Comparative 4D Label-Free Quantitative Proteomic Analysis of Bombus terrestris Provides Insights into Proteins and Processes Associated with Diapause
Abstract
:1. Introduction
2. Results
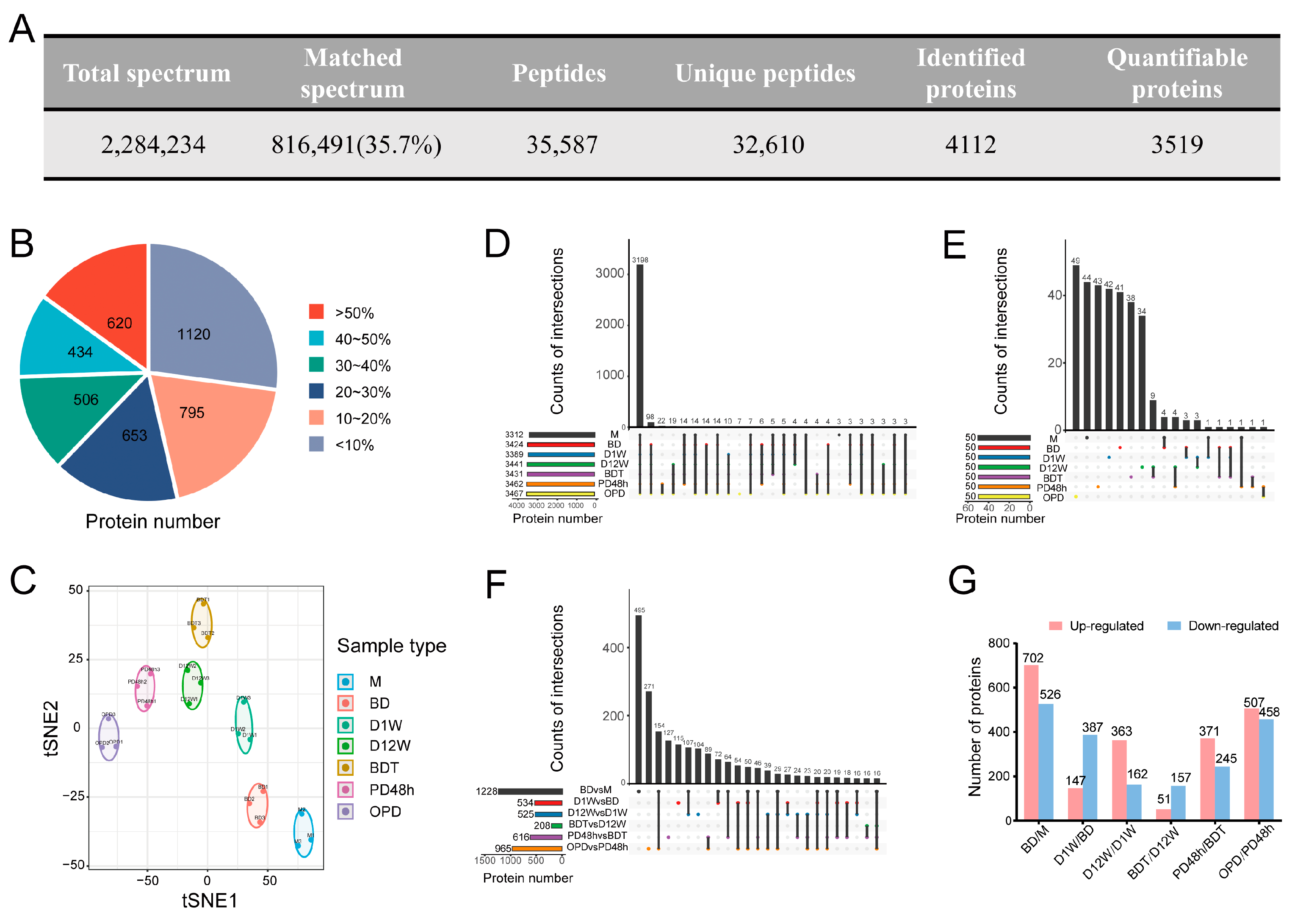
2.1. Related Protein Profiles in Seven Stages Regarding 4D-LFQ Proteomic Analyses of B. terrestris Queen Diapause
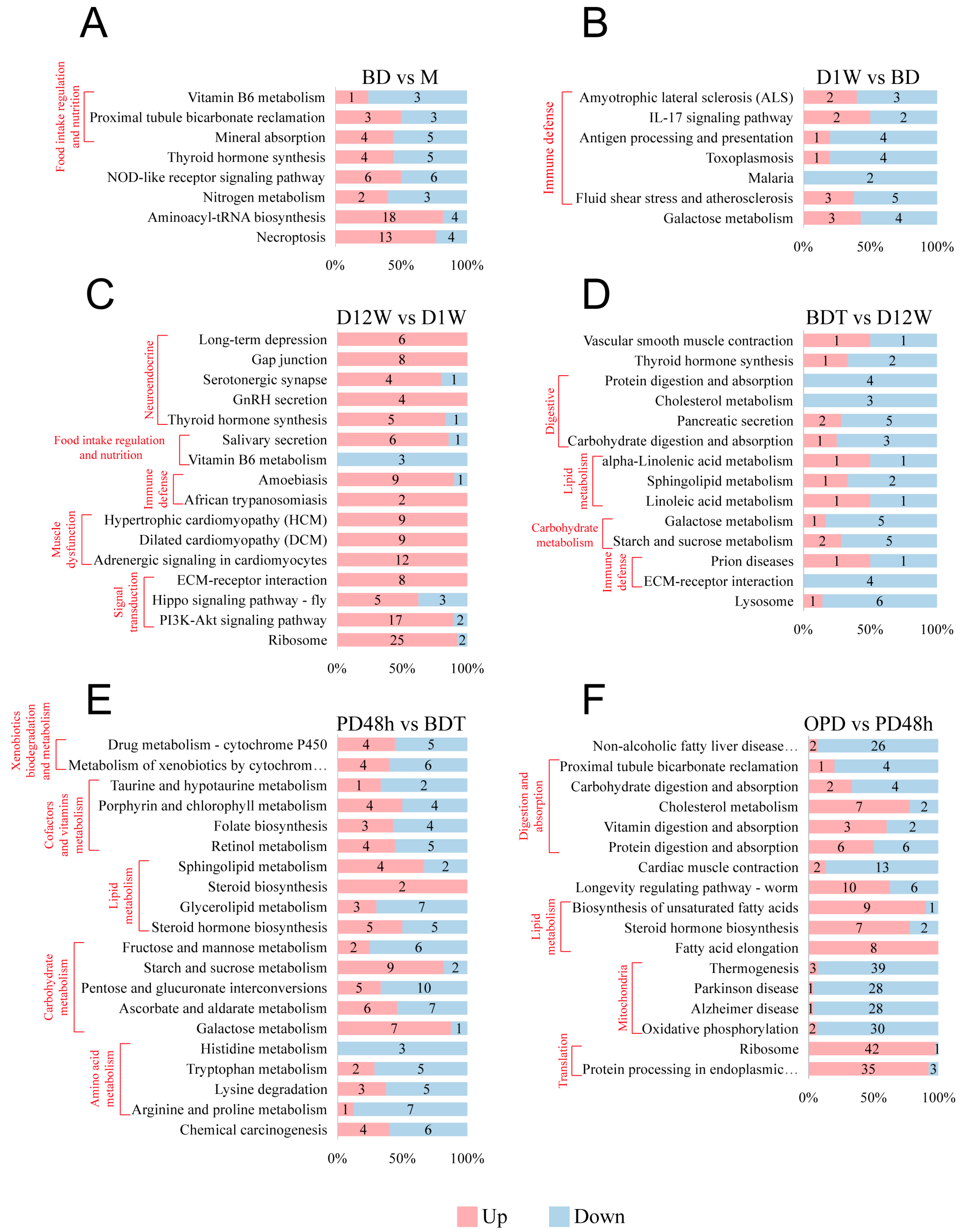
2.2. DAP Analysis
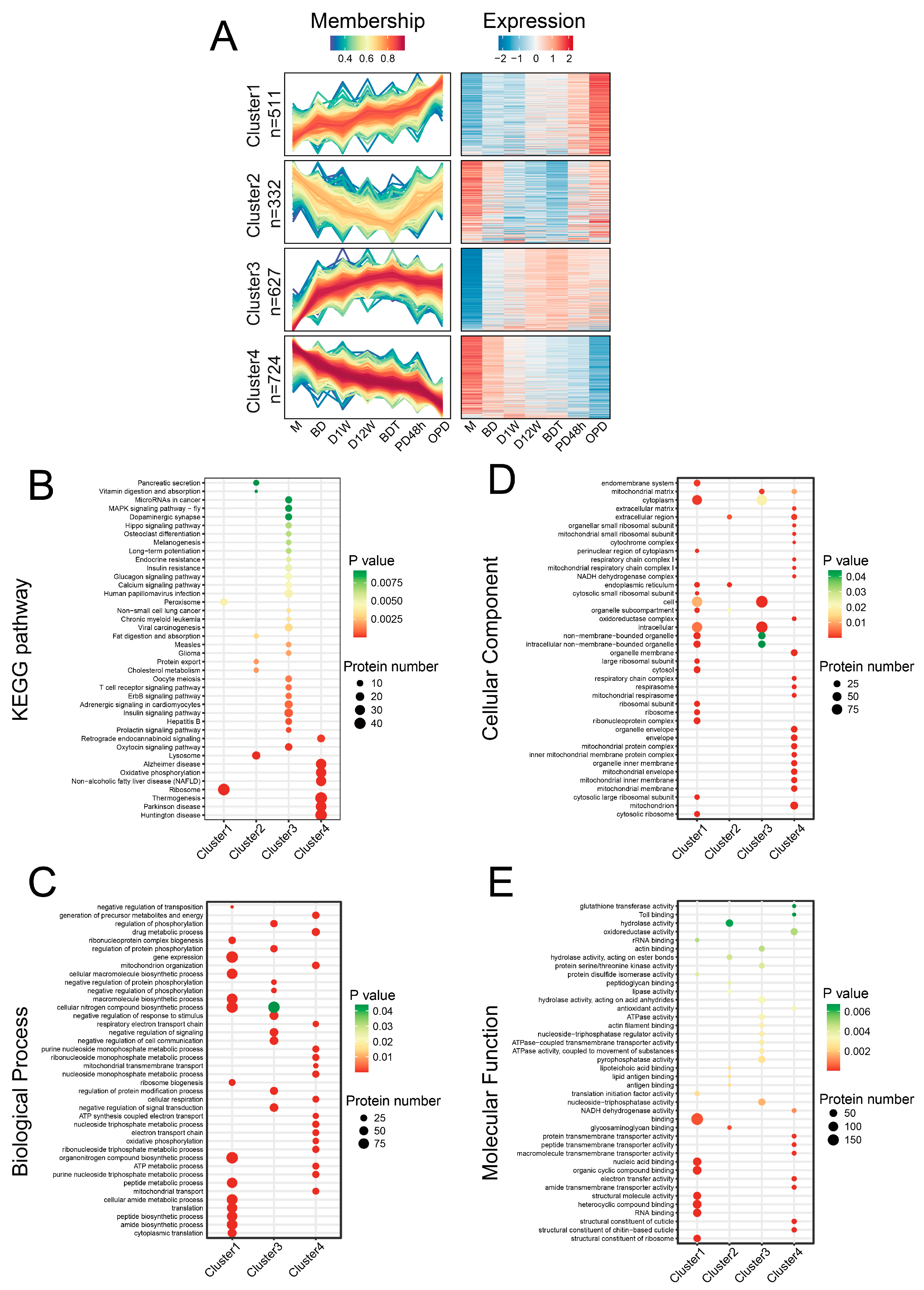
2.3. Temporal Expression Patterns of Proteins in Different Diapause Stages
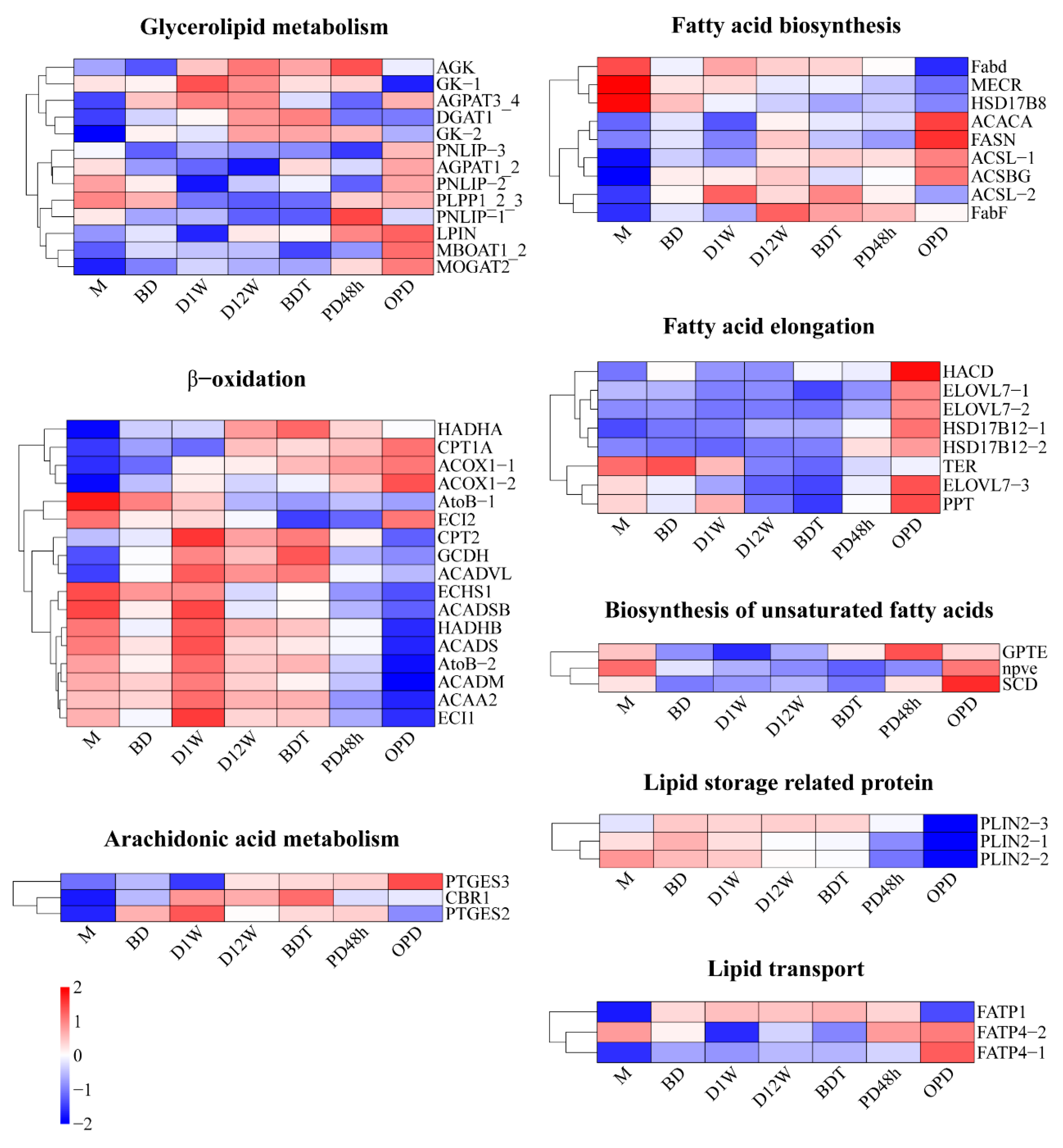
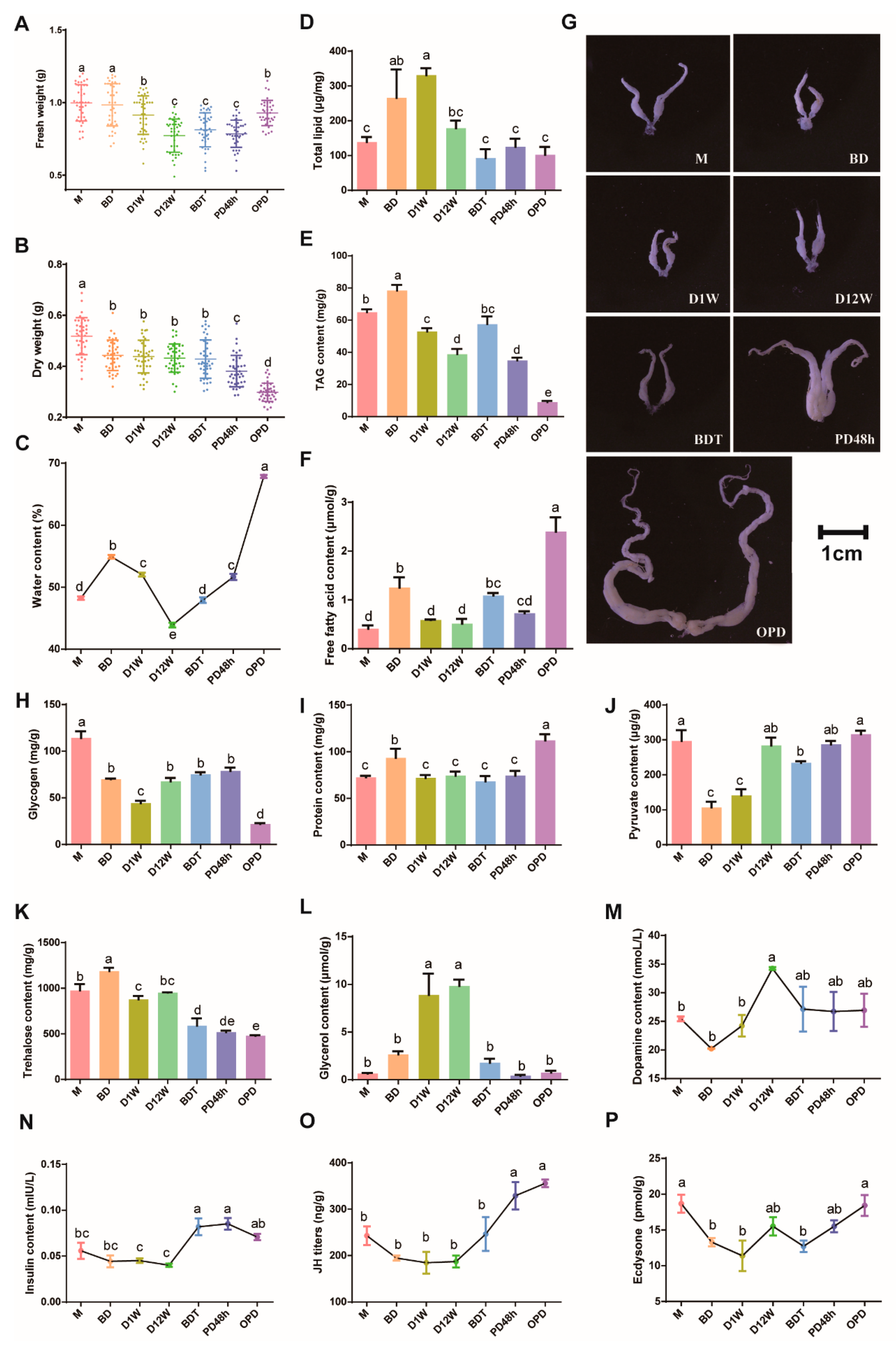
2.4. Lipid Metabolism during Diapause
2.5. Carbohydrate Metabolism during Diapause
2.6. Substance Content and Phenotypic Changes in Different Diapause Stages
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Protein Extraction and Trypsin Enzymatic Digestion
4.3. 4D-LFQ LC–MS Analysis
4.4. The Proteome Database Searching
4.5. Bioinformatics and Annotations
4.6. Measurement of Weight, Water, Total Lipids, Triacylglycerol (TAG), and Free Acid Content
4.7. Measurement of Other Substances
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacRae, T.H. Gene expression, metabolic regulation and stress tolerance during diapause. Cell. Mol. Life Sci. 2010, 67, 2405–2424. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, E.; Galbraith, D.A.; Cnaani, J.; Teal, P.E.A.; Grozinger, C.M. Conservation and modification of genetic and physiological toolkits underpinning diapause in bumble bee queens. Mol. Ecol. 2015, 24, 5596–5615. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F. Optimal switching to diapause in relation to the onset of winter. Theor. Popul. Biol. 1980, 18, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Koštál, V. Eco-physiological phases of insect diapause. J. Insect Physiol. 2006, 52, 113–127. [Google Scholar] [CrossRef]
