Chitosan-Based Dressing as a Sustained Delivery System for Bioactive Cytokines
Abstract
:1. Introduction
2. Results
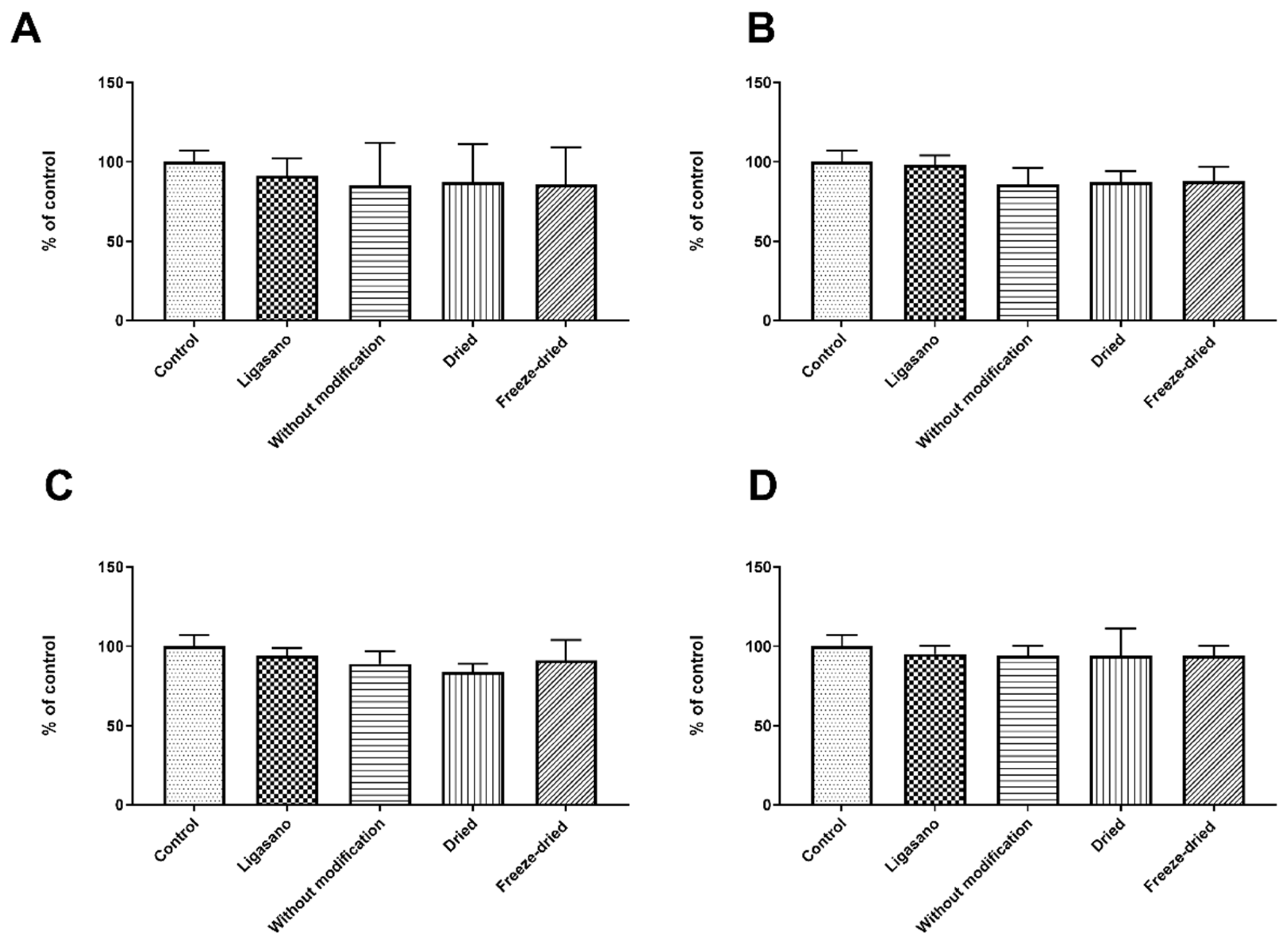
2.1. Effects of Different Preparation Techniques on Dressing
2.2. Kinetics of Cytokine Release from the Dressing
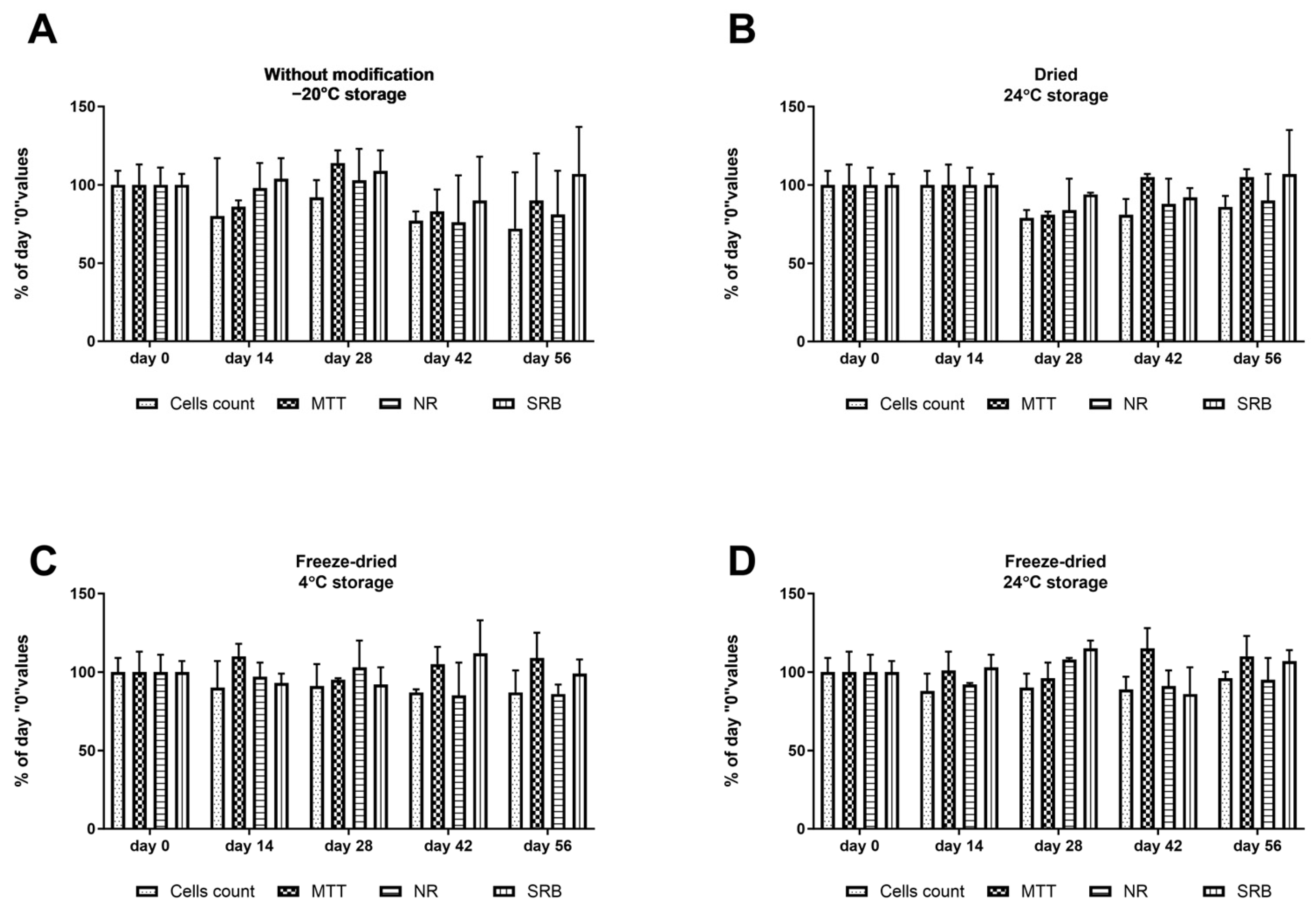
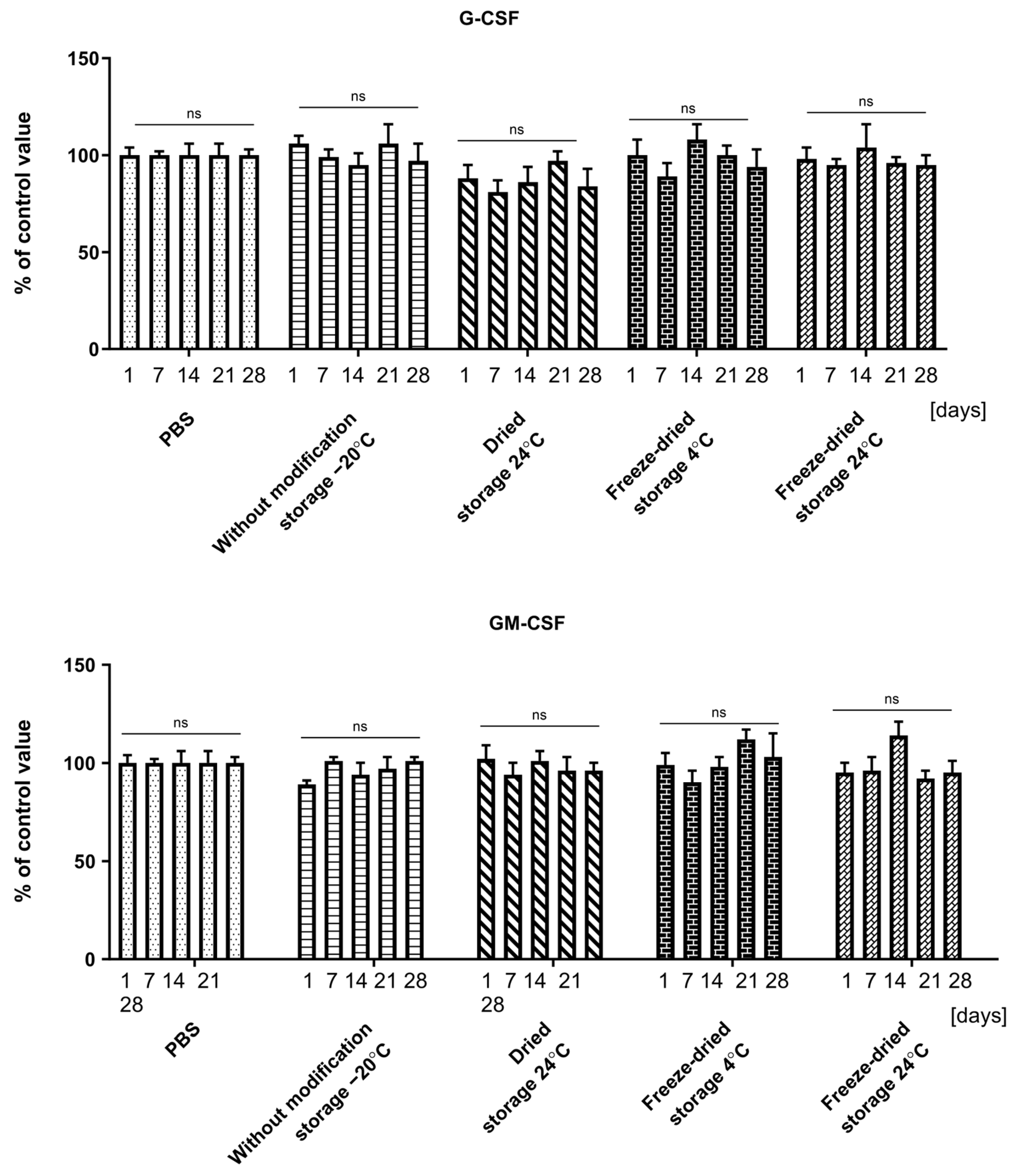
