Structure and Neuroprotector Properties of a Complex Compound of Lithium with Comenic Acid
Abstract
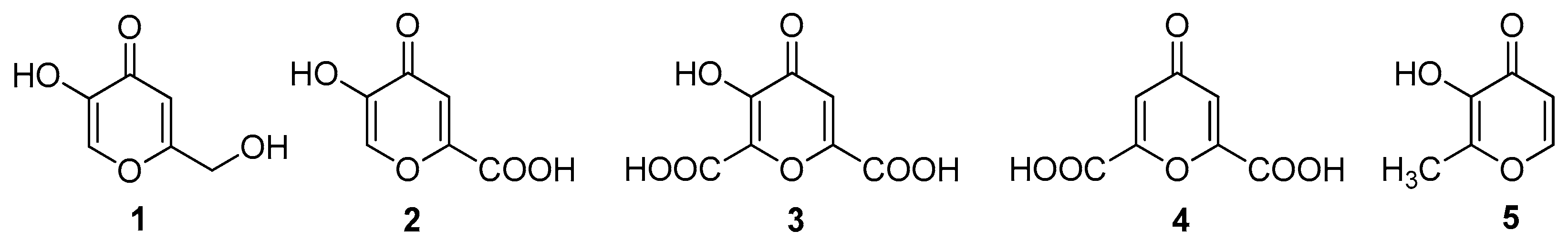
:1. Introduction
2. Results
2.1. Chemical Part
2.1.1. Thermal Analysis
2.1.2. Simultaneous Thermal Analysis/Mass Spectrometry
2.1.3. IR Spectroscopy
2.1.4. NMR Spectroscopy
2.1.5. Electron Spectroscopy in the UV Region
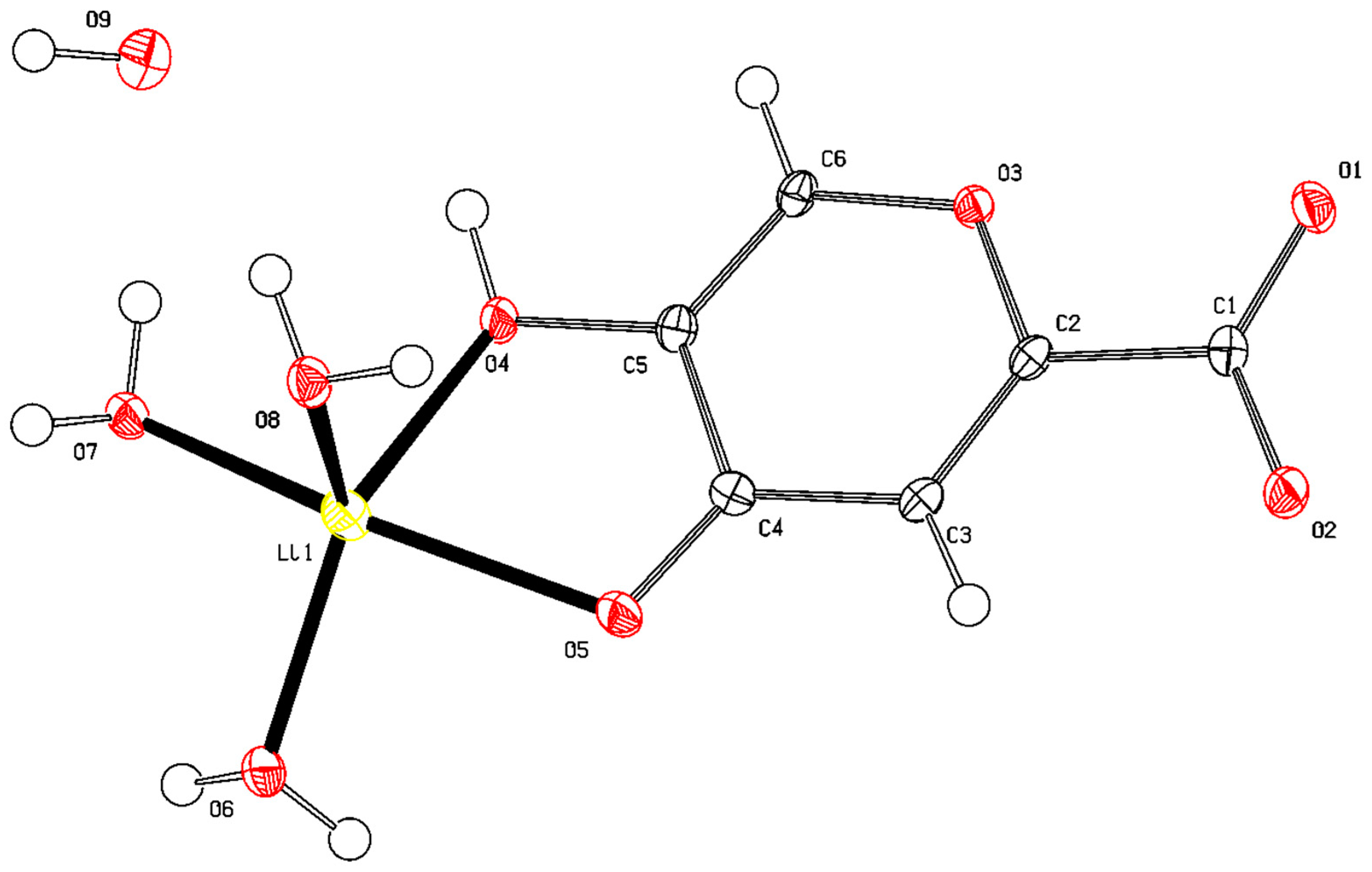
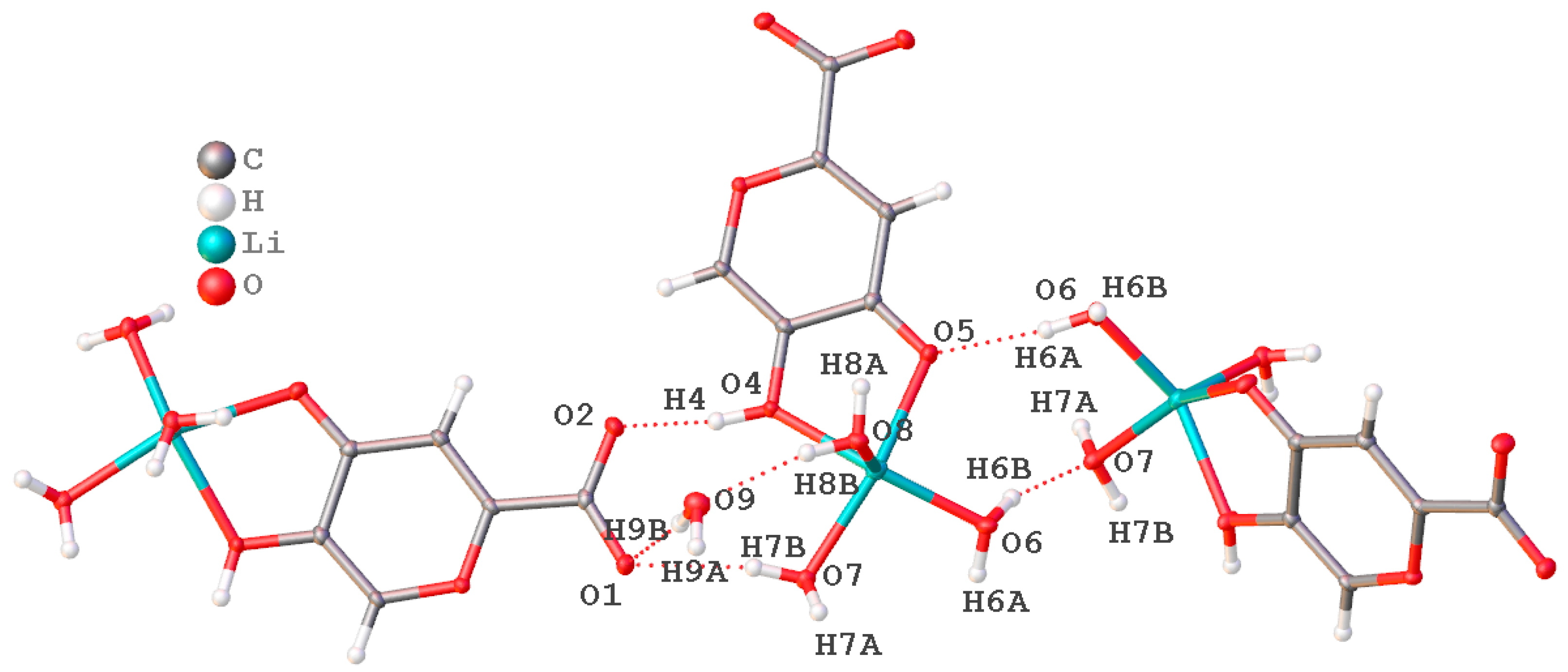
2.1.6. X-ray Diffraction Analysis
2.2. Biological Part
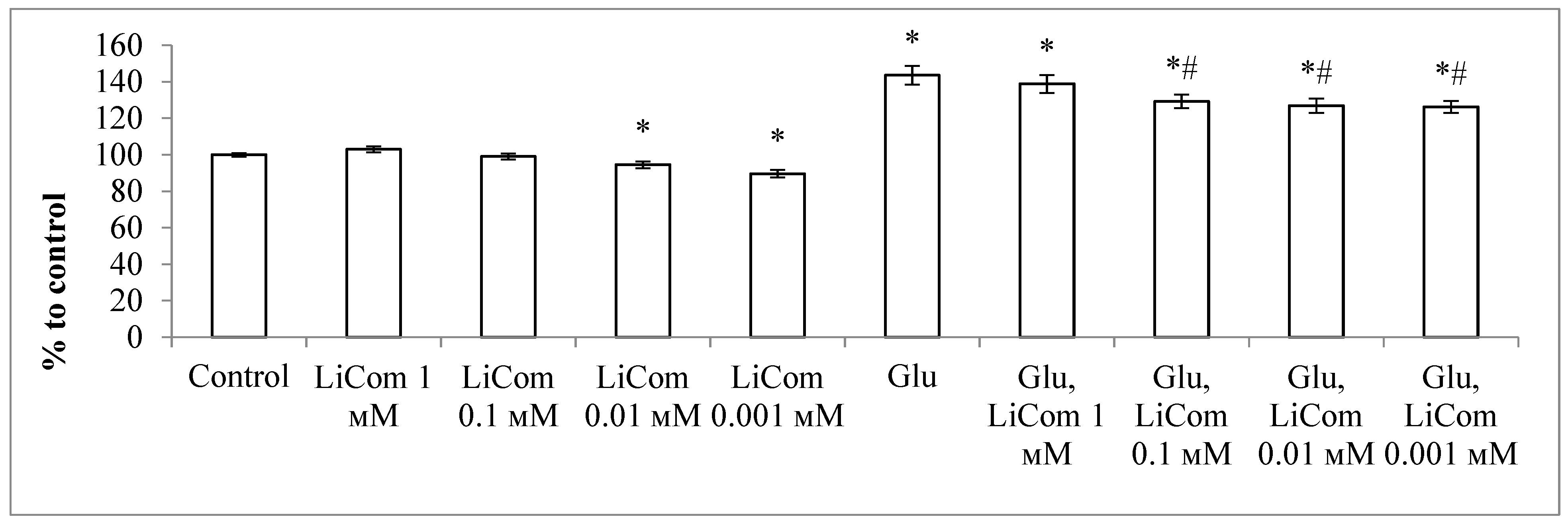
2.2.1. Neuroprotective Activity during Excitotoxic Exposure
2.2.2. Influence of Lithium Comenate on the Prooxidative and Antioxidant Status of the Brain under Stress
3. Discussion
4. Materials and Methods
4.1. Study of Neuroprotective Activity under Excitotoxic Exposure
4.2. Study of the Oxidative Processes in Brain Tissues under Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rybakowski, J. Lithium treatment–the state of the art for 2020. Psychiatr. Pol. 2020, 54, 1047–1066. [Google Scholar] [CrossRef] [PubMed]
- Rakofsky, J.J.; Lucido, M.J.; Dunlop, B.W. Lithium in the treatment of acute bipolar depression: A systematic review and meta-analysis. J. Affect. Disord. 2022, 308, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Sawmiller, D.; Wheeldon, B.; Tan, J. A Review for Lithium: Pharmacokinetics, Drug Design, and Toxicity. CNS Neurol. Disord. Drug. Targets 2019, 18, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, L.; Ott, M.; Oja, S.; Bergqvist, M.; Lundqvist, R.; Sandlund, M.; Salander Renberg, E.; Werneke, U. Reasons for lithium discontinuation in men and women with bipolar disorder: A retrospective cohort study. BMC Psychiatry 2018, 18, 37. [Google Scholar] [CrossRef]