- Denlinger, D.L. Basis fro a skewed sex ratio in diapause-destined flesh flies. Evolution 1981, 35, 1247–1248. [Google Scholar]
- Irwin, J.T.; Lee, R.E. Mild winter temperatures reduce survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis (Diptera: Tephritidae). J. Insect Physiol. 2000, 46, 655–661. [Google Scholar] [CrossRef]
- Velthuis, H.H.W.; Duchateau, M.J. Development and reproductive strategies in Bombus terrestris colonies. Behaviour 1988, 107, 186–207. [Google Scholar]
- Velthuis, H.H.W.; van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Su, L.; Zhao, S.; Dai, X.; Chen, H.; Wu, G.A.; Zhou, H.; Zheng, L.; Zhai, Y. Integrative Proteomic and Phosphoproteomic Analyses Revealed Complex Mechanisms Underlying Reproductive Diapause in Bombus terrestris Queens. Insects 2022, 13, 862. [Google Scholar] [CrossRef]
- Chen, H.; Wu, G.A.; Zhou, H.; Dai, X.; Steeghs, N.W.F.; Dong, X.; Zheng, L.; Zhai, Y. Hormonal Regulation of Reproductive Diapause That Occurs in the Year-Round Mass Rearing of Bombus terrestris Queens. J. Proteome Res. 2021, 20, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Walser, J.C. The biological limitations of transcriptomics in elucidating stress and stress responses. J. Evol. Biol. 2005, 18, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Zhang, L.S.; Han, Y.H.; An, T.; Liu, Y.; Li, Y.Y.; Chen, H.Y. Proteomic research on diapause-related proteins in the female ladybird, Coccinella septempunctata L. Bull. Entomol. Res. 2015, 106, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Y.; Zhou, X.