2.3. Effect of Dressing Storage on Cytokine Recovery
2.4. Sterility Assessment and Cytotoxicity
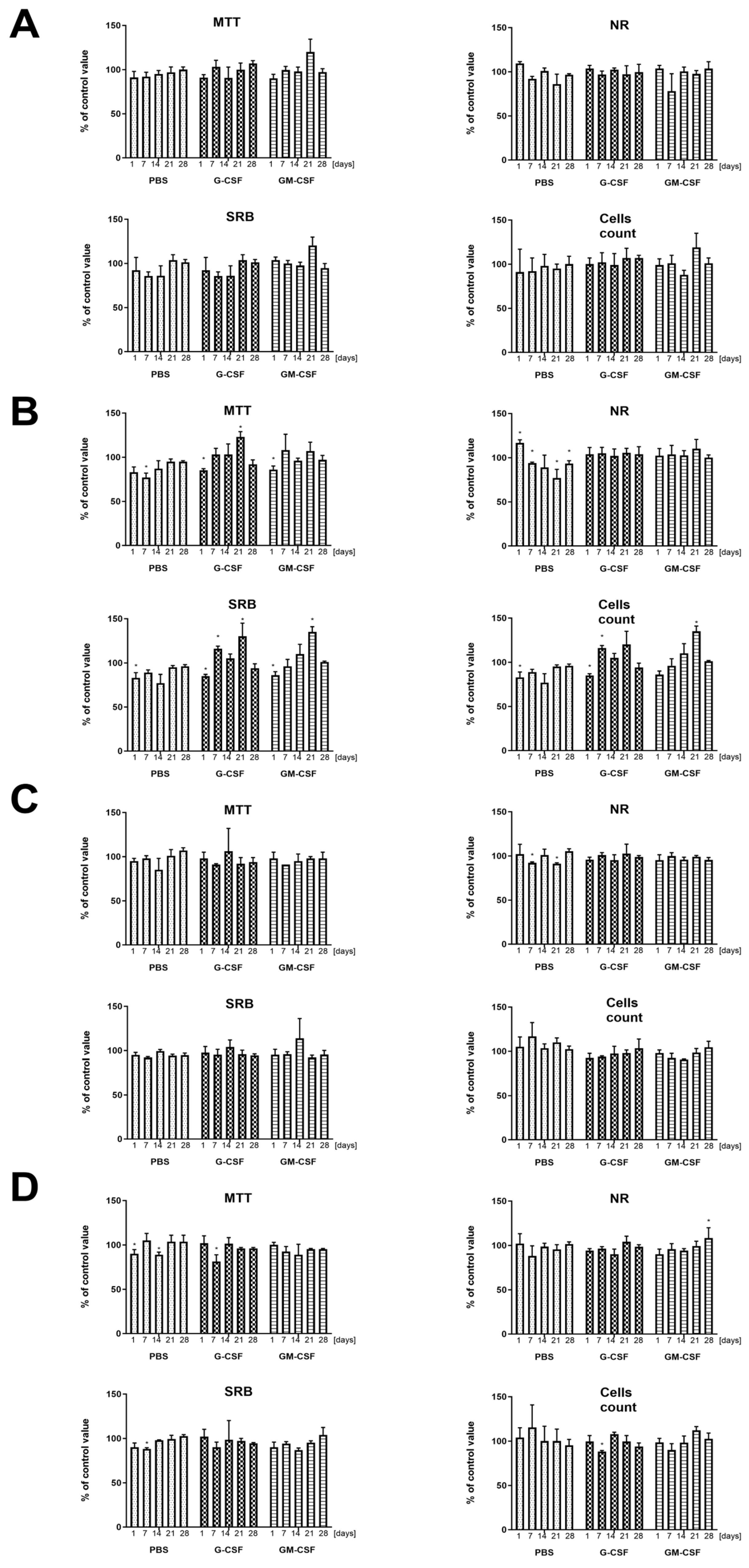
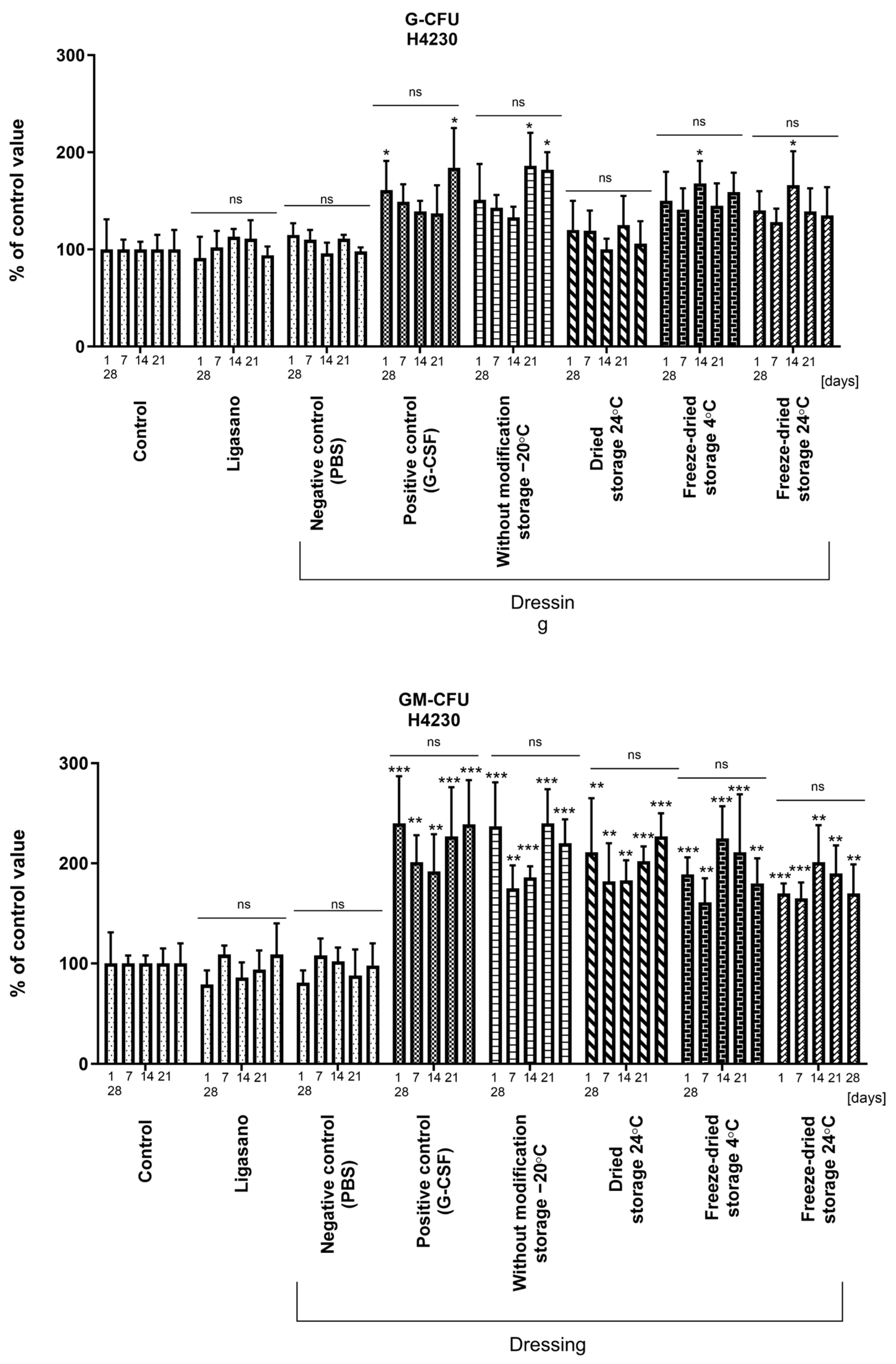
2.5. Biological Activity of Cytokines
3. Discussion
4. Materials and Methods
4.1. Cytokines
4.2. Dressing Carrier Fabrication
4.3. Incorporation of Cytokines into Dressing Carrier
4.4. Kinetics of Cytokine Release from the Dressing
4.5. Effect of Storage Conditions on Cytokine Recovery
- Dressings without modification: Dressings containing cytokines were placed in hermetically sealed containers, frozen at −80 °C, and preserved until analysis;
- Dried dressings: Dressings with cytokines were dried at 37 °C for 24 h, placed in hermetically sealed containers, and stored at room temperature in the dark;
- Freeze-dried dressings (4 °C): Dressings containing cytokines were lyophilized, placed in hermetically sealed containers, and stored at 4 °C;
- Freeze-dried dressings (24 °C): Dressings with cytokines were lyophilized, placed in hermetically sealed containers, and stored at 24 °C.