- Pacholko, A.G.; Bekar, L.K. Lithium orotate: A superior option for lithium therapy? Brain Behav. 2021, 11, e2262. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Kozin, S.; Kravtsov, A.; Ivashchenko, L.; Dotsenko, V.; Vasilyeva, L.; Vasilyev, A.; Tekutskaya, E.; Aksenov, N.; Baryshev, M.; Dorohova, A.; et al. Study of the Magnesium Comenate Structure, Its Neuroprotective and Stress-Protective Activity. Int. J. Mol. Sci. 2023, 24, 8046. [Google Scholar] [CrossRef]
- Mao, Y.; Du, J.; Chen, X.; Al Mamun, A.; Cao, L.; Yang, Y.; Mubwandarikwa, J.; Zaeem, M.; Zhang, W.; Chen, Y.; et al. Maltol Promotes Mitophagy and Inhibits Oxidative Stress via the Nrf2/PINK1/Parkin Pathway after Spinal Cord Injury. Oxid. Med. Cell. Longev. 2022, 2022, 1337630. [Google Scholar] [CrossRef]
- Xing, J.J.; Mi, X.J.; Hou, J.G.; Cai, E.B.; Zheng, S.W.; Wang, S.H.; Wang, Z.; Chen, C.; Li, W. Maltol mitigates cisplatin-evoked cardiotoxicity via inhibiting the PI3K/Akt signaling pathway in rodents in vivo and in vitro. Phytother. Res. 2022, 36, 1724–1735. [Google Scholar] [CrossRef]
- Kozin, S.V.; Kravtsov, A.A.; Kravchenko, S.V.; Ivashchenko, L.I. Cytoprotective and Antioxidant Effects of Meconic Acid in Model Systems. Bull. Exp. Biol. Med. 2021, 171, 619–622. [Google Scholar] [CrossRef]
- Shurygina, L.V.; Zlishcheva, E.I.; Kravtsov, A.A.; Kozin, S.V. Neurotrophic Effect of Magnesium Comenate in Normal and under Conditions of Oxidative Stress in Culture of Chicken Spinal Ganglia. Bul. Exper. Biol. Med. 2021, 171, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, S.J.; Kim, M.C.; Jeon, Y.D.; Um, J.Y.; Hong, S.H. The therapeutic eff ect of chelidonic acid on ulcerative colitis. Biol. Pharm. Bull. 2012, 35, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Yang, S.Y.; Kim, H.Y.; Kim, N.R.; Jang, J.B.; Kim, H.M. Chelidonic