Molecular and Medical Aspects of Psychedelics
Abstract
:1. Introduction
2. Classification of Psychedelics
3. The Mechanism of Action of Psychedelics
3.1. 5-HT2A Receptor as a Primary Target for Psychedelics
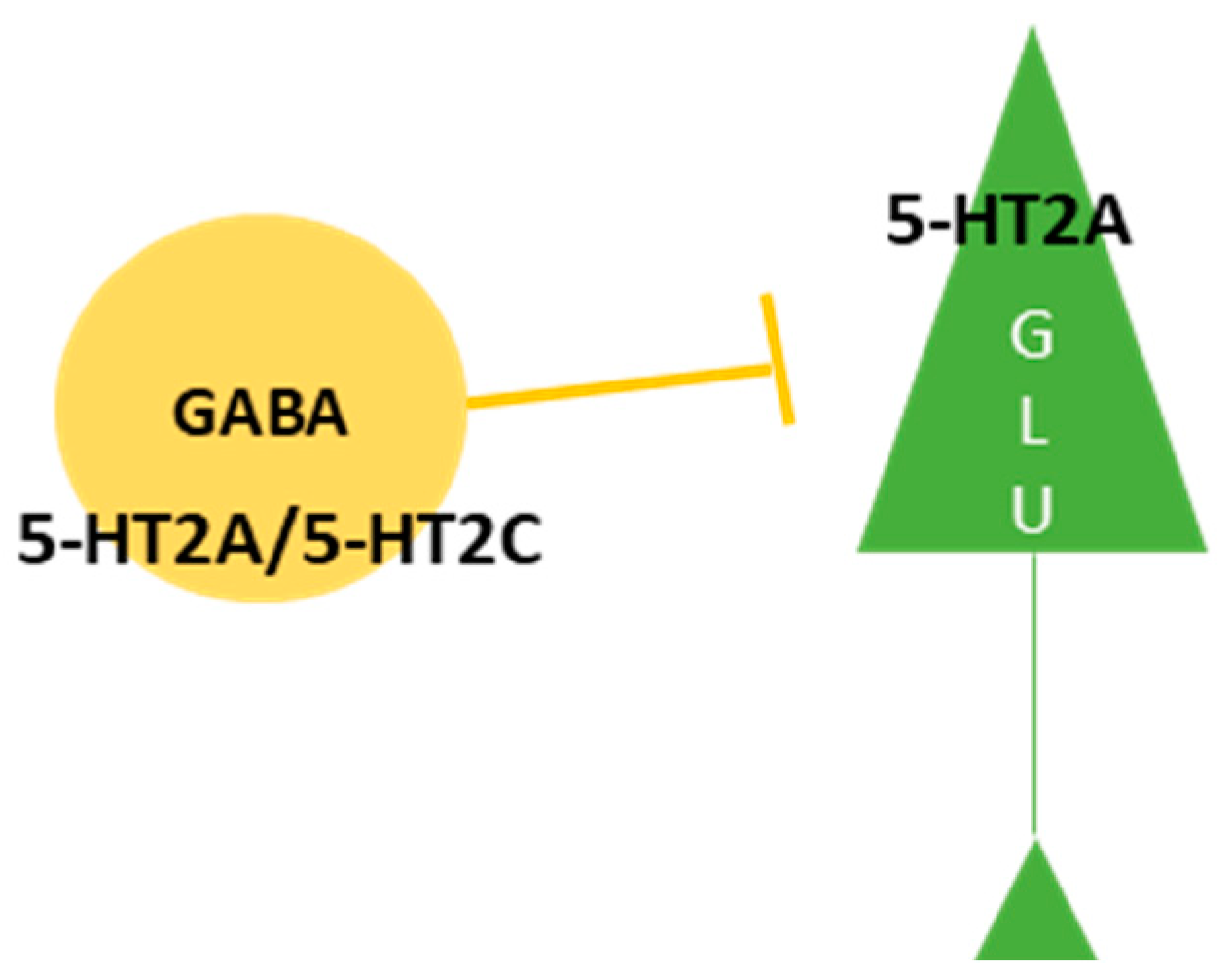
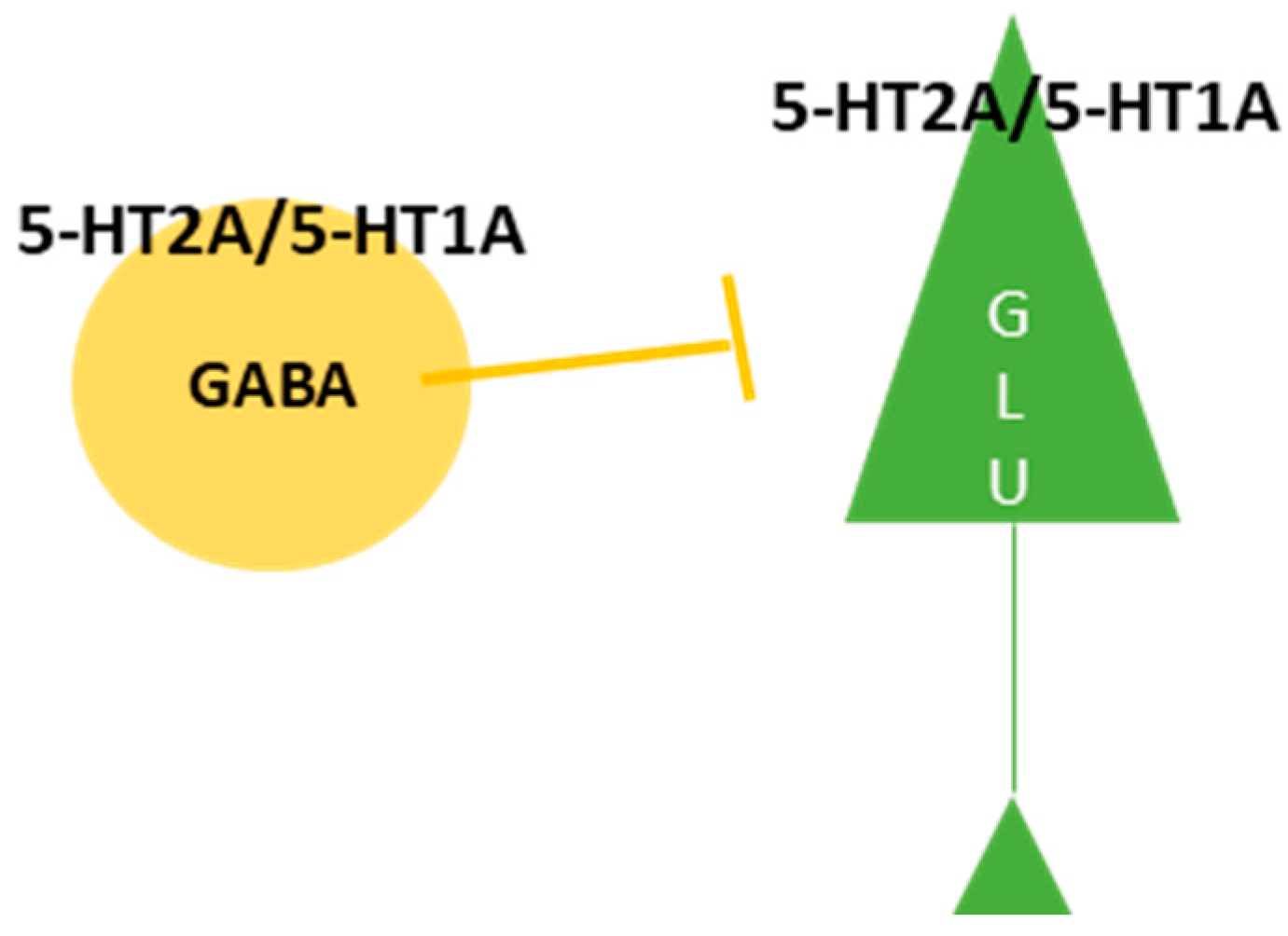
3.2. The Impact of 5-HT2A Receptor Activation on Behavior
4. NBOMes
4.1. The Effects of NBOMes on Behavior Observed in Rodents
4.2. The Effects of NBOMes Observed in Humans
4.3. The Effects Exerted on Neurotransmitter Release by a Single Exposure to NBOMes
4.4. The Consequences of Repeated Exposure to NBOMes
5. Psilocybin
5.1. History of Psilocybin
5.2. Pharmacology of Psilocybin
5.3. The Effects of Psilocybin Observed in Animal Studies
5.4. The Effects of Psilocybin Observed in Humans
5.5. The Effects of Psilocybin as a Fast-Acting Antidepressant
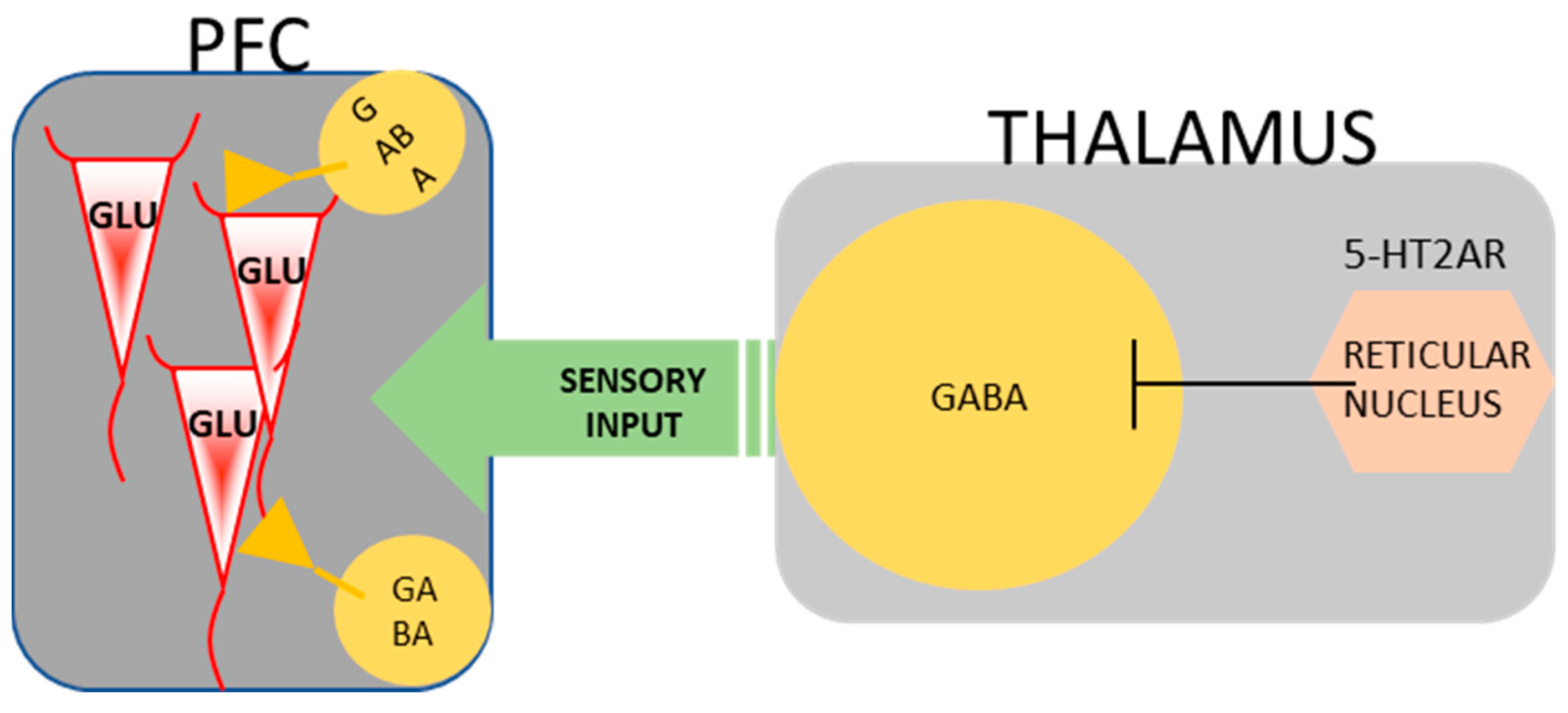
5.6. The Effect of Psilocybin Exerted on Cortical and Thalamic Neurotransmitters, Receptors, and Behavior
5.7. The Limbic Response to Psilocybin
5.8. The Therapeutic Potential of Psychedelics
6. Conclusions and Future Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schultes, R.E.; Hofmann, A.; Rätsch, C. Plants of the Gods: Their Sacred, Healing, and Hallucinogenic Powers. Heal. Art. Press. 1998, 1–208. [Google Scholar] [CrossRef]
- Nichols, D.E.; Walter, H. The History of Psychedelics in Psychiatry. Pharmacopsychiatry 2021, 54, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A. How LSD originated. J. Psychedelic Drugs 1979, 11, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.E. Modern Clinical Research on LSD. Neuropsychopharmacology 2017, 42, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Seybert, C.; Cotovio, G.; Madeira, L.; Ricou, M.; Pires, A.M.; Oliviera-Maia, A.J. Psychedelic treatments for mental health conditions pose challenges for informed consent. Nat. Med. Comment. 2023, 29, 2167–2170. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The neural basis of psychedelic action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef]
- Pierce, P.A.; Peroutka, S.J. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology 1989, 97, 118–122. [Google Scholar] [CrossRef]
- Titeler, M.; Lyon, R.A.; Glennon, R.A. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology 1988, 94, 213–216. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef]
- Nichols, D.E. Structure-activity relationships of serotonin 5-HT2A agonists. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 559–579. [Google Scholar] [CrossRef]
- Hill, S.L.; Thomas, S.H. Clinical toxicology of newer recreational drugs. Clin. Toxicol. 2011, 49, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A.; Titeler, M.; McKenney, J.D. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984, 35, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.M.; Carvalho, F.; Bastos, M.D.L.; Guedes de Pinho, P.; Carvalho, M. The hallucinogenic world of tryptamines: An updated review. Arch. Toxicol. 2015, 89, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Geyer, M.A. Effect of Hallucinogens on Unconditioned Behavior. Curr. Top. Behav. Neurosci. 2018, 36, 159–199. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.S. Psychedelics and the human receptorome. PLoS ONE 2010, 5, e9019. [Google Scholar] [CrossRef]
- Giacomelli, S.; Palmery, M.; Romanelli, L.; Cheng, C.Y.; Silvestrini, B. Lysergic acid diethylamide (LSD) is a partial agonist of D2 dopaminergic receptors and it potentiates dopamine-mediated prolactin secretion in lactotrophs in vitro. Life Sci. 1998, 63, 215–222. [Google Scholar] [CrossRef]
- Watts, V.J.; Lawler, C.P.; Fox, D.R.; Neve, K.A.; Nichols, D.E.; Mailman, R.B. LSD and structural analogs: Pharmacological evaluation at D1 dopamine receptors. Psychopharmacology 1995, 118, 401–409. [Google Scholar] [CrossRef]
- Rickli, A.; Luethi, D.; Reinisch, J.; Buchy, D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 2015, 99, 546–553. [Google Scholar] [CrossRef]