4.6. G-CSF and GM-CSF Concentration Analysis
4.7. Cell Isolation, Identification, and Culture
4.7.1. Umbilical Cord Stem Cells (UCSC)
4.7.2. Cord Blood Hematopoietic Stem Cells (CBHSC)
4.7.3. Granulocytes
4.8. Biological Assays
4.8.1. Preparation of Collected Aliquots of the Medium Obtained in the Cytokine Drug Release Assay for Biological Evaluation
4.8.2. Cytotoxicity
4.8.3. CBHSC Assay
4.8.4. Sterility Assessment
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Phillips, T.J. Wound Healing and Treating Wounds: Differential Diagnosis and Evaluation of Chronic Wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605; quiz 605–606. [Google Scholar] [CrossRef] [PubMed]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of Matrix Metalloproteinase in Wound Healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Boothby, I.C.; Cohen, J.N.; Rosenblum, M.D. Regulatory T Cells in Skin Injury: At the Crossroads of Tolerance and Tissue Repair. Sci. Immunol. 2020, 5, eaaz9631. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent Advances in the Controlled Release of Growth Factors and Cytokines for Improving Cutaneous Wound Healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Morris, L.M.; Papadopoulos, M.; Weinberg, L.; Filip, J.C.; Lang, S.A.; Vaikunth, S.S.; Crombleholme, T.M. Phase I study of H5.020CMV.PDGF-beta to treat venous leg ulcer disease. Mol Ther. 2009, 17, 1822–1829. [Google Scholar] [CrossRef]
- El Gazaerly, H.; Elbardisey, D.M.; Eltokhy, H.M.; Teaama, D. Effect of Transforming Growth Factor Beta 1 on Wound Healing in Induced Diabetic Rats. Int. J. Health Sci. 2013, 7, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Sallerfors, B.; Olofsson, T. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) and Granulocyte Colony-Stimulating Factor (G-CSF) Secretion by Adherent Monocytes Measured by Quantitative Immunoassays. Eur. J. Haematol. 1992, 49, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zsebo, K.M.; Yuschenkoff, V.N.; Schiffer, S.; Chang, D.; McCall, E.; Dinarello, C.A.; Brown, M.A.; Altrock, B.; Bagby, G.C. Vascular Endothelial Cells and Granulopoiesis: Interleukin-1 Stimulates Release of G-CSF and GM-CSF. Blood 1988, 71, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Baldi, L.; Zupo, S.; Dono, M.; Rinaldi, G.B.; Roncella, S.; Taborelli, G.; Truini, M.; Ferrarini, M.; Pistoia, V. Spontaneous Production of Granulocyte Colony-Stimulating Factor in Vitro by Human B-Lineage Lymphocytes Is a Distinctive Marker of Germinal Center Cells. J. Immunol. 1994, 153, 2868–2877. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Li, Y.; Rezakhanlou, A.M.; Ghahary, A. Keratinocyte-Releasable Factors Stimulate the Expression of Granulocyte Colony-Stimulating Factor in Human Dermal Fibroblasts. J. Cell. Biochem. 2017, 118, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Hannoush, E.J.; Sifri, Z.C.; Elhassan, I.O.; Mohr, A.M.; Alzate, W.D.; Offin, M.; Livingston, D.H. Impact of Enhanced Mobilization of Bone Marrow Derived Cells to Site of Injury. J. Trauma 2011, 71, 283–289. [Google Scholar] [CrossRef]
- Tanaka, M.; Dykes, P.J.; Marks, R. Keratinocyte Growth Stimulation by Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF). Keio J. Med. 1997, 46, 184–187. [Google Scholar] [CrossRef]
- Ead, J.K.; Armstrong, D.G. Granulocyte-Macrophage Colony-Stimulating Factor: Conductor of the Wound Healing Orchestra? Int. Wound J. 2023, 20, 1229–1234. [Google Scholar] [CrossRef]
- Fang, Y.; Gong, S.-J.; Xu, Y.-H.; Hambly, B.D.; Bao, S. Impaired Cutaneous Wound Healing in Granulocyte/Macrophage Colony-Stimulating Factor Knockout Mice. Br. J. Dermatol. 2007, 157, 458–465. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Novak, M.L.; Urao, N.; Sui, A.; Ennis, W.J.; Koh, T.J. Macrophage PPARγ and Impaired Wound Healing in Type 2 Diabetes. J. Pathol. 2015, 236, 433–444. [Google Scholar] [CrossRef]
