Prednisolone Targets Claudins in Mouse Brain Blood Vessels
Abstract
:1. Introduction
2. Results
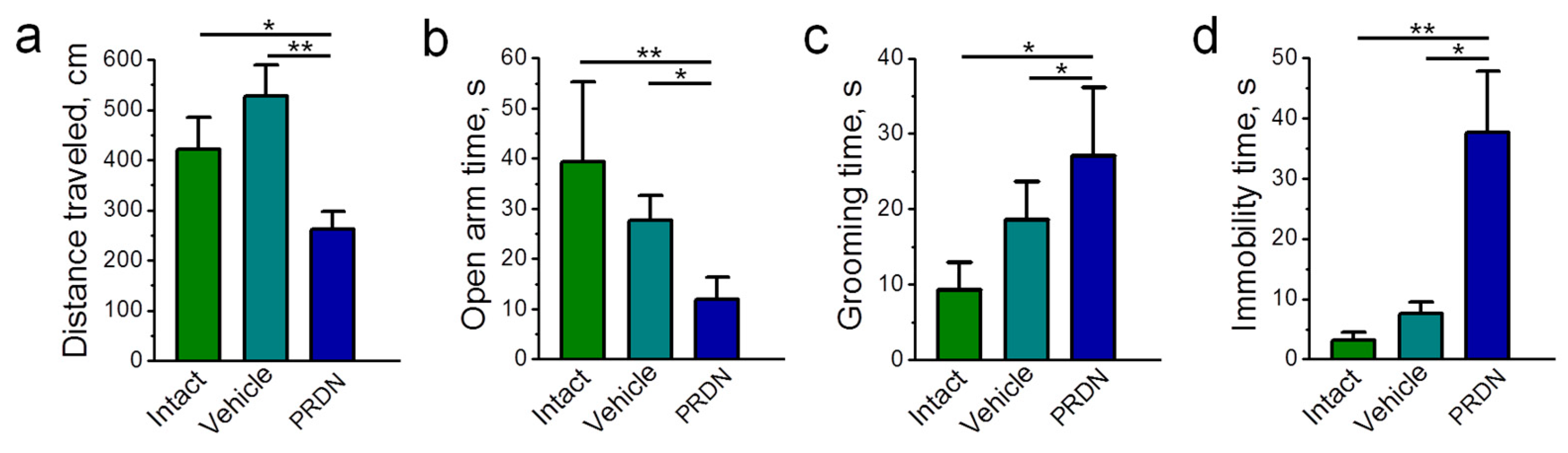
2.1. Physiological Effects of Prednisolone Administration
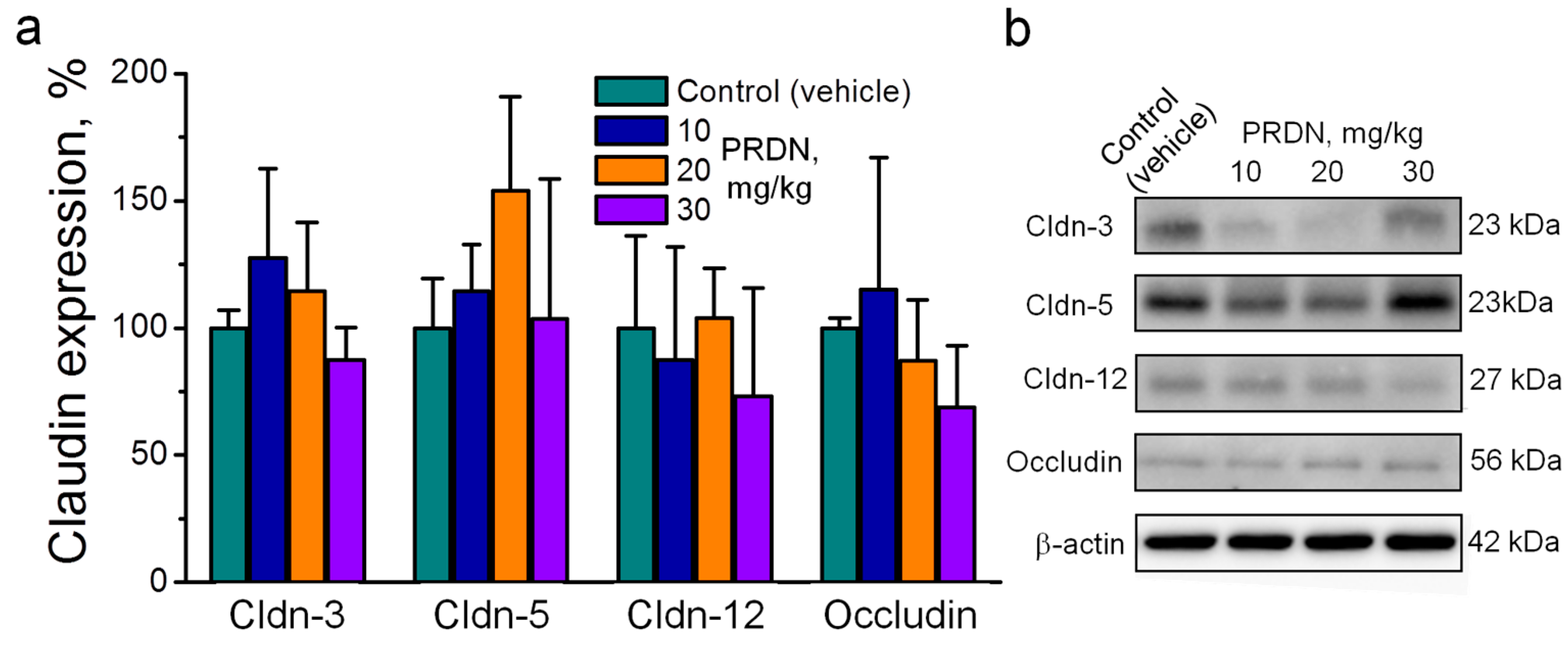
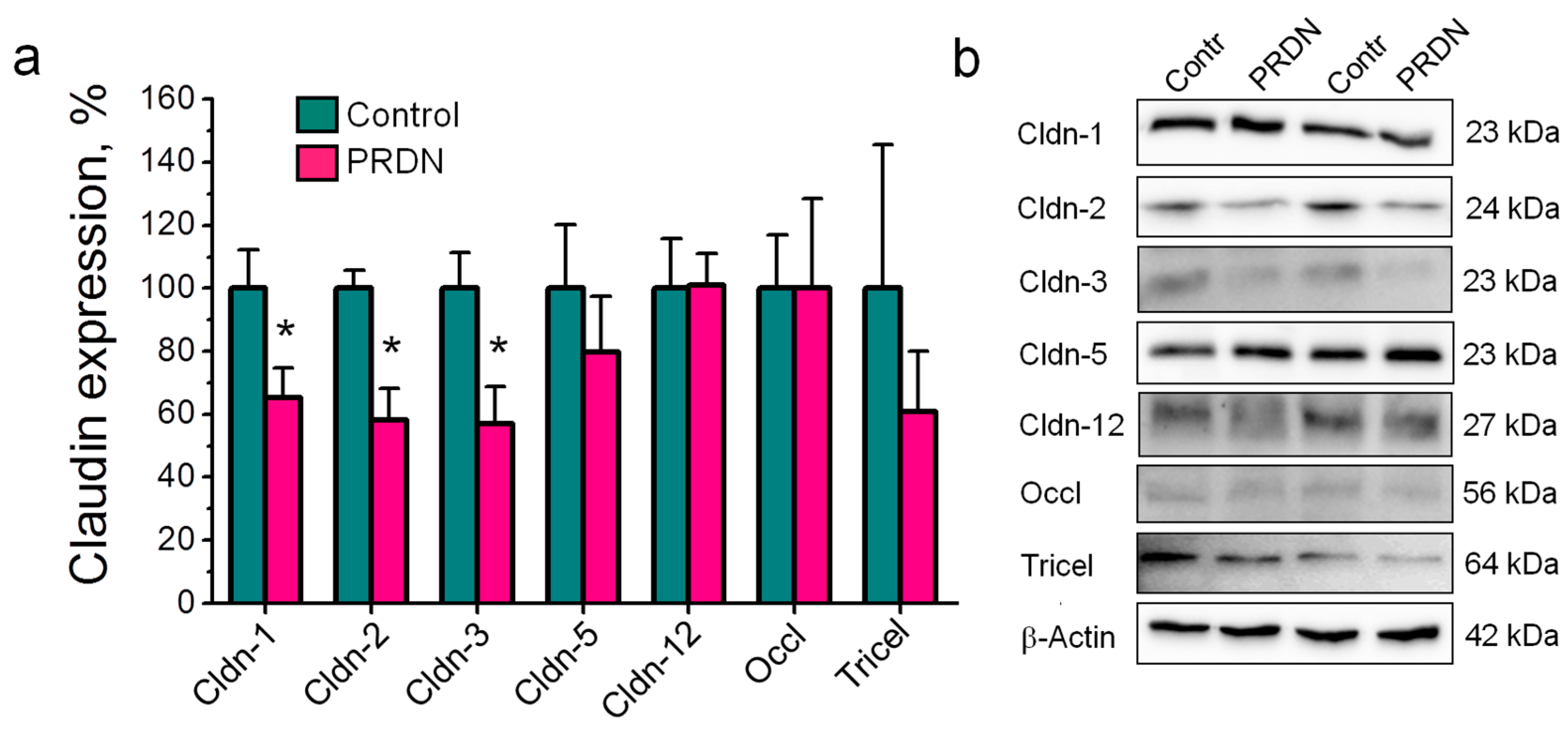
2.2. Prednisolone Administration Modulated Claudin Expression in the Mouse Brain
2.3. Prednisolone Administration Did Not Alter Claudin Localization in Cerebral Blood Vessels
3. Discussion
- (1)
- In addition to claudin-1, -3, -5, and -12, the expression of the channel-former claudin-2 in the vascular endothelium of the mouse brain was first shown at the protein level.
- (2)
- Prednisolone downregulated the channel-former claudin-2 and the tightening claudins claudin-1 and -3 without changes in the expression of claudin-5 and -12. These changes were accompanied by deviations in depression-related behavior.
- (3)
- Prednisolone modulated claudin expression without changes in their localization in cerebral blood vessels.
4. Materials and Methods
4.1. Animals
4.2. Behavioral Experiments
4.3. Western Blotting
4.4. Immunohistochemistry
4.5. Materials
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Ribatti, D. Morphofunctional aspects of the blood-brain barrier. Curr. Drug Metab. 2012, 13, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Tikiyani, V.; Babu, K. Claudins in the brain: Unconventional functions in neurons. Traffic 2019, 20, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Fromm, M. Claudins and other tight junction proteins. Compr. Physiol. 2012, 2, 1819–1852. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Wolburg-Buchholz, K.; Kraus, J.; Rascher-Eggstein, G.; Liebner, S.; Hamm, S.; Duffner, F.; Grote, E.-H.; Risau, W.; Engelhardt, B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003, 105, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.-P.; Fu, J.-S.; Zhang, S.; Bai, L.; Guo, L. Resveratrol Defends Blood-brain Barrier Integrity in Experimental Autoimmune Encephalomyelitis. J. Neurophysiol. 2016, 116, 2173–2179. [Google Scholar] [CrossRef]