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharm. 2016, 26, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, G.K.; Marek, G.J. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 1997, 36, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Sipes, T.E.; Geyer, M.A. DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT(2A) and not by 5-HT(2C) receptors. Behav. Pharmacol. 1995, 6, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Wing, L.L.; Tapson, G.S.; Geyer, M.A. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology 1990, 100, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Correction: Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 2019, 44, 1336–1337. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Smallridge, J.W. Classic Psychedelic Drugs: Update on Biological Mechanisms. Pharmacopsychiatry 2022, 55, 121–138. [Google Scholar] [CrossRef]
- Barrett, F.S.; Krimmel, S.R.; Griffiths, R.R.; Seminowicz, D.A.; Mathur, B.N. Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. Neuroimage 2020, 218, 116980. [Google Scholar] [CrossRef]
- Kraehenmann, R.; Prellerm, K.H.; Scheidegger, M.; Pokorny, T.; Bosch, O.G.; Seifritz, E.; Vollenweider, F.X. Psilocybin-Induced Decrease in Amygdala Reactivity Correlates with Enhanced Positive Mood in Healthy Volunteers. Biol. Psychiatry 2015, 78, 572–581. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Hellyer, P.J.; Shanahan, M.; Feilding, A.; Tagliazucchi, E.; Chialvo, D.R.; Nutt, D. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef]
- Aghajanian, G.K.; Marek, G.J. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain Res. Brain Res. Rev. 2000, 31, 302–312. [Google Scholar] [CrossRef]
- Béïque, J.C.; Imad, M.; Mladenovic, L.; Gingrich, J.A.; Andrade, R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 9870–9875. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Nichols, C.D. Psychedelics Recruit Multiple Cellular Types and Produce Complex Transcriptional Responses Within the Brain. EBioMedicine 2016, 11, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Geyer, M.A. Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology 2013, 227, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Braden, M.R.; Parrishm, J.C.; Naylor, J.C.; Nichols, D.E. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol. Pharmacol. 2006, 70, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, A.J.; Wolfrum, K.M.; Reed, J.F.; Kim, S.O.; Johnson, R.A.; Janowsky, A. Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors. Biochem. Pharmacol. 2018, 158, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Geyer, M.A. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 2014, 77, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, A.; Herian, M.; Skawski, M.; Sobocińska, M.; González-Marín, A.; Noworyta-Sokołowska, K.; Gołembiowska, K. Neurochemical and Behavioral Effects of a New Hallucinogenic Compound 25B-NBOMe in Rats. Neurotox. Res. 2021, 39, 305–326. [Google Scholar] [CrossRef]
- Custodio, R.J.P.; Sayson, L.V.; Botanas, C.J.; Abiero, A.; You, K.Y.; Kim, M.; Lee, H.J.; Yoo, S.Y.; Lee, K.W.; Lee, Y.S.; et al. 25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential. Addict. Biol. 2020, 25, e12850. [Google Scholar] [CrossRef]
- Gatch, M.B.; Dolan, S.B.; Forster, M.J. Locomotor and discriminative stimulus effects of four novel hallucinogens in rodents. Behav. Pharmacol. 2017, 28, 375–385. [Google Scholar] [CrossRef]
- Halberstadt, A.L. Pharmacology and Toxicology of N-Benzylphenethylamine (“NBOMe”) Hallucinogens. Curr. Top. Behav. Neurosci. 2017, 32, 283–311. [Google Scholar] [CrossRef]
- Tirri, M.; Bilel, S.; Arfè, R.; Corli, G.; Marchetti, B.; Bernardi, T.; Boccuto, F.; Serpelloni, G.; Botrè, F.; De-Giorgio, F.; et al. Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison with the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs. Front. Psychiatry 2022, 13, 875722. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; van der Heijden, I.; Ruderman, M.A.; Risbrough, V.B.; Gingrich, J.A.; Geyer, M.A.; Powell, S.B. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 2009, 34, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Kim, Y.H.; Kim, S.J.; Suh, S.K.; Cha, H.J. Abuse potential of 2-(4-iodo-2, 5-dimethoxyphenyl)N-(2-methoxybenzyl)ethanamine (25INBOMe); in vivo and ex vivo approaches. Neurochem. Int. 2019, 125, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Hur, K.H.; Ko, Y.H.; Kim, K.; Lee, B.R.; Kim, Y.J.; Kim, S.K.; Kim, S.E.; Lee, Y.S.; Kim, H.C.; et al. A novel designer drug, 25N-NBOMe, exhibits abuse potential via the dopaminergic system in rodents. Brain Res. Bull. 2019, 152, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Hur, K.H.; Hwang, S.B.; Lee, S.; Lee, S.Y.; Jang, C.G. Designer Drug, 25D-NBOMe, Has Reinforcing and Rewarding Effects through Change of a Dopaminergic Neurochemical System. ACS Chem. Neurosci. 2023, 14, 2658–2666. [Google Scholar] [CrossRef]