-R.; Tan, Y.; Pang, B.-P. Proteomic analysis of adult Galeruca daurica (Coleoptera: Chrysomelidae) at different stages during summer diapause. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 351–357. [Google Scholar] [CrossRef]
- Zhai, Y.; Dong, X.; Gao, H.; Chen, H.; Yang, P.; Li, P.; Yin, Z.; Zheng, L.; Yu, Y. Quantitative Proteomic and Transcriptomic Analyses of Metabolic Regulation of Adult Reproductive Diapause in Drosophila suzukii (Diptera: Drosophilidae) Females. Front. Physiol. 2019, 10, 344. [Google Scholar] [CrossRef]
- Colgan, T.J.; Finlay, S.; Brown, M.J.F.; Carolan, J.C. Mating precedes selective immune priming which is maintained throughout bumblebee queen diapause. BMC Genom. 2019, 20, 1–18. [Google Scholar] [CrossRef]
- Meier, F.; Brunner, A.D.; Koch, S.; Koch, H.; Lubeck, M.; Krause, M.; Goedecke, N.; Decker, J.; Kosinski, T.; Park, M.A.; et al. Online Parallel Accumulation-Serial Fragmentation (PASEF) with a Novel Trapped Ion Mobility Mass Spectrometer. Mol. Cell. Proteom. 2018, 17, 2534–2545. [Google Scholar] [CrossRef]
- Brzhozovskiy, A.; Kononikhin, A.; Bugrova, A.E.; Kovalev, G.I.; Schmit, P.-O.; Kruppa, G.; Nikolaev, E.N.; Borchers, C.H. The Parallel Reaction Monitoring-Parallel Accumulation–Serial Fragmentation (prm-PASEF) Approach for Multiplexed Absolute Quantitation of Proteins in Human Plasma. Anal. Chem. 2022, 94, 2016–2022. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Energetics of Insect Diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Marshall, K.E.; Suarez, R.K.; Hoppeler, H.H. The many roles of fats in overwintering insects. J. Exp. Biol. 2018, 221 (Suppl. 1), jeb161836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z.; Wang, X.; Kong, X.; Zhao, Y.M.; Xu, M.H.; Gao, Y.Q.; Huang, H.Y.; Liu, F.H.; Wang, S.; Xu, Y.Y.; et al. Quantitative transcriptomic and proteomic analyses reveal the potential maintenance mechanism of female adult reproductive diapause in Chrysoperla nipponensis. Pest Manag. Sci. 2023, 79, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, Y.; Goto, M. Physiological and biochemical changes in summer and winter diapause and non-diapause pupae of the cabbage armyworm, Mamestra brassicae L. during long-term cold acclimation. J. Insect Physiol. 2003, 49, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Miesfeld, R.L. Energy metabolism during diapause in Culex pipiens mosquitoes. J. Insect Physiol. 2009, 55, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.N. Trehalose—The Insect ‘Blood’ Sugar. Adv. Insect Physiol. 2003, 31 (Suppl. C), 205–285. [Google Scholar]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Liu, Z.; Torson, A.S. Energy Consumption and Cold Hardiness of Diapausing Fall Webworm Pupae. Insects 2022, 13, 853. [Google Scholar] [CrossRef]
- Xiao, Q.H.; He, Z.; Wu, R.W.; Zhu, D.H. Physiological and biochemical differences in diapause and non-diapause pupae of Sericinus montelus (Lepidoptera: Papilionidae). Front. Physiol. 2022, 13, 1031654. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Yocum, G.D.; Rinehart, J.P. Hormonal control of diapause. In Insect Endocrinology; Gilbert, L.I., Iatrou, K., Gill, S., Eds.; Academic Press: New York, NY, USA, 2012; Volume 8, pp. 430–463. [Google Scholar]