- Hanske, S.; Dyrna, F.; Bechmann, I.; Krueger, M. Different segments of the cerebral vasculature reveal specific endothelial specifications, while tight junction proteins appear equally distributed. Brain Struct. Funct. 2017, 222, 1179–1192. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Yamaguchi, H.; Katsukura, Y.; Asashima, T.; Terasaki, T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J. Neurochem. 2008, 104, 147–154. [Google Scholar] [CrossRef]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and Treatment of Cerebral Edema in Traumatic Brain Injury. Neuropharmacology 2019, 145, 230–246. [Google Scholar] [CrossRef]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Milatz, S.; Krug, S.M.; Oelrich, B.; Schulzke, J.-D.; Amasheh, S.; Günzel, D.; Fromm, M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010, 123, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S. Molecular basis for cation selectivity in claudin-2-based pores. Ann. N. Y. Acad. Sci. 2009, 1165, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Hanley, N.; Campbell, M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry 2020, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.A.; Dion-Albert, L.; Lebel, M.; LeClair, K.; Labrecque, S.; Tuck, E.; Perez, C.F.; Golden, S.A.; Tamminga, C.; Turecki, G.; et al. Molecular adaptations of the blood–brain barrier promote stress resilience vs. depression. Proc. Natl. Acad. Sci. USA 2020, 117, 3326–3336. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.; Burek, M.; Romero, I.A.; Weksler, B.; Couraud, P.-O.; Drenckhahn, D. Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood–brain barrier. J. Physiol. 2008, 586, 1937–1949. [Google Scholar] [CrossRef]
- Salvador, E.; Shityakov, S.; Förster, C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014, 355, 597–605. [Google Scholar] [CrossRef]
- Calabria, A.R.; Weidenfeller, C.; Jones, A.R.; Vries, H.E.; Shusta, E.V. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J. Neurochem. 2006, 97, 922–933. [Google Scholar] [CrossRef]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood-brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef]