acid evokes antidepressant-like effect through the up-regulation of BDNF in forced swimming test. Exp. Biol. Med. 2016, 241, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Gulati, K.; Ray, A. Effects of chelidonic acid, a secondary plant metabolite, on mast cell degranulation and adaptive immunity in rats. Int. Immunopharmacol. 2016, 40, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.A.; Kim, H.M.; Jeong, H.J. Beneficial effects of chelidonic acid on a model of allergic rhinitis. Int. Immunopharmacol. 2011, 11, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Demina, N.I.; Zlishcheva, L.I.; Shurygin, A.Y. The effect of Baliz-2 on the regeneration of rabbit ear skin after radiation-induced and mechanical injury. Dokl. Biol. Sci. 2000, 372, 293–295. [Google Scholar] [PubMed]
- Khrapov, A.A.; Chepkova, A.N.; Shurygin, A.Y.; Skrebitskii, V.G. Balysum-2 inhibits evoked activity of the pyramidal neurons in hippocampus. Bull. Exp. Biol. Med. 1998, 125, 53–55. [Google Scholar] [CrossRef]
- Kravtsov, A.A.; Shurygin, A.Y.; Skorokhod, N.S.; Khaspekov, L.G. Neuroprotector effect of comenic acid against cytotoxic action of glutamate in vitro in cultured neurons of lead-poisoned rat pups. Bull. Exp. Biol. Med. 2011, 150, 436–439. [Google Scholar] [CrossRef]
- Rogachevskii, I.V.; Plakhova, V.B.; Penniyaynen, V.A.; Terekhin, S.G.; Podzorova, S.A.; Krylov, B.V. New approaches to the design of analgesic medicinal substances. Can. J. Physiol. Pharmacol. 2022, 100, 43–52. [Google Scholar] [CrossRef]
- Kondratenko, R.V.; Chepkova, A.N.; Shurygin, A.Y.; Skrebitskii, V.G. Comenic acid prevents post-stress enhancement of long-term potentiation in rat hippocampus. Bull. Exp. Biol. Med. 2003, 136, 464–466. [Google Scholar] [CrossRef]
