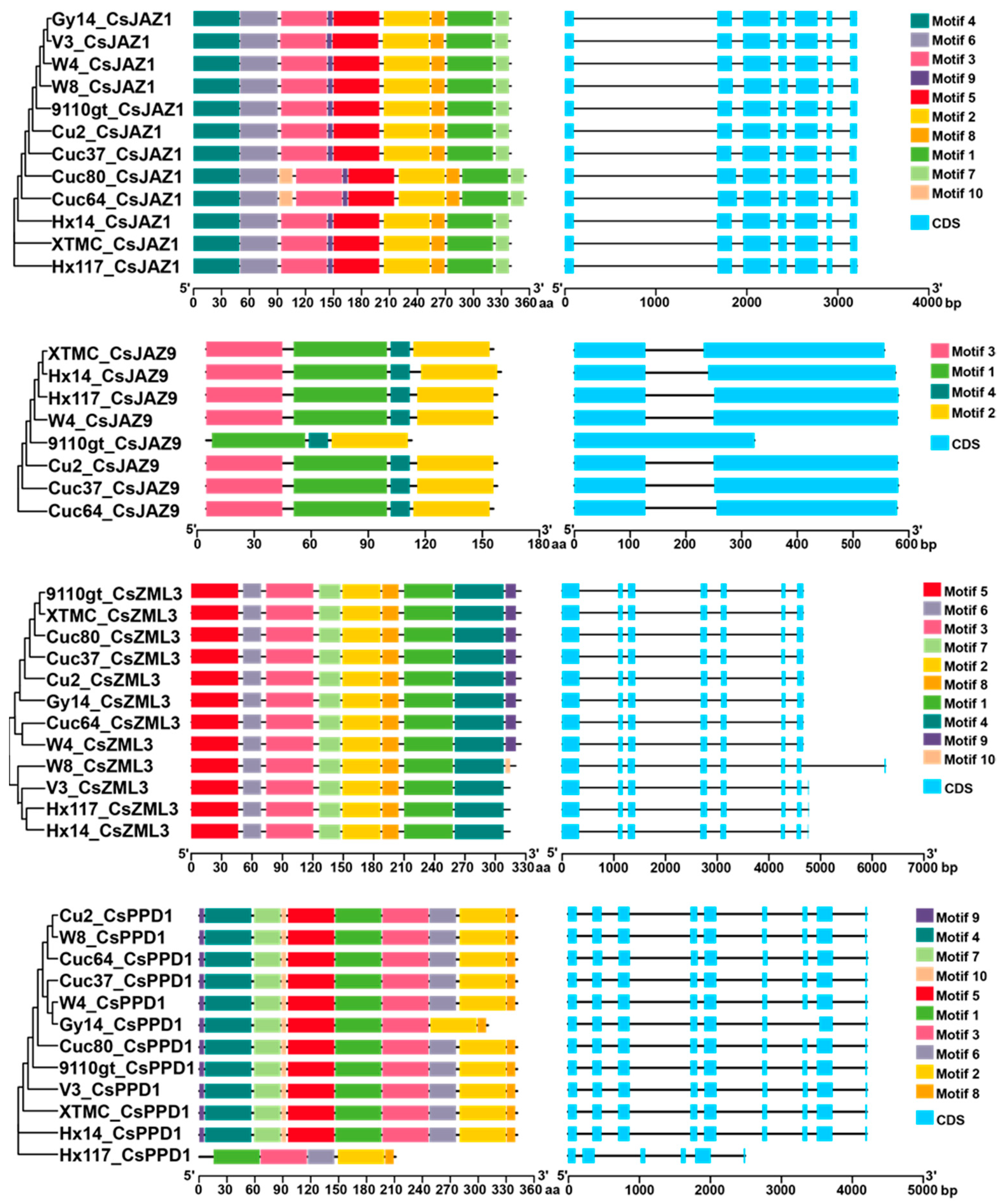
2.2. Gene Structure and Motif Composition of CsTIFYs
2.3. Chromosome Distribution and Synteny Analysis of CsTIFY Gene Family
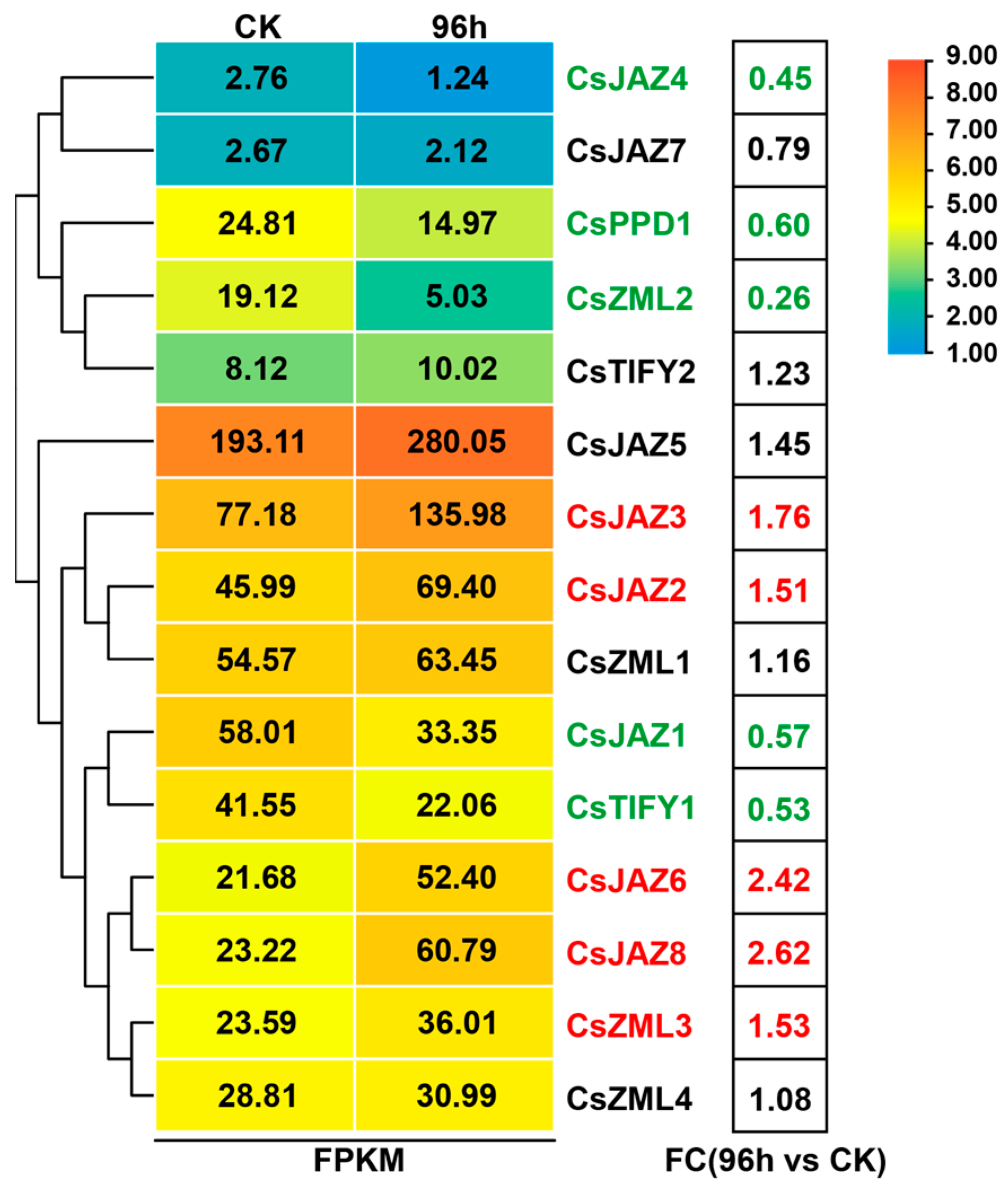
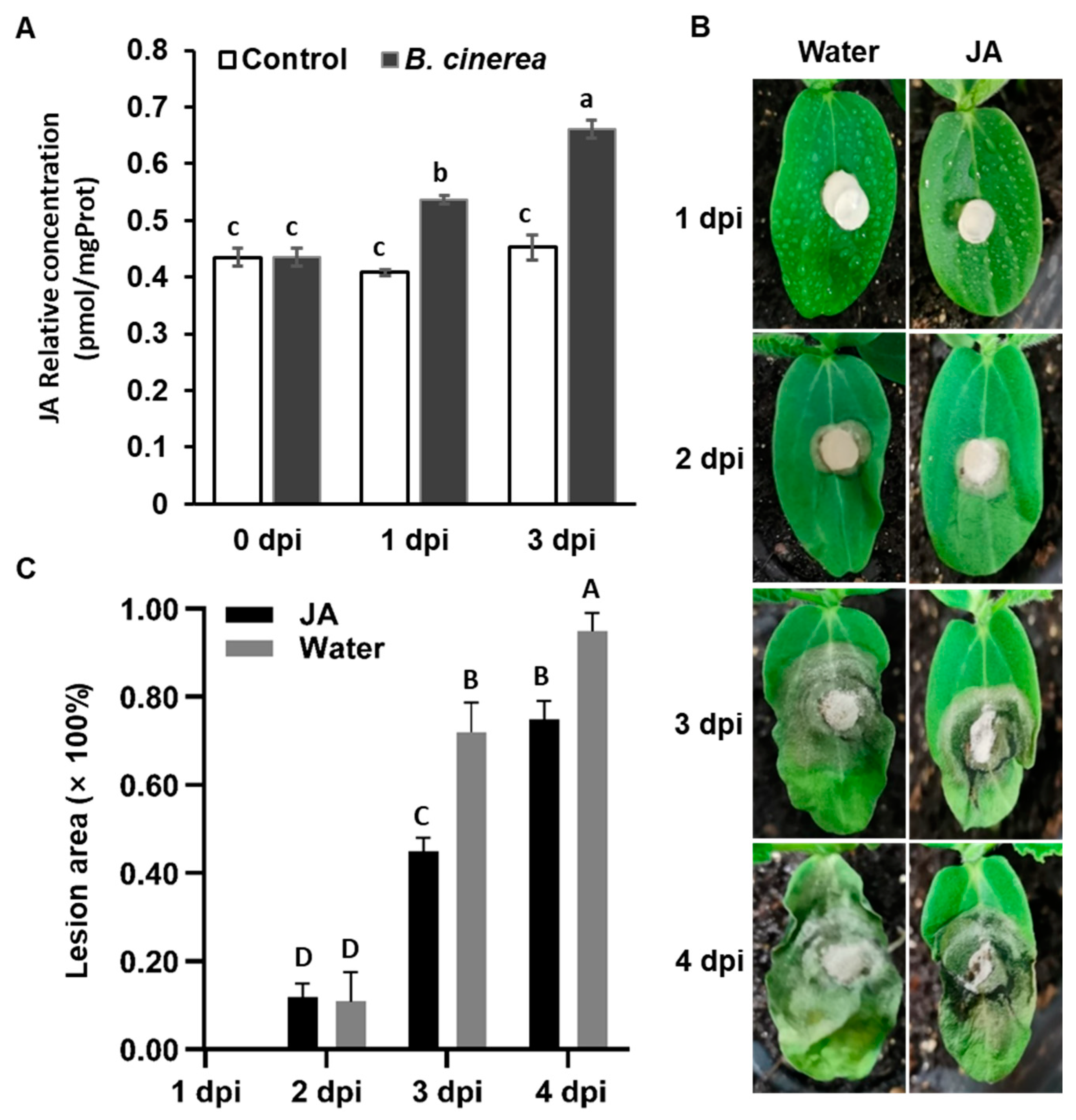
2.4. Responsive Analysis of CsTIFY Genes under Gray Mold Stress
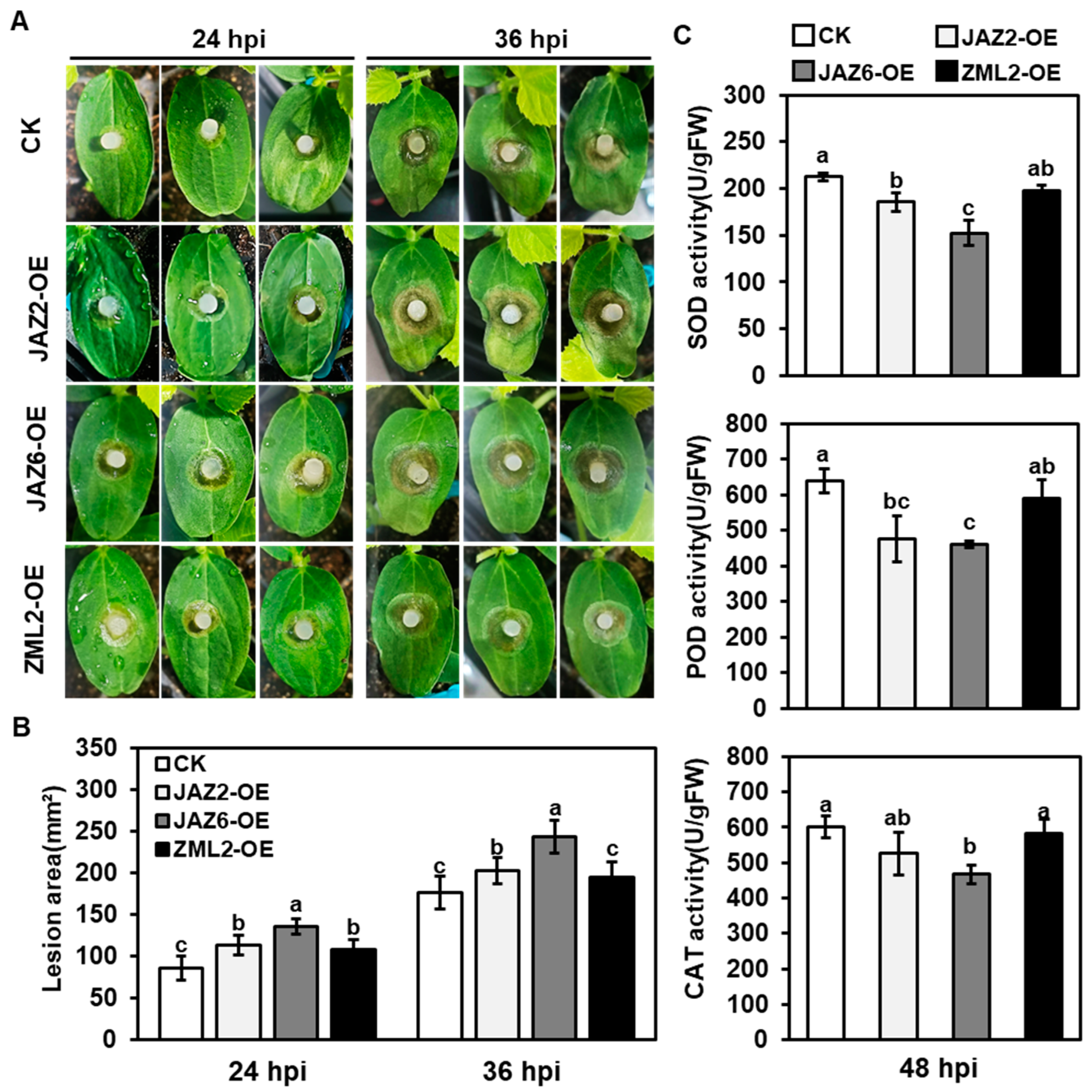
2.5. Functional Analysis of CsTIFY Genes in Resistance Response of Gray Mold
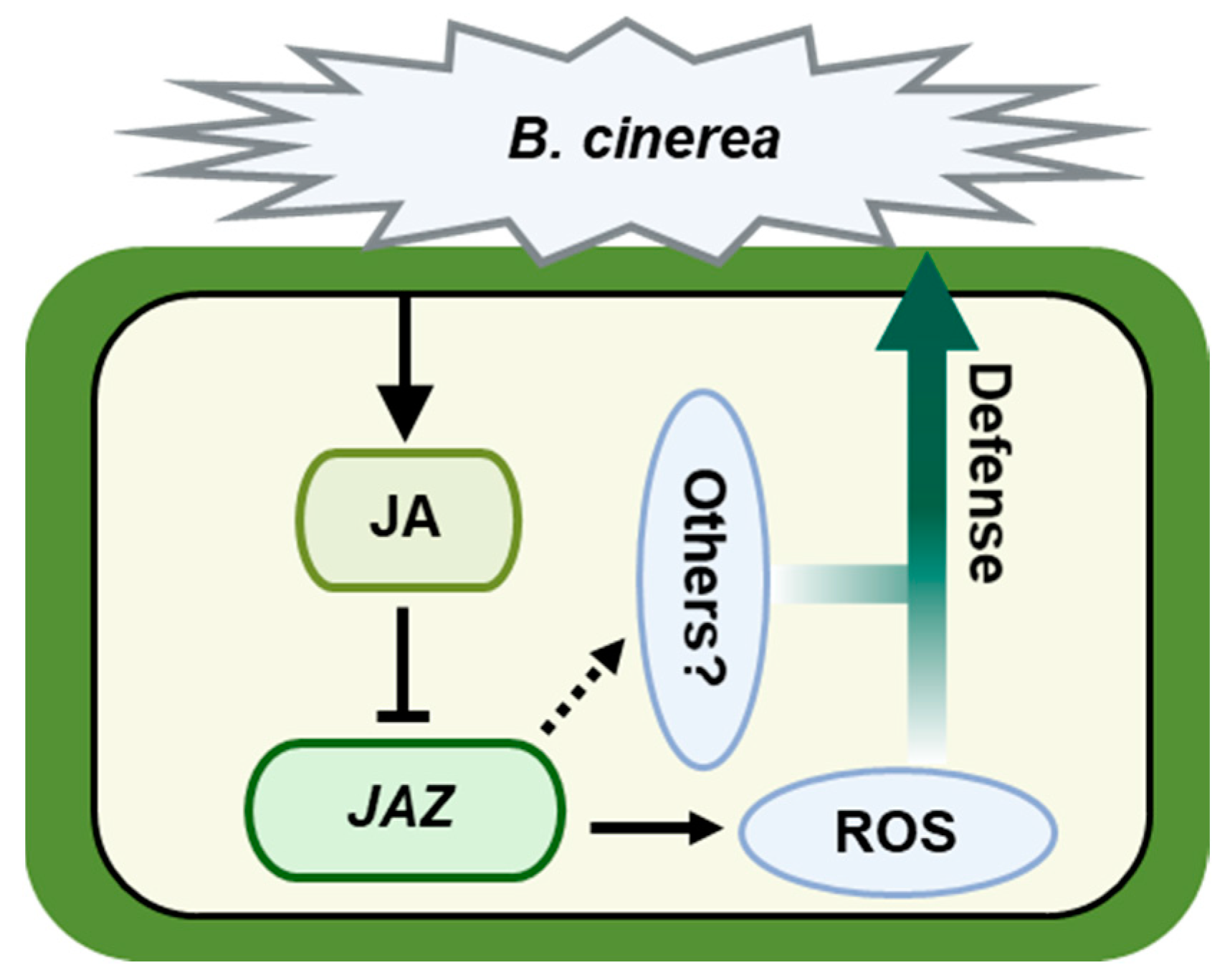
2.6. CsJAZs Regulate Resistance Response of Gray Mold via JA Pathway
3. Discussion
3.1. Bioinformatics Analysis of CsTIFYs Based on Cucumber Pan-Genome
3.2. Identification of CsTIFYs in Regulating Cucumber Resistance to Gray Mold
3.3. The Involvement of CsJAZ Genes in the JA Pathway Controls Cucumber’s Resistance to Gray Mold
4. Materials and Methods
4.1. Identification and Phylogenetic Tree Construction of TIFY Genes
4.2. Bioinformatics Analysis of CsTIFY Genes
4.3. Analysis of the Expression Pattern of CsTIFY Genes Based on Published Data
4.4. Real-Time PCR Used for Expression Analysis of CsJAZs
4.5. Construction of Recombinant Plasmids and Transient Infestation of Cucumber Cotyledons
4.6. Enzyme Activity Measurement of POD, SOD, and CAT
4.7. Determination of Plant Endogenous JA Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Y.; Kissoudis, C.; Yan, Z.; Visser, R.G.F.; van der Linden, G. Plant behaviour under combined stress: Tomato responses to combined salinity and pathogen stress. Plant J. 2018, 93, 781–793. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, M.; Zhou, S.; Guo, Q.; Chen, F.; Wu, J.; Wang, W. Overexpression of wheat ubiquitin gene, Ta-Ub2, improves abiotic stress tolerance of Brachypodium distachyon. Plant Sci. 2016, 248, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, X.; Han, J.; Lu, W.; Ren, Z. Genome-wide analysis of the WRKY gene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses. BMC Plant Biol. 2020, 20, 443. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kang, Y.; Li, W.; Liu, W.; Xie, P.; Liao, L.; Huang, L.; Yao, M.; Qian, L.; Liu, Z.; et al. Genome-wide identification and functional analysis of the TIFY gene family in the response to multiple stresses in Brassica napus L. BMC Genom. 2020, 21, 736. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.F.; Ferreira, P.C.G.; Kobayashi, A.K.; Harmon, F.G.; Nepomuceno, A.L.; Molinari, H.B.C.; Grossi-de-Sa, M.F. MicroRNAs and new biotechnological tools for its modulation and improving stress tolerance in plants. Plant Biotechnol. J. 2019, 17, 1482–1500. [Google Scholar] [CrossRef]
- Guo, T.T.; Mao, X.G.; Zhang, H.; Zhang, Y.; Fu, M.D.; Sun, Z.F.; Kuai, P.; Lou, Y.G.; Fang, Y.D. Lamin-like Proteins Negatively Regulate Plant Immunity through NAC WITH TRANSMEMBRANE MOTIF1-LIKE9 and NONEXPRESSOR OF PR GENES1 in Arabidopsis thaliana. Mol. Plant 2017, 10, 1334–1348. [Google Scholar] [CrossRef]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The transcription factor MYB115 contributes to the regulation of proanthocyanidin biosynthesis and enhances fungal resistance in poplar. New Phytol. 2017, 215, 351–367. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, M.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. Interactions of WRKY15 and WRKY33 transcription factors and their roles in the resistance of oilseed rape to Sclerotinia infection. Plant Biotechnol. J. 2018, 16, 911–925. [Google Scholar] [CrossRef]
- Fernandez-Calvo, P.; Chini, A.; Fernandez-Barbero, G.; Chico, J.M.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Pre, M.; Atallah, M.; Champion, A.; De Vos, M.; Pieterse, C.M.; Memelink, J. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 2008, 147, 1347–1357. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Ou, S.; Wang, W.; Liu, L.; Wu, Y.; Chu, C.; Wang, X. Correction to: OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol. Biol. 2018, 97, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Meng, Y.; Huang, D.; Qi, Y.; Chen, M. Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics 2011, 98, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, B.; Grunewald, W.; Bateman, A.; Kohchi, T.; Gheysen, G. The tify family previously known as ZIM. Trends Plant Sci. 2007, 12, 239–244. [Google Scholar] [CrossRef]