- Neuhaus, W.; Schlunt, M.; Fehrholz, M.; Ehrke, A.; Kunzmann, S.; Liebner, S.; Speer, C.P.; Förster, C.Y. Multiple antenatal dexamethasone treatment alters brain vessel differentiation in newborn mouse pups. PLoS ONE 2015, 10, e0136221. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.G.; Fedorova, A.A.; Kravtsova, V.V.; Bikmurzina, A.E.; Okorokova, L.S.; Matchkov, V.V.; Cornelius, V.; Amasheh, S.; Krivoi, I.I. Circulating Ouabain Modulates Expression of Claudins in Rat Intestine and Cerebral Blood Vessels. Int. J. Mol. Sci. 2020, 21, 5067. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.; Giusti, H.; Souza, A.P.; Franci, C.R.; Saia, R.S. Dexamethasone prevents lipopolysaccharide-induced epithelial barrier dysfunction in rat ileum. Shock 2018, 49, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Bastin, M.E.; Wardlaw, J.M.; Armitage, P.A.; Whittle, I.R. Effects of dexamethasone on peritumoral oedematous brain: A DT-MRI study. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Vicuña, E.A.; Kuttappan, V.F.; Galarza-Seeber, R.; Latorre, J.D.; Faulkner, O.B.; Hargis, B.M.; Tellez, G.; Bielke, L.R. Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poult. Sci. 2015, 94, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.; Du, L. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008, 581, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, Y.; Iijima, Y.; Chiba, S.; Furuta, M.; Ninomiya, M.; Izumi, A.; Shibata, S.; Kunugi, H. Prednisolone causes anxiety- and depression-like behaviors and altered expression of apoptotic genes in mice hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 159–165. [Google Scholar] [CrossRef]
- Maia Oliveira, I.C.; Vasconcelos Mallmann, A.S.; Adelvane de Paula Rodrigues, F.; Teodorio Vidal, L.M.; Lopes Sales, I.S.; Rodrigues, G.C.; Ferreira de Oliveira, N.; Rodrigues, G.C.; de Oliveira, N.F.; de Castro Chaves, R.; et al. Neuroprotective and Antioxidant Effects of Riparin I in a Model of Depression Induced by Corticosterone in Female Mice. Neuropsychobiology 2022, 81, 28–38. [Google Scholar] [CrossRef]
- Rosenthal, R.; Günzel, D.; Krug, S.M.; Schulzke, J.-D.; Fromm, M.; Yu, A.S.L. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2017, 219, 521–536. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Pathogenesis of Brain Edema and Investigation into Anti-Edema Drugs. Int. J. Mol. Sci. 2015, 16, 9949–9975. [Google Scholar] [CrossRef]