- Herian, M.; Skawski, M.; Wojtas, A.; Sobocińska, M.K.; Noworyta, K.; Gołembiowska, K. Tolerance to neurochemical and behavioral effects of the hallucinogen 25I-NBOMe. Psychopharmacology 2021, 238, 2349–2364. [Google Scholar] [CrossRef]
- Ford, C.P. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef]
- Poklis, J.L.; Rasom, S.A.; Alfordm, K.N.; Poklism, A.; Peacem, M.R. Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper. J. Anal. Toxicol. 2015, 39, 617–623. [Google Scholar] [CrossRef]
- Zuba, D.; Sekuła, K.; Buczek, A. 25C-NBOMe—New potent hallucinogenic substance identified on the drug market. Forensic Sci. Int. 2013, 227, 7–14. [Google Scholar] [CrossRef]
- Al-Imam, A.; AbdulMajeed, B.A. NBOMe Compounds: Systematic Review and Data Crunching of the Surface Web. Glob. J. Health Sci. 2017, 9, 126. [Google Scholar] [CrossRef]
- Gee, P.; Schep, L.J.; Jensen, B.P.; Moore, G.; Barrington, S. Case series: Toxicity from 25B-NBOMe—A cluster of N-bomb cases. Clin. Toxicol. 2016, 54, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, P.; Papoutsis, I.; Stefanidou, M.; Spiliopoulou, C.; Athanaselis, S. 2C-I-NBOMe, an “N-bomb” that kills with “Smiles”. Toxicological and legislative aspects. Drug Chem. Toxicol. 2015, 38, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Muschamp, J.W.; Regina, M.J.; Hull, E.M.; Winter, J.C.; Rabin, R.A. Lysergic acid diethylamide and [-]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004, 1023, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Noworyta-Sokołowska, K.; Kamińska, K.; Kreiner, G.; Rogóż, Z.; Gołembiowska, K. Neurotoxic Effects of 5-MeO-DIPT: A Psychoactive Tryptamine Derivative in Rats. Neurotox. Res. 2016, 30, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Herian, M.; Wojtas, A.; Kamińska, K.; Świt, P.; Wach, A.; Gołembiowska, K. Hallucinogen-Like Action of the Novel Designer Drug 25I-NBOMe and Its Effect on Cortical Neurotransmitters in Rats. Neurotox. Res. 2019, 36, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Poulie, C.B.M.; Jensen, A.A.; Halberstadt, A.L.; Kristensen, J.L. DARK Classics in Chemical Neuroscience: NBOMes. ACS Chem. Neurosci. 2020, 11, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Kacela, M.; Adamowicz, P. NBOMes-Highly Potent and Toxic Alternatives of LSD. Front. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Miliano, C.; Marti, M.; Pintori, N.; Castelli, M.P.; Tirri, M.; Arfè, R.; De Luca, M.A. Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe. Front. Pharmacol. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Miner, L.A.; Backstrom, J.R.; Sanders-Bush, E.; Sesack, S.R. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience 2003, 116, 107–117. [Google Scholar] [CrossRef]
- Scruggs, J.L.; Schmidt, D.; Deutch, A.Y. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci. Lett. 2003, 346, 137–140. [Google Scholar] [CrossRef]
- Abi-Saab, W.M.; Bubser, M.; Roth, R.H.; Deutch, A.Y. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology 1999, 20, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, A.; Bysiek, A.; Wawrzczak-Bargiela, A.; Szych, Z.; Majcher-Maślanka, I.; Herian, M.; Maćkowiak, M.; Gołembiowska, K. Effect of Psilocybin and Ketamine on Brain Neurotransmitters, Glutamate Receptors, DNA and Rat Behavior. Int. J. Mol. Sci. 2022, 23, 6713. [Google Scholar] [CrossRef] [PubMed]
- Herian, M.; Wojtas, A.; Maćkowiak, M.; Wawrzczak-Bargiela, A.; Solarz, A.; Bysiek, A.; Madej, K.; Gołembiowska, K. Neurotoxicological profile of the hallucinogenic compound 25I-NBOMe. Sci. Rep. 2022, 12, 2939. [Google Scholar] [CrossRef] [PubMed]
- Quirion, R.; Richard, J.; Dam, T.V. Evidence for the existence of serotonin type-2 receptors on cholinergic terminals in rat cortex. Brain Res. 1985, 333, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.G.; Gudelsky, G.A. Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse 2004, 53, 202–207. [Google Scholar] [CrossRef]