- White, D.W. PEAPOD regulates lamina size and curvature in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 13238–13243. [Google Scholar] [CrossRef] [PubMed]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E. JAZing up jasmonate signaling. Trends Plant Sci. 2008, 13, 66–71. [Google Scholar] [CrossRef]
- Chung, H.S.; Howe, G.A. A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell 2009, 21, 131–145. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, X.; Luo, X.; Chen, Q.; Cai, H.; Ji, W.; Zhu, Y. Identification of wild soybean (Glycine soja) TIFY family genes and their expression profiling analysis under bicarbonate stress. Plant Cell Rep. 2013, 32, 263–272. [Google Scholar] [CrossRef]
- Chini, A.; Ben-Romdhane, W.; Hassairi, A.; Aboul-Soud, M.A.M. Identification of TIFY/JAZ family genes in Solanum lycopersicum and their regulation in response to abiotic stresses. PLoS ONE 2017, 12, e0177381. [Google Scholar] [CrossRef]
- Ma, Y.; Shu, S.; Bai, S.; Tao, R.; Qian, M.; Teng, Y. Genome-wide survey and analysis of the TIFY gene family and its potential role in anthocyanin synthesis in Chinese sand pear (Pyrus pyrifolia). Tree Genet. Genomes 2018, 14, 25. [Google Scholar] [CrossRef]
- Ye, H.; Du, H.; Tang, N.; Li, X.; Xiong, L. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol. Biol. 2009, 71, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Ebel, C.; BenFeki, A.; Hanin, M.; Solano, R.; Chini, A. Characterization of wheat (Triticum aestivum) TIFY family and role of Triticum Durum TdTIFY11a in salt stress tolerance. PLoS ONE 2018, 13, e0200566. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernandez, G.; Adie, B.; Chico, J.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.; Ponce, M. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Ibanez, S.; Chini, A.; Solano, R. How Microbes Twist Jasmonate Signaling around Their Little Fingers. Plants 2016, 5, 9. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Cevik, V.; Grant, M.; Zhai, B.; Jones, J.D.; Manners, J.M.; Kazan, K. Characterization of a JAZ7 activation-tagged Arabidopsis mutant with increased susceptibility to the fungal pathogen Fusarium oxysporum. J. Exp. Bot. 2016, 67, 2367–2386. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, T.; Geng, S.; Scott, P.B.; Li, H.; Chen, S. Comparative proteomics and metabolomics of JAZ7-mediated drought tolerance in Arabidopsis. J. Proteom. 2019, 196, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Bai, M.; Sun, J.; Liu, J.; Ren, M.; Dong, Y.; Wang, N.; Ning, G.; Wang, C. RcMYB84 and RcMYB123 mediate jasmonate-induced defense responses against Botrytis cinerea in rose (Rosa chinensis). Plant J. 2020, 103, 1839–1849. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Pan, X.W.; Yu, Y.; Zhu, Y.M.; Kong, F.J.; Sun, X.; Wang, F.F. Overexpression of a TIFY family gene, GsJAZ2, exhibits enhanced tolerance to alkaline stress in soybean. Mol. Breed. 2020, 40, 1–13. [Google Scholar] [CrossRef]
- Olczak-Woltman, H.; Schollenberger, M.; Niemirowicz-Szczytt, K. Genetic background of host-pathogen interaction between Cucumis sativus L. and Pseudomonas syringae pv. lachrymans. J. Appl. Genet. 2009, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Savory, E.A.; Granke, L.L.; Quesada-Ocampo, L.M.; Varbanova, M.; Hausbeck, M.K.; Day, B. The cucurbit downy mildew pathogen Pseudoperonospora cubensis. Mol. Plant Pathol. 2011, 12, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.B.; Guo, P.; Wang, Z.Y.; Chen, C.H.; Ren, Z.H. Transcriptome profiling reveals response genes for downy mildew resistance in cucumber. Planta 2021, 253, 112. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis spp. and Diseases They Cause in Agricultural Systems—An Introduction; Springer: Dordrecht, The Netherlands, 2007; pp. 1–8. [Google Scholar]