- Morgan, T.D.; Chippendale, G.M. Free amino acids of the haemolymph of the southwestern corn borer and the European corn borer in relation to their diapause. J. Insect Physiol. 1983, 29, 735–740. [Google Scholar] [CrossRef]
- Tan, Q.Q.; Feng, L.; Liu, W.; Zhu, L.; Lei, C.L.; Wang, X.P. Differences in the pre-diapause and pre-oviposition accumulation of critical nutrients in adult females of the beetle Colaphellus bowringi. Entomol. Exp. Appl. 2016, 160, 117–125. [Google Scholar] [CrossRef]
- Patil, Y.N.; Gnaiger, E.; Landry, A.P.; Leno, Z.J.; Hand, S.C. OXPHOS capacity is diminished and the phosphorylation system inhibited during diapause in an extremophile, embryos of Artemia franciscana. J. Exp. Biol. 2023, jeb.245828. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Palli, S.R.; Guo, S.; Tian, Z.; Wu, Q.-W.; King-Jones, K.; Liu, W.; Zhu, F.; Wang, X.-P. Steroid hormone ecdysone deficiency stimulates preparation for photoperiodic reproductive diapause. PLoS Genet. 2021, 17, e1009352. [Google Scholar]
- Sim, C.; Denlinger, D.L. Insulin signaling and the regulation of insect diapause. Front. Physiol. 2013, 4, 189. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, J.J.; Liu, M.Y.; He, W.W.; Reynolds, J.A.; Wang, Y.N.; Wang, M.Q.; Zhang, L.S. Enhanced Degradation of Juvenile Hormone Promotes Reproductive Diapause in the Predatory Ladybeetle Coccinella Septempunctata. Front. Physiol. 2022, 13, 877153. [Google Scholar] [CrossRef]