- Aliper, A.M.; Frieden-Korovkina, V.P.; Buzdin, A.; Roumiantsev, S.A.; Zhavoronkov, A. A Role for G-CSF and GM-CSF in Nonmyeloid Cancers. Cancer Med. 2014, 3, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Tanha, S.; Rafiee-Tehrani, M.; Abdollahi, M.; Vakilian, S.; Esmaili, Z.; Naraghi, Z.S.; Seyedjafari, E.; Javar, H.A. G-CSF Loaded Nanofiber/Nanoparticle Composite Coated with Collagen Promotes Wound Healingn Vivo. J. Biomed. Mater. Res. Part A 2017, 105, 2830–2842. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.B.; Águas, A.P.; Barbosa, M.A.; Barbosa, J.N. Immunomodulatory Biomaterial-Based Wound Dressings Advance the Healing of Chronic Wounds via Regulating Macrophage Behavior. Regen. Biomater. 2022, 9, rbac065. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, L.; Zhang, H.; Sun, X.; Liu, D.; Zhang, J.; Zhang, Y.; Cheng, L.; Santos, H.A.; Cui, W. Programmable Immune Activating Electrospun Fibers for Skin Regeneration. Bioact. Mater. 2021, 6, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, G.; Majid, M.; Sherman, M.B.; Mukhopadhyay, S.; Wright, C.S.; Martin, P.E.; Dunn, A.K.; Baker, A.B. Syndesome Therapeutics for Enhancing Diabetic Wound Healing. Adv. Healthc. Mater. 2016, 5, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.E.; Sun, L.T.; Natesan, S.; Zamora, D.O.; Christy, R.J.; Washburn, N.R. Effects of Hyaluronic Acid Conjugation on Anti-TNF-α Inhibition of Inflammation in Burns. J. Biomed. Mater. Res. A 2014, 102, 1527–1536. [Google Scholar] [CrossRef]
- Xuan, X.; Zhou, Y.; Chen, A.; Zheng, S.; An, Y.; He, H.; Huang, W.; Chen, Y.; Yang, Y.; Li, S.; et al. Silver Crosslinked Injectable bFGF-Eluting Supramolecular Hydrogels Speed up Infected Wound Healing. J. Mater. Chem. B 2020, 8, 1359–1370. [Google Scholar] [CrossRef]
- Wang, P.; Huang, S.; Hu, Z.; Yang, W.; Lan, Y.; Zhu, J.; Hancharou, A.; Guo, R.; Tang, B. In Situ Formed Anti-Inflammatory Hydrogel Loading Plasmid DNA Encoding VEGF for Burn Wound Healing. Acta Biomater. 2019, 100, 191–201. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, H.; Qian, M.; Ji, G.; Wei, Y.; He, J.; Tian, H.; Zhao, Q. MSC-Derived sEV-Loaded Hyaluronan Hydrogel Promotes Scarless Skin Healing by Immunomodulation in a Large Skin Wound Model. Biomed. Mater. 2022, 17, 034104. [Google Scholar] [CrossRef]
- Hatibie, M.J.; Islam, A.A.; Hatta, M.; Moenadjat, Y.; Susilo, R.H.; Rendy, L. Hyperbaric Oxygen Therapy for Second-Degree Burn Healing: An Experimental Study in Rabbits. Adv. Ski. Wound Care 2019, 32, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, B.; Hu, J.; Xu, B.; Ni, P.; Hou, L.; Xie, T. Consensus on the Health Education of Home-Based Negative Pressure Wound Therapy for Patients with Chronic Wounds: A Modified Delphi Study. Burn. Trauma 2021, 9, tkab046. [Google Scholar] [CrossRef] [PubMed]
- Occleston, N.L.; Laverty, H.G.; O’Kane, S.; Ferguson, M.W.J. Prevention and Reduction of Scarring in the Skin by Transforming Growth Factor Beta 3 (TGFbeta3): From Laboratory Discovery to Clinical Pharmaceutical. J. Biomater. Sci. Polym. Ed. 2008, 19, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Tofigh, A.; Tajik, M. Comparing the Standard Surgical Dressing with Dehydrated Amnion and Platelet-Derived Growth Factor Dressings in the Healing Rate of Diabetic Foot Ulcer: A Randomized Clinical Trial. Diabetes Res. Clin. Pract. 2022, 185, 109775. [Google Scholar] [CrossRef]
- Grzybowski, J.; Ołdak, E.; Janiak, M.K. Local application of G-CSF, GM-CSF and EGF in treatment of wounds. Postepy Hig. Med. Dosw. 1999, 53, 75–86. [Google Scholar]
- Söderlund, Z.; Ibáñez-Fonseca, A.; Hajizadeh, S.; Rodríguez-Cabello, J.C.; Liu, J.; Ye, L.; Tykesson, E.; Elowsson, L.; Westergren-Thorsson, G. Controlled Release of Growth Factors Using Synthetic Glycosaminoglycans in a Modular Macroporous Scaffold for Tissue Regeneration. Commun. Biol. 2022, 5, 1349. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Li, J.; Du, N.; Li, D.; Li, F.; Man, J. Application Status and Technical Analysis of Chitosan-Based Medical Dressings: A Review. RSC Adv. 2020, 10, 34308–34322. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Nakayama, R.; Katsumata, K.; Niwa, Y.; Namiki, N. Dependence of Water-Permeable Chitosan Membranes on Chitosan Molecular Weight and Alkali Treatment. Membranes 2020, 10, 351. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan Based Hydrogels: Characteristics and Pharmaceutical Applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Fletes-Vargas, G.; Espinosa-Andrews, H.; Cervantes-Uc, J.M.; Limón-Rocha, I.; Luna-Bárcenas, G.; Vázquez-Lepe, M.; Morales-Hernández, N.; Jiménez-Ávalos, J.A.; Mejía-Torres, D.G.; Ramos-Martínez, P.; et al. Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties. Polymers 2023, 15, 2203. [Google Scholar] [CrossRef] [PubMed]