- Kong, W.; Chen, N.; Liu, T.; Zhu, J.; Wang, J.; He, X.; Jin, Y. Large-Scale Transcriptome Analysis of Cucumber and Botrytis cinerea during Infection. PLoS ONE 2015, 10, e0142221. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.N.; Dong, S.Y.; Miao, H.; Liu, X.P.; Han, J.A.; Li, C.X.; Gu, X.F.; Zhang, S.P. Genome-Wide Identification of TIFY Genes and Their Response to Various Pathogen Infections in Cucumber (Cucumis sativus L.). Sci. Hortic. 2022, 295, 110814. [Google Scholar] [CrossRef]
- Bayer, P.E.; Golicz, A.A.; Scheben, A.; Batley, J.; Edwards, D. Plant pan-genomes are the new reference. Nat. Plants 2020, 6, 914–920. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Zheng, H.; Luo, R.; Zhu, H.; Li, Q.; Qian, W.; Ren, Y.; Tian, G.; Li, J.; et al. Building the sequence map of the human pan-genome. Nat. Biotechnol. 2010, 28, 57–63. [Google Scholar] [CrossRef]
- Gao, L.; Gonda, I.; Sun, H.H.; Ma, Q.Y.; Bao, K.; Tieman, D.M.; Burzynski-Chang, E.A.; Fish, T.L.; Stromberg, K.A.; Sacks, G.L.; et al. The tomato pan-genome uncovers new genes and a rare allele regulating fruit flavor. Nat. Genet. 2019, 51, 1044–1051. [Google Scholar] [CrossRef]
- Tao, Y.F.; Luo, H.; Xu, J.B.; Cruickshank, A.; Zhao, X.R.; Teng, F.; Hathorn, A.; Wu, X.Y.; Liu, Y.M.; Shatte, T.; et al. Extensive variation within the pan-genome of cultivated and wild sorghum. Nat. Plants 2021, 7, 766–773. [Google Scholar] [CrossRef]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S.; et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: Implications for the microbial “pan-genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Chai, S.; Yang, Z.; Zhang, Z. Graph-based pan-genome reveals structural and sequence variations related to agronomic traits and domestication in cucumber. Nat. Commun. 2022, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Qi, G.; Kong, Y.; Kong, D.; Gao, Q.; Zhou, G. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol. 2010, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Wang, N.A.; Xiang, Y.; Fang, L.C.; Wang, Y.J.; Xin, H.P.; Li, S.H. Patterns of Gene Duplication and Their Contribution to Expansion of Gene Families in Grapevine. Plant Mol. Biol. Rep. 2013, 31, 852–861. [Google Scholar] [CrossRef]