- Mukai, A.; Mano, G.; Des Marteaux, L.; Shinada, T.; Goto, S.G. Juvenile hormone as a causal factor for maternal regulation of diapause in a wasp. Insect Biochem. Mol. Biol. 2022, 144, 103758. [Google Scholar] [CrossRef]
- de Kort, C.A.D. Thirty-five years of diapause research with the Colorado potato beetle. Entomol. Exp. Appl. 1990, 56, 1–13. [Google Scholar] [CrossRef]
- Kim, M.; Denlinger, D.L. A potential role for ribosomal protein S2 in the gene network regulating reproductive diapause in the mosquito Culex pipiens. J. Comp. Physiol. B 2010, 180, 171–178. [Google Scholar] [CrossRef]
- Kim, M.; Sim, C.; Denlinger, D.L. RNA interference directed against ribosomal protein S3a suggests a link between this gene and arrested ovarian development during adult diapause in Culex pipiens. Insect Mol. Biol. 2010, 19, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Swevers, L.; Iatrou, K. The ecdysone regulatory cascade and ovarian development in lepidopteran insects: Insights from the silkmoth paradigm. Insect Biochem. Mol. Biol. 2003, 33, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Telfer, W.H. Egg formation in lepidoptera. J. Insect Sci. 2009, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Sheng, Z.; Sun, Z.; Palli, S.R. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Xu, J.; Bai, H.; Zhu, F.; Palli, S.R. Juvenile Hormone Regulates Vitellogenin Gene Expression through Insulin-like Peptide Signaling Pathway in the Red Flour Beetle, Tribolium castaneum. J. Biol. Chem. 2011, 286, 41924–41936. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Serafin, R.B.; Watkins, N.L.; Richard, D.S. Ecdysteroids regulate yolk protein uptake by Drosophila melanogaster oocytes. J. Insect Physiol. 1998, 44, 637–644. [Google Scholar]