- Lusiana, R.A.; Protoningtyas, W.P.; Wijaya, A.R.; Siswanta, D.; Mudasir; Santosa, S.J. Chitosan-Tripoly Phosphate (CS-TPP) Synthesis through Cross-Linking Process: The Effect of Concentration towards Membrane Mechanical Characteristic and Urea Permeation. Orient. J. Chem. 2017, 33, 2913–2919. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of Ionic and Covalent Crosslinking Agents on Properties of Chitosan Beads and Sorption Effectiveness of Reactive Black 5 Dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. Evaluation of the Mucoadhesive Properties of Chitosan Nanoparticles Prepared Using Different Chitosan to Tripolyphosphate (CS:TPP) Ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, M.L.; Ismail, I.A.-N.; Refi, D.M.; Almeman, A.; Yaakob, N.C.; Saman, K.M.; Mansor, N.F.; Noordin, N.; Babar, Z.-U.-D. Management of COVID-19 Vaccines Cold Chain Logistics: A Scoping Review. J. Pharm. Policy Pract. 2022, 15, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Pritchard, E.; Hu, X.; Valentin, T.; Panilaitis, B.; Omenetto, F.G.; Kaplan, D.L. Stabilization of Vaccines and Antibiotics in Silk and Eliminating the Cold Chain. Proc. Natl. Acad. Sci. USA 2012, 109, 11981–11986. [Google Scholar] [CrossRef]
- Price, D.N.; Kunda, N.K.; Ellis, R.; Muttil, P. Design and Optimization of a Temperature-Stable Dry Powder BCG Vaccine. Pharm. Res. 2019, 37, 11. [Google Scholar] [CrossRef]
- Grzybowski, J.; Ołdak, E.; Antos-Bielska, M.; Janiak, M.K.; Pojda, Z. New Cytokine Dressings. I. Kinetics of the in Vitro rhG-CSF, rhGM-CSF, and rhEGF Release from the Dressings. Int. J. Pharm. 1999, 184, 173–178. [Google Scholar] [CrossRef]
- Power, G.; Moore, Z.; O’Connor, T. Measurement of pH, Exudate Composition and Temperature in Wound Healing: A Systematic Review. J. Wound Care 2017, 26, 381–397. [Google Scholar] [CrossRef]
- Paleczny, J.; Junka, A.; Brożyna, M.; Dydak, K.; Oleksy-Wawrzyniak, M.; Ciecholewska-Juśko, D.; Dziedzic, E.; Bartoszewicz, M. The High Impact of Staphylococcus aureus Biofilm Culture Medium on in Vitro Outcomes of Antimicrobial Activity of Wound Antiseptics and Antibiotic. Pathogens 2021, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Expert Working Group; Satellite Expert Working Group. Would Exudate and the Role of Dressings. Int. Wound J. 2008, 5, iii–12. [Google Scholar] [CrossRef]
- Iizaka, S.; Sanada, H.; Nakagami, G.; Koyanagi, H.; Konya, C.; Sugama, J. Quantitative Estimation of Exudate Volume for Full-thickness Pressure Ulcers: The ESTimation Method. J. Wound Care 2011, 20, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Open Resources for Nursing (Open RN); Ernstmeyer, K.; Christman, E. Table 10.6b—Wound Assessment. Available online: https://www.ncbi.nlm.nih.gov/books/NBK591822/table/ch10integumentary.T.wound_assessment/ (accessed on 6 December 2023).
- Modern Exudate Management: A Review of Wound Treatments. Available online: http://www.worldwidewounds.com/2006/september/White/Modern-Exudate-Mgt.html (accessed on 6 December 2023).
- Wiegand, C.; Tittelbach, J.; Hipler, U.-C.; Elsner, P. Clinical Efficacy of Dressings for Treatment of Heavily Exuding Chronic Wounds. Chronic Wound Care Manag. Res. 2015, 2, 101–111. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Qinna, N.A.; Karwi, Q.G.; Al-Jbour, N.; Al-Remawi, M.A.; Alhussainy, T.M.; Al-So’ud, K.A.; Al Omari, M.M.H.; Badwan, A.A. Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Mar. Drugs 2015, 13, 1710–1725. [Google Scholar] [CrossRef]
- Pieróg, M.; Gierszewska-Drużyńska, M.; Ostrowska-Czubenko, J. Effect of Ionic Crosslinking Agents on Swelling Behaviour of Chitosan Hydrogel Membranes. Prog. Chem. Appl. Chitin Deriv. 2009, XIV, 75–82. [Google Scholar]
- Omrani, M.; Naimi-Jamal, M.R.; Far, B.F. The Design of Multi-Responsive Nanohydrogel Networks of Chitosan for Controlled Drug Delivery. Carbohydr. Polym. 2022, 298, 120143. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan Solution Enhances the Immunoadjuvant Properties of GM-CSF. Vaccine 2007, 25, 8673–8686. [Google Scholar] [CrossRef]
- Noh, K.H.; Park, Y.M.; Kim, H.S.; Kang, T.H.; Song, K.-H.; Lee, Y.-H.; Byeon, Y.; Jeon, H.N.; Jung, I.D.; Shin, B.C.; et al. GM-CSF-Loaded Chitosan Hydrogel as an Immunoadjuvant Enhances Antigen-Specific Immune Responses with Reduced Toxicity. BMC Immunol. 2014, 15, 48. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Petrova, V.A.; Romanov, D.P.; Skorik, Y.A. pH-Sensitive Drug Delivery System Based on Chitin Nanowhiskers-Sodium Alginate Polyelectrolyte Complex. Materials 2022, 15, 5860. [Google Scholar] [CrossRef] [PubMed]