- Che, G.; Zhang, X. STAYGREEN, STAY HEALTHY: A loss-of-susceptibility mutation in the STAYGREEN gene provides durable, broad-spectrum disease resistances for over 50 years of US cucumber production. New Phytol. 2019, 47, 38–46. [Google Scholar]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Bellin, D.; Asai, S.; Delledonne, M.; Yoshioka, H. Nitric Oxide as a Mediator for Defense Responses. Mol. Plant Microbe 2013, 26, 271–277. [Google Scholar] [CrossRef]
- Zhu, Z.; An, F.; Feng, Y.; Li, P.; Xue, L.; Mu, A.; Jiang, Z.; Kim, J.M.; To, T.K.; Li, W.; et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 12539–12544. [Google Scholar] [CrossRef]
- Johnson, L.Y.D.; Major, I.T.; Chen, Y.; Yang, C.; Vanegas-Cano, L.J.; Howe, G.A. Diversification of JAZ-MYC signaling function in immune metabolism. New Phytol. 2023, 239, 2277–2291. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Gene ID 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9930-V3 | 9930-V2 | XTMC | Cu2 | Cuc37 | Cuc64 | Cuc80 | W4 | W8 | Hx14 | Hx117 | Gy14 | 9110gt | |
| CsJAZ1 | 1G007260 | 1G042920 | 1G007320 | 1G007500 | 1G007370 | 1G007340 | 1G007330 | 1G007340 | 1G007380 | 1G013550 | 1G010560 | 1G012580 | 1G007620 |
| CsJAZ2 | 1G041270 | 1G597690 | 1G046320 | 1G037640 | 1G041300 | 1G052450 | — | 1G062110 | 1G039100 | — | 1G058680 | 1G049580 | 1G041850 |
| CsJAZ3 | 3G030830 | 3G645940 | 3G047350 | 3G036860 | 3G046720 | 3G052270 | 3G042000 | 3G036650 | 3G035010 | 1G044590 | 3G055960 | 3G046570 | 3G037230 |
| CsJAZ4 | 4G002460 | 4G009880 | 4G002440 | 4G005390 | 4G002460 | 4G017990 | 4G002380 | 4G002450 | 4G002460 | 1G056790 | 4G002460 | 4G003410 | 4G003440 |
| CsJAZ5 | 5G037080 | 5G628650 | 5G058190 | 5G053120 | 5G055380 | 5G042390 | 5G061610 | 5G039340 | 5G044750 UNG162140 | 2G021820 | 5G066040 | 5G060960 | 5G045250 |
| CsJAZ6 | 6G007840 | 6G091930 | 6G009050 | 6G008870 | 6G007910 | 6G008010 | 6G012070 | 6G007970 | 6G009990 | 2G038620 | 6G008030 | 6G012110 | 6G007990 |