- Abramson, H.; Jarvik, M.; Govin, M.; Hirsch, M. Lysergic acid diethylamide (LSD-25) antagonists. II. Development of tolerance in man to LSD-25 by prior administration of MLD-41 (1-methyl-d-lysergic acid diethylamide). AMA Arch. Neurol. Psychiatry 1958, 79, 201–207. [Google Scholar] [CrossRef]
- Angrist, B.; Rotrosen, J.; Gershon, S. Assessment of tolerance to the hallucinogenic effects of DOM. Psychopharmacologia 1974, 36, 203–207. [Google Scholar] [CrossRef]
- Wojtas, A.; Herian, M.; Maćkowiak, M.; Solarz, A.; Wawrzczak-Bargiela, A.; Bysiek, A.; Noworyta, K.; Gołembiowska, K. Hallucinogenic activity, neurotransmitters release, anxiolytic and neurotoxic effects in Rat’s brain following repeated administration of novel psychoactive compound 25B-NBOMe. Neuropharmacology 2023, 240, 109713. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Wrona, M.Z.; Dryhurst, G. Oxidation of serotonin by superoxide radical: Implications to neurodegenerative brain disorders. Chem. Res. Toxicol. 1998, 11, 639–650. [Google Scholar] [CrossRef]
- Xu, P.; Qiu, Q.; Li, H.; Yan, S.; Yang, M.; Naman, C.B.; Wang, Y.; Zhou, W.; Shen, H.; Cui, W. 25C-NBOMe, a Novel Designer Psychedelic, Induces Neurotoxicity 50 Times More Potent Than Methamphetamine In Vitro. Neurotox. Res. 2019, 35, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, G.; Maeda, H.; Kikura-Hanajiri, R.; Yoshida, K.I.; Hayashi, Y.K. The psychoactive drug 25B-NBOMe recapitulates rhabdomyolysis in zebrafish larvae. Forensic Toxicol. 2017, 35, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, G.; Jacobs, J.Q.; Ramírez-Guillén, F.; Murrieta, D.; Gándara, E. The taxonomy of Psilocybe fagicola-complex. J. Microbiol. 2005, 43, 158–165. [Google Scholar] [PubMed]
- Wasson, R.G. Secret of “Divine Mushrooms”. Life . 13 May 1957, pp. 100–120. Available online: https://www.cuttersguide.com/pdf/Periodical-Publications/life-by-time-inc-published-may-13-1957.pdf (accessed on 16 November 2023).
- Carod-Artal, F.J. Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurologia 2015, 30, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Griffiths, R.R.; Hendricks, P.S.; Henningfield, J.E. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 2018, 142, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Frey, A.; Ott, H.; Petrzilka, T.; Troxler, F. Elucidation of the structure and the synthesis of psilocybin. Experientia 1958, 14, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Horita, A.; Weber, L.J. Dephosphorylation of psilocybin to psilocin by alkaline phosphatase. Proc. Soc. Exp. Biol. Med. 1961, 106, 32–34. [Google Scholar] [CrossRef]
- Horita, A.; Weber, L.J. The enzymic dephosphorylation and oxidation of psilocybin and psilocin by mammalian tissue homogenates. Biochem. Pharmacol. 1961, 7, 47–54. [Google Scholar] [CrossRef]
- Hasler, F.; Bourquin, D.; Brenneisen, R.; Bär, T.; Vollenweider, F.X. Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm. Acta Helv. 1997, 72, 175–184. [Google Scholar] [CrossRef]
- Eivindvik, K.; Rasmussen, K.E.; Sund, R.B. Handling of psilocybin and psilocin by everted sacs of rat jejunum and colon. Acta Pharm. Nord. 1989, 1, 295–302. [Google Scholar]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Horita, A. Some biochemical studies on psilocybin and psilogin. J. Neuropsychiatry 1963, 4, 270–273. [Google Scholar]
- Tylš, F.; Páleníček, T.; Horáček, J. Psilocybin—Summary of knowledge and new perspectives. Eur. Neuropsychopharmacol. 2014, 24, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Kalberer, F.; Kreis, W.; Rutschmann, J. The fate of psilocin in the rat. Biochem. Pharmacol. 1962, 11, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lindenblatt, H.; Krämer, E.; Holzmann-Erens, P.; Gouzoulis-Mayfrank, E.; Kovar, K.A. Quantitation of psilocin in human plasma by high-performance liquid chromatography and electrochemical detection: Comparison of liquid-liquid extraction with automated on-line solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 1998, 709, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Preller, K.H.; Pokorny, T.; Hock, A.; Kraehenmann, R.; Stämpfli, P.; Seifritz, E.; Scheidegger, M.; Vollenweider, F.X. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc. Natl. Acad. Sci. USA 2016, 113, 5119–5124. [Google Scholar] [CrossRef]