- Leng, T.; Shi, Y.; Xiong, Z.-G.; Sun, D. Proton-sensitive cation channels and ion exchangers in ischemic brain injury: New therapeutic targets for stroke? Prog. Neurobiol. 2014, 115, 189–209. [Google Scholar] [CrossRef]
- Slivka, A.P.; Murphy, E.J. High-dose methylprednisolone treatment in experimental focal cerebral ischemia. Exp. Neurol. 2001, 167, 166–172. [Google Scholar] [CrossRef]
- Kozler, P.; Marešová, D.; Pokorný, J. Methylprednisolone modulates intracranial pressure in the brain cellular edema induced by water intoxication. Physiol. Res. 2017, 66, S511–S516. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Segovia, J.; López-Ornelas, A.; Puerta-Guardo, H.; Ludert, J.; Chávez, B.; Meraz-Cruz, N.; González-Mariscal, L. Transmigration of neural stem cells across the blood brain barrier induced by glioma cells. PLoS ONE 2013, 8, e60655. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Calderón-Peláez, M.A.; Castellanos, J.E. In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium. PLoS ONE 2016, 11, e0157786. [Google Scholar] [CrossRef]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005, 565, 475–486. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Milatz, S.; Krug, S.M.; Rosenthal, R.; Günzel, D.; Müller, D.; Schulzke, J.-D.; Amasheh, S.; Fromm, M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim. Biophys. Acta 2010, 1798, 2048–2057. [Google Scholar] [CrossRef]
- Coisne, C.; Dehouck, L.; Faveeuw, C.; Delplace, Y.; Miller, F.; Landry, C.; Morissette, C. Mouse syngenic in vitro blood-brain barrier model: A new tool to examine inflammatory events in cerebral endothelium. Lab. Investig. 2005, 85, 734–746. [Google Scholar] [CrossRef]
- Schrade, A.; Sade, H.; Couraud, P.-O.; Romero, I.A.; Weksler, B.B.; Niewoehner, J. Expression and localization of claudins-3 and -12 in transformed human brain endothelium. Fluids Barriers CNS 2012, 29, 6. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.; Waschke, J.; Burek, M.; Leers, J.; Drenckhahn, D. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J. Physiol. 2006, 573, 413–425. [Google Scholar] [CrossRef]