- Domnin, I.N.; Remizova, L.A.; Misharev, A.D.; Takhistov, V.V. Fragmentation of some 4H-pyran-4-one and pyridin-4-one derivatives under electron impact. Russ. J. Org. Chem. 2008, 44, 1369–1373. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, X.; Chen, L.; Lenahan, C.; Fu, Z.; Fang, Y.; Yu, W. Crosstalk Between the Oxidative Stress and Glia Cells After Stroke: From Mechanism to Therapies. Front. Immunol. 2022, 13, 852416. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Hough, C.J.; Chuang, D.M. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc. Natl. Acad. Sci. USA 1998, 95, 2642–2647. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcellos, A.P.; Nieto, F.B.; Crema, L.M.; Diehl, L.A.; de Almeida, L.M.; Prediger, M.E.; da Rocha, E.R.; Dalmaz, C. Chronic lithium treatment has antioxidant properties but does not prevent oxidative damage induced by chronic variate stress. Neurochem. Res. 2006, 31, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Grimes, C.A.; Jope, R.S. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 2001, 65, 391–426. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, M.; Ji, M.; Zhang, D.; Chen, B.; Gong, W.; Li, X.; Zhou, Y.; Dong, C.; Wen, G.; et al. The neuroprotective mechanism of lithium after ischaemic stroke. Commun. Biol. 2022, 5, 105. [Google Scholar] [CrossRef]
- Chiu, C.T.; Chuang, D.M. Neuroprotective action of lithium in disorders of the central nervous system. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011, 36, 461–476. [Google Scholar] [CrossRef]
- Hashimoto, R.; Fujimaki, K.; Jeong, M.R.; Christ, L.; Chuang, D.M. Lithium-induced inhibition of Src tyrosine kinase in rat cerebral cortical neurons: A role in neuroprotection against N-methyl-D-aspartate receptor-mediated excitotoxicity. FEBS Lett. 2003, 538, 145–148. [Google Scholar] [CrossRef]