- Preller, K.H.; Herdener, M.; Pokorny, T.; Planzer, A.; Kraehenmann, R.; Stämpfli, P.; Liechti, M.E.; Seifritz, E.; Vollenweider, F.X. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr. Biol. 2017, 27, 451–457. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vontobel, P.; Hell, D.; Leenders, K.L. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man—A PET study with [11C]raclopride. Neuropsychopharmacology 1999, 20, 424–433. [Google Scholar] [CrossRef]
- Marona-Lewicka, D.; Thisted, R.A.; Nichols, D.E. Distinct temporal phases in the behavioral pharmacology of LSD: Dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology 2005, 180, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Kometer, M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat. Rev. Neurosci. 2010, 11, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Podrebarac, S.K.; Duane, J.H.; Amegadzie, S.S.; Malone, T.C.; Owens, L.T.; Ross, S.; Mennenga, S.E. Clinical Interpretations of Patient Experience in a Trial of Psilocybin-Assisted Psychotherapy for Alcohol Use Disorder. Front. Pharmacol. 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.A.; Vollenweider, F.X. Serotonin research: Contributions to understanding psychoses. Trends Pharmacol. Sci. 2008, 29, 445–453. [Google Scholar] [CrossRef]
- Corne, S.J.; Pickering, R.W. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 1967, 11, 65–78. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Koedood, L.; Powell, S.B.; Geyer, M.A. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 2011, 25, 1548–1561. [Google Scholar] [CrossRef]
- Winter, J.C.; Rice, K.C.; Amorosi, D.J.; Rabin, R.A. Psilocybin-induced stimulus control in the rat. Pharmacol. Biochem. Behav. 2007, 87, 472–480. [Google Scholar] [CrossRef]
- Fantegrossi, W.E.; Murnane, A.C.; Reissig, J.C. The behavioral pharmacology of hallucinogens. Behav. Biol. 2008, 75, 17–33. [Google Scholar] [CrossRef]
- Sakashita, Y.; Abe, K.; Katagiri, N.; Kambe, T.; Saitoh, T.; Utsunomiya, I.; Horiguchi, Y.; Taguchi, K. Effect of psilocin on extracellular dopamine and serotonin levels in the mesoaccumbens and mesocortical pathway in awake rats. Biol. Pharm. Bull. 2015, 38, 134–138. [Google Scholar] [CrossRef]
- Wang, J.; Liang, M.; Shang, Q.; Qian, H.; An, R.; Liu, H.; Shao, G.; Li, T.; Liu, X. Psilocin suppresses methamphetamine-induced hyperlocomotion and acquisition of conditioned place preference via D2R-mediated ERK signaling. CNS Neurosci. Ther. 2023, 29, 831–841. [Google Scholar] [CrossRef]
- Sakloth, F.; Leggett, E.; Moerke, M.J.; Townsend, E.A.; Banks, M.L.; Negus, S.S. Effects of acute and repeated treatment with serotonin 5-HT2A receptor agonist hallucinogens on intracranial self-stimulation in rats. Exp. Clin. Psychopharmacol. 2019, 27, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Jaster, A.M.; Elder, H.; Marsh, S.A.; de la Fuente Revenga, M.; Negus, S.S.; González-Maeso, J. Effects of the 5-HT2A receptor antagonist volinanserin on head-twitch response and intracranial self-stimulation depression induced by different structural classes of psychedelics in rodents. Psychopharmacology 2022, 239, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, J.; Yao, Y.; Yan, H.; Su, R. Rearing behaviour in the mouse behavioural pattern monitor distinguishes the effects of psychedelics from those of lisuride and TBG. Front. Pharmacol. 2023, 14, 1021729. [Google Scholar] [CrossRef] [PubMed]
- Hibicke, M.; Landry, A.N.; Kramer, H.M.; Talman, Z.K.; Nichols, C.D. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci. 2020, 11, 864–871. [Google Scholar] [CrossRef]
- Catlow, B.J.; Song, S.; Paredes, D.A.; Kirstein, C.L.; Sanchez-Ramos, J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp. Brain Res. 2013, 228, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Hagsäter, S.M.; Pettersson, R.; Pettersson, C.; Atanasovski, D.; Näslund, J.; Eriksson, E. A Complex Impact of Systemically Administered 5-HT2A Receptor Ligands on Conditioned Fear. Int. J. Neuropsychopharmacol. 2021, 24, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Jefsen, O.; Højgaard, K.; Christiansen, S.L.; Elfving, B.; Nutt, D.J.; Wegener, G.; Müller, H.K. Psilocybin lacks antidepressant-like effect in the Flinders Sensitive Line rat. Acta Neuropsychiatry 2019, 31, 213–219. [Google Scholar] [CrossRef]