| CsJAZ7 | 6G051810 | 6G523460 | 6G066600 | 6G047700 | 6G048270 | 6G046340 | — | 6G045210 | 6G045460 | 2G038630 | 6G057100 | 6G058030 | 6G049490 |
| CsJAZ8 | 7G034270 | 7G448810 | 7G042890 | 7G033210 | 7G046090 | 7G037890 | 7G045520 | 7G031980 | 7G045180 | 2G041060 | 7G049570 | 7G041480 | 7G034610 |
| CsJAZ9 | — | — | 1G035110 | 1G028480 | 1G033150 | 1G042040 | — | 1G029160 | — | 3G052170 | 1G042630 | 1G038520 | 1G030630 |
| CsZML1 | 2G030170 | 2G370420 | 2G031610 | 2G030370 | 2G100170 | 2G070540 | 2G103280 | 2G035530 | 2G042710 | 3G075320 | 2G041600 | 2G038450 | 2G031870 |
| CsZML2 | 2G030180 | 2G370430 | 2G031620 | 2G030380 | 2G100180 | 2G070550 | 2G103290 | 2G035540 | 2G042720 | 5G059690 | 2G041610 | 2G038460 | 2G031880 |
| CsZML3 | 7G006810 | 7G064580 | 7G010060 | 7G006730 | 7G005610 | 7G002370 | 7G005770 | 7G005620 | 7G010880 | 6G011910 | 7G010800 | 7G006710 | 7G007870 |
| CsZML4 | 7G033740 | 7G447800 | 7G042380 | 7G032680 | 7G045570 | 7G037360 | 7G044990 | 7G031470 | 7G044650 | 6G064770 | 7G049040 | 7G040950 | 7G034070 |
| CsTIFY1 | 2G031660 | 2G379290 | 2G034120 | 2G032830 | 2G101610 | 2G071990 | 2G104740 | 2G037020 | 2G044220 | 7G008850 | 2G044080 | 2G039930 | 2G033380 |
| CsTIFY2 | 3G046630 | 3G878900 | 3G069390 | 3G057580 | 3G062760 | 3G068300 | 3G058900 | 3G053660 | 3G052280 | 7G043050 | 3G073290 | — | 3G054610 |
| CsPPD1 | 2G013410 | 2G222060 | 2G013060 | 2G014060 | 2G011860 | 2G011850 | 2G015800 | 2G014790 | 2G020910 | 7G043570 | 2G017780 | 2G019980 | 2G015010 |
| Protein Number | 9930-V3 | XTMC | Cu2 | Cuc80 | Cuc64 | W4 | W8 | Hx14 | Hx117 | Cuc37 | Gy14 | 9110gt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CsJAZ1 | 339 | 340 | 340 | 356 | 356 | 340 | 340 | 340 | 340 | 340 | 340 | 340 |
| CsJAZ2 | 231 | 231 | 169 | — | 231 | 231 | 231 | 231 | 231 | 231 | 231 | 231 |
| CsJAZ3 | 209 | 209 | 209 | 209 | 209 | 209 | 209 | 209 | 209 | 209 | 209 | 209 |
| CsJAZ4 | 200 | 200 | 200 | 200 | 190 | 200 * | 200 | — | 190 | 200 | 190 | 200 |
| CsJAZ5 | 381 | 381 | 381 | 381 | 381 | 381 | 381 | 381 | 381 | 381 | 381 | 381 |