- Cioni, C.; Turlizzi, E.; Zanelli, U.; Oliveri, G.; Annunziata, P. Expression of Tight Junction and Drug Efflux Transporter Proteins in an in vitro Model of Human Blood-Brain Barrier. Front. Psychiatry 2012, 3, 47. [Google Scholar] [CrossRef]
- Liebner, S.; Kniesel, U.; Kalbacher, H.; Wolburg, H. Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur. J. Cell Biol. 2000, 79, 707–717. [Google Scholar] [CrossRef]
- Campbell, M.; Kiang, A.-S.; Kenna, P.F.; Kerskens, C.; Blau, C.; O’Dwyer, L.; Tivnan, A.; Kelly, J.A.; Brankin, B.; Farrar, G.-J.; et al. RNAi-mediated reversible opening of the blood-brain barrier. J. Gene Med. 2008, 10, 930–947. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, 1054–1064. [Google Scholar] [CrossRef]
- Krug, S.M.; Amasheh, S.; Richter, J.F.; Milatz, S.; Günzel, D.; Westphal, J.K.; Huber, O.; Schulzke, J.D.; Fromm, M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 2009, 20, 3713–3724. [Google Scholar] [CrossRef]
- Krug, S.M.; Schulzke, J.D.; Fromm, M. Tight junction, selective permeability, and related diseases. Semin. Cell Dev. Biol. 2014, 36, 166–176. [Google Scholar] [CrossRef]
- Buschman, M.M.; Shen, L.; Rajapakse, H.; Raleigh, D.R.; Wang, Y.; Wang, Y.; Lingaraju, A.; Zha, J.; Abbott, E.; McAuley, E.M.; et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell 2013, 24, 3056–3068. [Google Scholar] [CrossRef]
- Lorén, V.; Cabré, E.; Ojanguren, I.; Domènech, E.; Pedrosa, E.; García-Jaraquemada, A.; Mañosa, M.; Manyé, J. Interleukin-10 Enhances the Intestinal Epithelial Barrier in the Presence of Corticosteroids through p38 MAPK Activity in Caco-2 Monolayers: A Possible Mechanism for Steroid Responsiveness in Ulcerative Colitis. PLoS ONE 2015, 10, e0130921. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.A.; Radewicz, K.; Jubin, E.; Michel, C.C.; Greenwood, J.; Couraud, P.-O.; Adamson, P. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci. Lett. 2003, 344, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, C.; Alvarez, J.I.; Prat, A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011, 585, 3770–3780. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Luo, Y.; Hun, Z. Crosstalk between inflammation and the BBB in stroke. Curr. Neuropharmacol. 2020, 18, 1227–1236. [Google Scholar] [CrossRef]