- Shao, L.X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021, 109, 2535–2544. [Google Scholar] [CrossRef]
- Hesselgrave, N.; Troppoli, T.A.; Wulff, A.B.; Cole, A.B.; Thompson, S.M. Harnessing psilocybin: Antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2022489118. [Google Scholar] [CrossRef]
- Gable, R.S. Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 2004, 99, 686–696. [Google Scholar] [CrossRef]
- Morgan, C.J.; Noronha, L.A.; Muetzelfeldt, M.; Feilding, A.; Curran, H.V. Harms and benefits associated with psychoactive drugs: Findings of an international survey of active drug users. J. Psychopharmacol. 2013, 27, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.; King, L.A.; Saulsbury, W.; Blakemore, C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet 2007, 369, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Hasler, F.; Grimberg, U.; Benz, M.A.; Huber, T.; Vollenweider, F.X. Acute psychological and physiological effects of psilocybin in healthy humans: A double-blind, placebo-controlled dose-effect study. Psychopharmacology 2004, 172, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Hollister, L.E. Clinical, biochemical and psychologic effects of psilocybin. Arch. Int. Pharmacodyn. Ther. 1961, 130, 42–52. [Google Scholar] [PubMed]
- Dittrich, A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 1998, 31 (Suppl. 2), 80–84. [Google Scholar] [CrossRef] [PubMed]
- Studerus, E.; Kometer, M.; Hasler, F.; Vollenweider, F.X. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J. Psychopharmacol. 2011, 25, 1434–1452. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Geyer, M.A. A systems model of altered consciousness: Integrating natural and drug-induced psychoses. Brain Res. Bull. 2001, 56, 495–507. [Google Scholar] [CrossRef]
- Savalia, N.K.; Shao, L.X.; Kwan, A.C. A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics. Trends Neurosci. 2021, 44, 260–275. [Google Scholar] [CrossRef]
- Borsellino, P.; Krider, R.I.; Chea, D.; Grinnell, R.; Vida, T.A. Ketamine and the Disinhibition Hypothesis: Neurotrophic Factor-Mediated Treatment of Depression. Pharmaceuticals 2023, 16, 742. [Google Scholar] [CrossRef]
- De Gregorio, D.; Enns, J.P.; Nuñez, N.A.; Posa, L.; Gobbi, G. d-Lysergic acid diethylamide, psilocybin, and other classic hallucinogens: Mechanism of action and potential therapeutic applications in mood disorders. Prog. Brain Res. 2018, 242, 69–96. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.; Artigas, F. Expression of Serotonin2C Receptors in Pyramidal and GABAergic Neurons of Rat Prefrontal Cortex: A Comparison with Striatum. Cereb. Cortex 2017, 27, 3125–3139. [Google Scholar] [CrossRef] [PubMed]
- Amargós-Bosch, M.; Bortolozzi, A.; Puig, M.V.; Serrats, J.; Adel, A.; Celada, P.; Totch, M.; Mengod, G.; Artigas, F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb. Cortex 2004, 14, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Santana, N. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex 2004, 14, 1100–1109. [Google Scholar] [CrossRef]
- Sheldon, P.W.; Aghajanian, G.K. Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: Evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse 1991, 9, 208–218. [Google Scholar] [CrossRef]
- Béïque, J.C.; Campbell, B.; Perring, P.; Hamblin, M.V.; Walker, P.; Mladenovic, L.; Andrade, R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: Coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J. Neurosci. 2004, 24, 4807–4817. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Lövdén, M.; Rosenthal, G.; Feilding, A.; Nutt, D.J.; Carhart-Harris, R.L. Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Hum. Brain Mapp. 2015, 36, 3137–3153. [Google Scholar] [CrossRef]