- Hashimoto, R.; Hough, C.; Nakazawa, T.; Yamamoto, T.; Chuang, D.M. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: Involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J. Neurochem. 2002, 80, 589–597. [Google Scholar] [CrossRef]
- Choi, J.E.; Carpena, N.T.; Lee, J.H.; Chang, S.Y.; Lee, M.Y.; Jung, J.Y.; Chung, W.H. Round-window delivery of lithium chloride regenerates cochlear synapses damaged by noise-induced excitotoxic trauma via inhibition of the NMDA receptor in the rat. PLoS ONE 2023, 18, e0284626. [Google Scholar] [CrossRef] [PubMed]
- Sibarov, D.A.; Abushik, P.A.; Poguzhelskaya, E.E.; Bolshakov, K.V.; Antonov, S.M. Inhibition of Plasma Membrane Na/Ca-Exchanger by KB-R7943 or Lithium Reveals Its Role in Ca-Dependent N-methyl-d-aspartate Receptor Inactivation. J. Pharmacol. Exp. Ther. 2015, 355, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Boikov, S.I.; Sibarov, D.A.; Stepanenko, Y.D.; Karelina, T.V.; Antonov, S.M. Calcium-Dependent Interplay of Lithium and Tricyclic Antidepressants, Amitriptyline and Desipramine, on N-methyl-D-aspartate Receptors. Int. J. Mol. Sci. 2022, 23, 16177. [Google Scholar] [CrossRef] [PubMed]
- Shurygin, A.Y.; Shurygina, L.V.; Lobova, N.N. Method for Producing Comenic Acid. RU Patent No. 2459623C1, 27 August 2012. Available online: https://patents.google.com/patent/RU2459623C1/ru (accessed on 27 March 2023).
- Shurygin, A.Y. The Bacterial Strain Gluconobacter Oxydans-03 is a Balise Producer and a Method for Producing Balise. RU Patent No. 2287583C1, 20 November 2006. Available online: https://patents.google.com/patent/RU2287583C1/ru?oq=2287583 (accessed on 27 March 2023).