- Calvello, R.; Lofrumento, D.D.; Perrone, M.G.; Cianciulli, A.; Salvatore, R.; Vitale, P.; Nuccio, F.; Giannotti, L.; Nicolardi , G.; Panaro, M.A.; et al. Highly selective cyclooxygenase-1 inhibitors P6 and mofezolac counteract inflammatory state both in vitro and in vivo models of neuroinflammation. Front. Neurol. 2017, 8, 251. [Google Scholar] [CrossRef]
- Coisne, C.; Engelhardt, B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid. Redox Signal. 2011, 15, 1285–1303. [Google Scholar] [CrossRef]
- Burek, M.; Arias-Loza, P.A.; Roewer, N.; Förster, C.Y. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; pp. 1–246.
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatment. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Li, Z.; Langhans, S.A. Transcriptional regulators of Na,K-ATPase subunits. Front. Cell Dev. Biol. 2015, 3, 66. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markov, A.G.; Bikmurzina, A.E.; Fedorova, A.A.; Vinogradova, E.P.; Kruglova, N.M.; Krivoi, I.I.; Amasheh, S. Prednisolone Targets Claudins in Mouse Brain Blood Vessels. Int. J. Mol. Sci. 2024, 25, 276. https://doi.org/10.3390/ijms25010276
Markov AG, Bikmurzina AE, Fedorova AA, Vinogradova EP, Kruglova NM, Krivoi II, Amasheh S. Prednisolone Targets Claudins in Mouse Brain Blood Vessels. International Journal of Molecular Sciences. 2024; 25(1):276. https://doi.org/10.3390/ijms25010276
Chicago/Turabian StyleMarkov, Alexander G., Anastasia E. Bikmurzina, Arina A. Fedorova, Ekaterina P. Vinogradova, Natalia M. Kruglova, Igor I. Krivoi, and Salah Amasheh. 2024. "Prednisolone Targets Claudins in Mouse Brain Blood Vessels" International Journal of Molecular Sciences 25, no. 1: 276. https://doi.org/10.3390/ijms25010276
APA StyleMarkov, A. G., Bikmurzina, A. E., Fedorova, A. A., Vinogradova, E. P., Kruglova, N. M., Krivoi, I. I., & Amasheh, S. (2024). Prednisolone Targets Claudins in Mouse Brain Blood Vessels. International Journal of Molecular Sciences, 25(1), 276. https://doi.org/10.3390/ijms25010276