- Millan, M.J.; Gobert, A.; Lejeune, F.; Dekeyne, A.; Newman-Tancredi, A.; Pasteau, V.; Rivet, J.M.; Cussac, D. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J. Pharmacol. Exp. Ther. 2003, 306, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Frånberg, O.; Marcus, M.M.; Svensson, T.H. Involvement of 5-HT2A receptor and α2-adrenoceptor blockade in the asenapine-induced elevation of prefrontal cortical monoamine outflow. Synapse 2012, 66, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.L.; Kuypers, K.P.C.; Müller, F.; Reckweg, J.; Tse, D.H.Y.; Toennes, S.W.; Hutten, N.R.P.W.; Jansen, J.F.A.; Stiers, P.; Feilding, A.; et al. Me, myself, bye: Regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology 2020, 45, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.M.; Hablitz, J.J. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J. Neurophysiol. 1999, 82, 2989–2999. [Google Scholar] [CrossRef]
- Rodrigues, N.B.; McIntyre, R.S.; Lipsitz, O.; Cha, D.S.; Lee, Y.; Gill, H.; Majeed, A.; Phan, L.; Nasri, F.; Ho, R.; et al. Changes in symptoms of anhedonia in adults with major depressive or bipolar disorder receiving IV ketamine: Results from the Canadian Rapid Treatment Center of Excellence. J. Affect. Disord. 2020, 276, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Altinay, M.; Malone, D.A., Jr.; Anand, A. Where in the brain is depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Gado, M.H.; Price, J.L. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport 1998, 9, 2023–2028. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Jackowski, A.; Salas, R.; Gupta, S.; Sato, J.R.; Mao, X.; Coplan, J.D.; Shungu, D.C.; Mathew, S.J. The Nucleus Accumbens and Ketamine Treatment in Major Depressive Disorder. Neuropsychopharmacology 2017, 42, 1739–1746. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Wu, F.C.; Wang, C.Y.; Zheng, W.; Lan, X.F.; Deng, X.R.; Ning, Y.P. Relationship between hippocampal volume and inflammatory markers following six infusions of ketamine in major depressive disorder. J. Affect. Disord. 2020, 276, 608–615. [Google Scholar] [CrossRef]
- Wojtas, A.; Bysiek, A.; Wawrzczak-Bargiela, A.; Maćkowiak, M.; Gołembiowska, K. Limbic system response to psilocybin and ketamine administration in rats: A neurochemical and behavioral study. Int. J. Mol. Sci. 2024, 25, 100. [Google Scholar] [CrossRef]
- Guan, X.M.; McBride, W.J. Serotonin microinfusion into the ventral tegmental area increases accumbens dopamine release. Brain Res. Bull. 1989, 23, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L.H.; Justice, J.B., Jr. Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J. Neurochem. 1993, 61, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdere, P.; Di Giovanni, G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Prog. Neurobiol. 2017, 151, 175–236. [Google Scholar] [CrossRef] [PubMed]
- Celada, P.; Puig, M.V.; Artigas, F. Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 2013, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Cornea-Hébert, V.; Riad, M.; Wu, C.; Singh, S.K.; Descarries, L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J. Comp. Neurol. 1999, 409, 187–209. [Google Scholar] [CrossRef]
- Izumi, J.; Washizuka, M.; Miura, N.; Hiraga, Y.; Ikeda, Y. Hippocampal serotonin 5-HT1A receptor enhances acetylcholine release in conscious rats. J. Neurochem. 1994, 62, 1804–1808. [Google Scholar] [CrossRef]
- Dasari, S.; Gulledge, A.T. M1 and M4 receptors modulate hippocampal pyramidal neurons. J. Neurophysiol. 2011, 105, 779–792. [Google Scholar] [CrossRef]
- Bombardi, C.; Di Giovanni, G. Functional anatomy of 5-HT2A receptors in the amygdala and hippocampal complex: Relevance to memory functions. Exp. Brain Res. 2013, 230, 427–439. [Google Scholar] [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 1994, 23, 163–178. [Google Scholar] [CrossRef]
- Palchaudhuri, M.; Flügge, G. 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell Tissue Res. 2005, 321, 159–172. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Sanacora, G.; Woolley, J.; Heinzerling, K.; Dunlop, B.W.; Brown, R.T.; Kakar, R.; Hassman, M.; Trivedi, R.P.; Robison, R.; et al. Single-dose psilocybin treatment for major depressive disorder. A randomized clinical trial. JAMA 2023, 330, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Gukasyan, N.; Davis, A.K.; Barrett, F.S.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Griffiths, R.R. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up. J. Psychopharmacol. 2022, 36, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Barba, T.; Buehler, S.; Kettner, H.; Radu, C.; Cunha, B.G.; Nutt, D.J.; Erritzoe, D.; Roseman, L.; Carhart-Harris, R. Effects of psilocybin versus escitalopram on rumination and thought suppression in depression. BJPsych Open 2022, 8, e163. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Forcehimes, A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.C.R.; Strassman, R.J. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef]