- Richard, D.S.; Jones, J.M.; Barbarito, M.R.; Cerula, S.; Detweiler, J.P.; Fisher, S.J.; Brannigan, D.M.; Scheswohl, D.M. Vitellogenesis in diapausing and mutant Drosophila melanogaster: Further evidence for the relative roles of ecdysteroids and juvenile hormones. J. Insect Physiol. 2001, 47, 905–913. [Google Scholar] [CrossRef]
- Noguchi, H.; Hayakawa, Y. Dopamine is a key factor for the induction of egg diapause of the silkworm, Bombyx mori. Eur. J. Biochem. 2001, 268, 774–780. [Google Scholar] [CrossRef]
- Noguchi, H.; Hayakawa, Y. Role of dopamine at the onset of pupal diapause in the cabbage armyworm Mamestra brassicae. FEBS Lett. 1997, 413, 157–161. [Google Scholar] [CrossRef]
- Jakšić, A.M.; Karner, J.; Nolte, V.; Hsu, S.K.; Barghi, N.; Mallard, F.; Otte, K.A.; Svečnjak, L.; Senti, K.A.; Schlötterer, C. Neuronal Function and Dopamine Signaling Evolve at High Temperature in Drosophila. Mol. Biol. Evol. 2020, 37, 2630–2640. [Google Scholar] [CrossRef]
- Uefune, F.; Aonishi, T.; Kitaguchi, T.; Takahashi, H.; Seino, S.; Sakano, D.; Kume, S. Dopamine Negatively Regulates Insulin Secretion Through Activation of D1-D2 Receptor Heteromer. Diabetes 2022, 71, 1946–1961. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Vernon, P.; Hance, T. Does thermal-related plasticity in size and fat reserves influence supercooling abilities and cold-tolerance in Aphidius colemani (Hymenoptera: Aphidiinae) mummies? J. Therm. Biol. 2007, 32, 374–382. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ma, J.; Liu, Y.; Chen, Z.; Xiao, N.; Lu, Y.; Fu, Y.; Yang, C.; Li, M.; Wu, S.; et al. iProX in 2021: Connecting proteomics data sharing with big data. Nucleic Acids Res. 2022, 50, D1522–D1527. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Su, L.; Wang, R.; Dai, X.; Li, X.; Chang, Y.; Zhao, S.; Chen, H.; Yin, Z.; Wu, G.; et al. Comparative 4D Label-Free Quantitative Proteomic Analysis of Bombus terrestris Provides Insights into Proteins and Processes Associated with Diapause. Int. J. Mol. Sci. 2024, 25, 326. https://doi.org/10.3390/ijms25010326
Liu Y, Su L, Wang R, Dai X, Li X, Chang Y, Zhao S, Chen H, Yin Z, Wu G, et al. Comparative 4D Label-Free Quantitative Proteomic Analysis of Bombus terrestris Provides Insights into Proteins and Processes Associated with Diapause. International Journal of Molecular Sciences. 2024; 25(1):326. https://doi.org/10.3390/ijms25010326
Chicago/Turabian StyleLiu, Yan, Long Su, Ruijuan Wang, Xiaoyan Dai, Xiuxue Li, Yuqing Chang, Shan Zhao, Hao Chen, Zhenjuan Yin, Guang’an Wu, and et al. 2024. "Comparative 4D Label-Free Quantitative Proteomic Analysis of Bombus terrestris Provides Insights into Proteins and Processes Associated with Diapause" International Journal of Molecular Sciences 25, no. 1: 326. https://doi.org/10.3390/ijms25010326
APA StyleLiu, Y., Su, L., Wang, R., Dai, X., Li, X., Chang, Y., Zhao, S., Chen, H., Yin, Z., Wu, G., Zhou, H., Zheng, L., & Zhai, Y. (2024). Comparative 4D Label-Free Quantitative Proteomic Analysis of Bombus terrestris Provides Insights into Proteins and Processes Associated with Diapause. International Journal of Molecular Sciences, 25(1), 326. https://doi.org/10.3390/ijms25010326