| CsJAZ6 | 184 | 184 | 184 | 184 | 184 | 184 | 184 | 184 | 184 | 184 | 184 | 184 |
| CsJAZ7 | 132 | 132 | 132 | — | 130 | 130 | 130 | 132 | 132 | 132 | 132 | 132 |
| CsJAZ8 | 295 | 295 | 295 | 295 | 295 | 295 | 295 | 295 | 295 | 295 | 295 | 295 |
| CsJAZ9 | — | 150 | 152 | — | 150 | 152 | — | 154 | 154 | 152 | 152 | 107 |
| CsZML1 | 352 | 352 | 352 | 352 * | 352 | 352 | 352 | 352 | 352 | 352 | 352 | 352 |
| CsZML2 | 279 | 293 | 293 | 293 | 293 | 293 | 293 | 293 | 293 | 293 | 293 * | 293 |
| CsZML3 | 313 | 321 * | 321 | 321 | 321 | 321 | 316 | 313 | 313 | 321 | 321 | 321 |
| CsZML4 | 303 | 303 | 303 | 332 | 303 | 303 | 303 | 303 | 303 | 303 | 303 | 303 |
| CsTIFY1 | 274 | 274 * | 274 * | 274 * | 274 * | 274 * | 274 * | 274 * | 274 * | 274 * | 274 * | 274 * |
| CsTIFY2 | 376 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 376 | 400 | — | 400 |
| CsPPD1 | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 336 | 207 | 336 | 305 | 336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Xu, H.; Gao, X.; Lu, Y.; Wang, L.; Ren, Z.; Chen, C. Pan-Genome Analysis of TIFY Gene Family and Functional Analysis of CsTIFY Genes in Cucumber. Int. J. Mol. Sci. 2024, 25, 185. https://doi.org/10.3390/ijms25010185
Liu K, Xu H, Gao X, Lu Y, Wang L, Ren Z, Chen C. Pan-Genome Analysis of TIFY Gene Family and Functional Analysis of CsTIFY Genes in Cucumber. International Journal of Molecular Sciences. 2024; 25(1):185. https://doi.org/10.3390/ijms25010185
Chicago/Turabian StyleLiu, Kun, Haiyu Xu, Xinbin Gao, Yinghao Lu, Lina Wang, Zhonghai Ren, and Chunhua Chen. 2024. "Pan-Genome Analysis of TIFY Gene Family and Functional Analysis of CsTIFY Genes in Cucumber" International Journal of Molecular Sciences 25, no. 1: 185. https://doi.org/10.3390/ijms25010185
APA StyleLiu, K., Xu, H., Gao, X., Lu, Y., Wang, L., Ren, Z., & Chen, C. (2024). Pan-Genome Analysis of TIFY Gene Family and Functional Analysis of CsTIFY Genes in Cucumber. International Journal of Molecular Sciences, 25(1), 185. https://doi.org/10.3390/ijms25010185