- Johnson, M.W.; Garcia-Romeu, A.; Cosimano, M.P.; Griffiths, R.R. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014, 28, 983–992. [Google Scholar] [CrossRef]
- Johnson, M.W.; Garcia-Romeu, A.; Griffiths, R.R. Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol Abus. 2017, 43, 55–60. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Griffiths, R.R.; Johnson, M.W. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr. Drug Abus. Rev. 2014, 7, 157–164. [Google Scholar] [CrossRef]
- Foldi, C.; Liknaitzky, P.; Williams, M.; Oldfield, B.J. Rethinking therapeutic strategies for anorexia nervosa, insights from psychedelic medicine and animal models. Front. Neurosci. 2020, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Griffits, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer. A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Grob, C.S.; Danforth, A.L.; Chopra, G.S.; Hagerty, M.; McKay, C.R.; Halberstadt, A.L.; Greer, G.R. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch. Gen. Psychiatry 2011, 68, 71–78. [Google Scholar] [CrossRef]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef]
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatry 2006, 67, 1735–1740. [Google Scholar] [CrossRef]
- Krediet, E.; Bostoen, T.; Breeksema, J.; Van Schagen, A.; Passie, T.; Vermetten, E. Reviewing the potential of psychedelics for treatment of PTSD. Int. J. Neuropsychopharmacol. 2020, 23, 385–400. [Google Scholar] [CrossRef]
- González-Maeso, J.; Sealfon, S.C. Psychedelics and schizophrenia. Trends Neurosci. 2009, 32, 225–232. [Google Scholar] [CrossRef]
- Celada, P.; Lladó-Pelfort, L.; Santana, N.; Kargieman, L.; Troyano-Rodriguez, E.; Riga, M.S.; Artigas, F. Disruption of thalamocortical activity in schizophrenia models: Relevance to antipsychotic drug action. Int. J. Neuropsychopharmacol. 2013, 16, 2145–2163. [Google Scholar] [CrossRef]
- Mahmood, D.; Alenzi, S.K.; Anwar, M.J.; Azam, F.; Qureshi, K.A.; Jaremko, M. New paradigms of old psychedelics in schizophrenia. Pharmaceuticals 2022, 15, 640. [Google Scholar] [CrossRef]
- Olson, D.E. The Subjective Effects of Psychedelics May Not Be Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci. 2020, 4, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef] [PubMed]
| Compound/Receptor Subtype | Ki ± SD (nM) | |||
|---|---|---|---|---|
| 5-HT1A | 5-HT2A | 5-HT2B | 5-HT2C | |
| Phenethylamines | ||||
| Mescaline | 4600 ± 400 | 6300 ± 1800 | >2000 | 17,000 ± 2000 |
| 25I-NBOMe | 1800 ± 300 | 0.6 ± 0.2 | 130 ± 80 | 4.6 ± 2 |
| 25B-NBOMe | 3600 ± 300 | 0.5 ± 0 | 10 ± 10 | 6.2 ± 2.2 |
| Indoleamines | ||||
| Psilocin | 123 ± 20 | 49 ± 10 | >20,000 | 94 ± 9 |
| DMT | 75 ± 20 | 237 ± 40 | 34 ± 32 | 424 ± 150 |
| LSD | 3 ± 0.5 | 4 ± 1 | 12,000 ± 400 | 15 ± 3 |
| Abbreviation | Chemical Name |
|---|---|
| 25B-NBOMe | 2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine |
| 25C-NBOMe | 2-(4-chloro-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine |
| 25D-NBOMe | 2-(2,5-dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine |
| 25I-NBOMe | 2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine |
| 25N-NBOMe | 2-(2,5-dimethoxy-4-nitrophenyl)-N-(2-methoxybenzyl)ethanamine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtas, A.; Gołembiowska, K. Molecular and Medical Aspects of Psychedelics. Int. J. Mol. Sci. 2024, 25, 241. https://doi.org/10.3390/ijms25010241
Wojtas A, Gołembiowska K. Molecular and Medical Aspects of Psychedelics. International Journal of Molecular Sciences. 2024; 25(1):241. https://doi.org/10.3390/ijms25010241
Chicago/Turabian StyleWojtas, Adam, and Krystyna Gołembiowska. 2024. "Molecular and Medical Aspects of Psychedelics" International Journal of Molecular Sciences 25, no. 1: 241. https://doi.org/10.3390/ijms25010241