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Kravtsov, A.; Kozin, S.; Basov, A.; Butina, E.; Baryshev, M.; Malyshko, V.; Moiseev, A.; Elkina, A.; Dzhimak, S. Reduction of deuterium level supports resistance of neurons to glucose deprivation and hypoxia: Study in cultures of neurons and on animals. Molecules 2022, 27, 243. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozin, S.; Kravtsov, A.; Ivashchenko, L.; Dotsenko, V.; Dzhimak, S.; Aksenov, N.; Vashurin, A.; Ivlev, V.; Baryshev, M.; Bespalov, A.; et al. Structure and Neuroprotector Properties of a Complex Compound of Lithium with Comenic Acid. Int. J. Mol. Sci. 2024, 25, 286. https://doi.org/10.3390/ijms25010286
Kozin S, Kravtsov A, Ivashchenko L, Dotsenko V, Dzhimak S, Aksenov N, Vashurin A, Ivlev V, Baryshev M, Bespalov A, et al. Structure and Neuroprotector Properties of a Complex Compound of Lithium with Comenic Acid. International Journal of Molecular Sciences. 2024; 25(1):286. https://doi.org/10.3390/ijms25010286
Chicago/Turabian StyleKozin, Stanislav, Alexandr Kravtsov, Lev Ivashchenko, Victor Dotsenko, Stepan Dzhimak, Nicolai Aksenov, Arthur Vashurin, Vasily Ivlev, Mikhail Baryshev, Alexandr Bespalov, and et al. 2024. "Structure and Neuroprotector Properties of a Complex Compound of Lithium with Comenic Acid" International Journal of Molecular Sciences 25, no. 1: 286. https://doi.org/10.3390/ijms25010286
APA StyleKozin, S., Kravtsov, A., Ivashchenko, L., Dotsenko, V., Dzhimak, S., Aksenov, N., Vashurin, A., Ivlev, V., Baryshev, M., Bespalov, A., Fedulova, L., Dorohova, A., & Anashkina, A. (2024). Structure and Neuroprotector Properties of a Complex Compound of Lithium with Comenic Acid. International Journal of Molecular Sciences, 25(1), 286. https://doi.org/10.3390/ijms25010286