- Vakilian, S.; Mashayekhan, S.; Shabani, I.; Khorashadizadeh, M.; Fallah, A.; Soleimani, M. Structural Stability and Sustained Release of Protein from a Multilayer Nanofiber/Nanoparticle Composite. Int. J. Biol. Macromol. 2015, 75, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Olschläger, V.; Schrader, A.; Hockertz, S. Comparison of Primary Human Fibroblasts and Keratinocytes with Immortalized Cell Lines Regarding Their Sensitivity to Sodium Dodecyl Sulfate in a Neutral Red Uptake Cytotoxicity Assay. Arzneimittelforschung 2009, 59, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Nickel, A. Toxic or Not Toxic? The Specifications of the Standard ISO 10993-5 Are Not Explicit Enough to Yield Comparable Results in the Cytotoxicity Assessment of an Identical Medical Device. Front. Med. Technol. 2023, 5, 1195529. [Google Scholar] [CrossRef] [PubMed]
- Rustad, K.C.; Gurtner, G.C. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv. Wound Care 2012, 1, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and Cytotoxicity of Chitosan-Based Hydrogels Modified with Silver Nanoparticles. Colloids Surf. B Biointerfaces 2017, 160, 325–330. [Google Scholar] [CrossRef]
- Wiegand, C.; Winter, D.; Hipler, U.-C. Molecular-Weight-Dependent Toxic Effects of Chitosans on the Human Keratinocyte Cell Line HaCaT. Skin Pharmacol. Physiol. 2010, 23, 164–170. [Google Scholar] [CrossRef]
- Schuettpelz, L.G.; Borgerding, J.N.; Christopher, M.J.; Gopalan, P.K.; Romine, M.P.; Herman, A.C.; Woloszynek, J.R.; Greenbaum, A.M.; Link, D.C. G-CSF Regulates Hematopoietic Stem Cell Activity, in Part, through Activation of Toll-Like Receptor Signaling. Leukemia 2014, 28, 1851–1860. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, Z. Surgical Wound Healing Using Hemostatic Gauze Scaffold Loaded with Nanoparticles Containing Sustained-Release Granulocyte Colony-Stimulating Factor. Int. J. Nanomed. 2011, 6, 3139–3149. [Google Scholar] [CrossRef]
- Huang, G.; Sun, T.; Zhang, L.; Wu, Q.; Zhang, K.; Tian, Q.; Huo, R. Combined Application of Alginate Dressing and Human Granulocyte-Macrophage Colony Stimulating Factor Promotes Healing in Refractory Chronic Skin Ulcers. Exp. Ther. Med. 2014, 7, 1772–1776. [Google Scholar] [CrossRef]
- Jaschke, E.; Zabernigg, A.; Gattringer, C. Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor Applied Locally in Low Doses Enhances Healing and Prevents Recurrence of Chronic Venous Ulcers. Int. J. Dermatol. 1999, 38, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Şalva, E.; Alan, S.; Karakoyun, B.; Çakalağaoğlu, F.; Özbaş, S.; Akbuğa, J. Investigation of Therapeutic Effects in the Wound Healing of Chitosan/pGM-CSF Complexes. Braz. J. Pharm. Sci. 2022, 58, e19668. [Google Scholar] [CrossRef]
- Karimi Dehkordi, N.; Minaiyan, M.; Talebi, A.; Akbari, V.; Taheri, A. Nanocrystalline Cellulose-Hyaluronic Acid Composite Enriched with GM-CSF Loaded Chitosan Nanoparticles for Enhanced Wound Healing. Biomed. Mater. 2019, 14, 035003. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, M.; Zdanowski, R.; Suska, M.; Brewczyńska, A.; Stankiewicz, W.; Kloc, M.; Kubiak, J.Z.; Lewicki, S. Effects of Hexachlorophene, a Chemical Accumulating in Adipose Tissue, on Mouse and Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 211–222. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewicki, S.; Zwoliński, M.; Hovagimyan, A.; Stelmasiak, M.; Szarpak, Ł.; Lewicka, A.; Pojda, Z.; Szymański, Ł. Chitosan-Based Dressing as a Sustained Delivery System for Bioactive Cytokines. Int. J. Mol. Sci. 2024, 25, 30. https://doi.org/10.3390/ijms25010030
Lewicki S, Zwoliński M, Hovagimyan A, Stelmasiak M, Szarpak Ł, Lewicka A, Pojda Z, Szymański Ł. Chitosan-Based Dressing as a Sustained Delivery System for Bioactive Cytokines. International Journal of Molecular Sciences. 2024; 25(1):30. https://doi.org/10.3390/ijms25010030
Chicago/Turabian StyleLewicki, Sławomir, Michał Zwoliński, Adrian Hovagimyan, Marta Stelmasiak, Łukasz Szarpak, Aneta Lewicka, Zygmunt Pojda, and Łukasz Szymański. 2024. "Chitosan-Based Dressing as a Sustained Delivery System for Bioactive Cytokines" International Journal of Molecular Sciences 25, no. 1: 30. https://doi.org/10.3390/ijms25010030
APA StyleLewicki, S., Zwoliński, M., Hovagimyan, A., Stelmasiak, M., Szarpak, Ł., Lewicka, A., Pojda, Z., & Szymański, Ł. (2024). Chitosan-Based Dressing as a Sustained Delivery System for Bioactive Cytokines. International Journal of Molecular Sciences, 25(1), 30. https://doi.org/10.3390/ijms25010030





