Molecular Therapies in Cardiovascular Diseases: Small Interfering RNA in Atherosclerosis, Heart Failure, and Hypertension
Abstract
1. Introduction
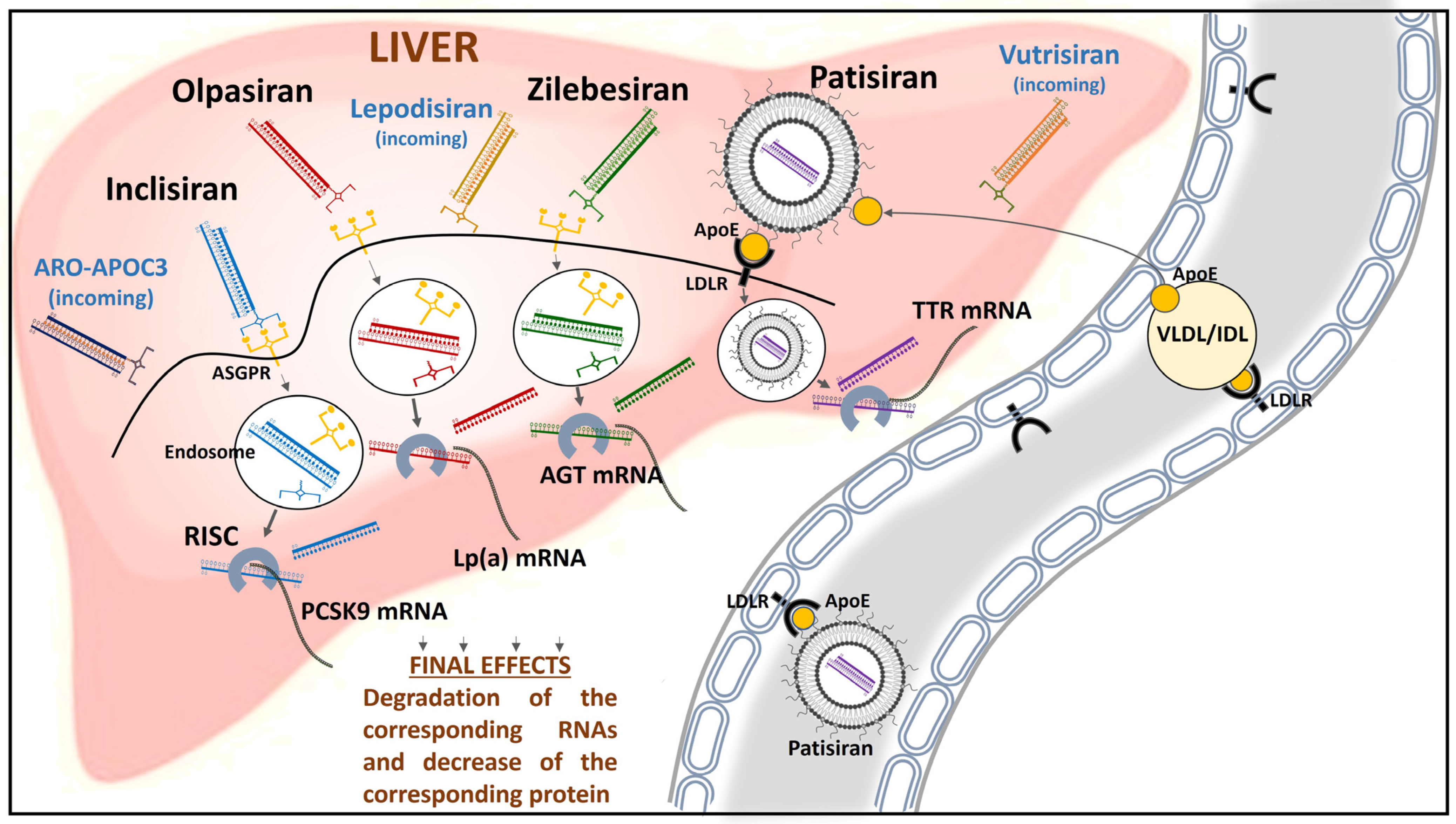
2. Inclisiran
3. Olpasiran
4. Patisiran (and Vutrisiran)
5. Zilebesiran
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levin, A.A. Targeting Therapeutic Oligonucleotides. N. Engl. J. Med. 2017, 376, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Bernards, R. The Nobel Prize in Physiology or Medicine for 2006 for the discovery of RNA interference. Ned. Tijdschr. Geneeskd. 2006, 150, 2849–2853. [Google Scholar]
- Ranasinghe, P.; Addison, M.L.; Dear, J.W.; Webb, D.J. Small interfering RNA: Discovery, pharmacology and clinical development-An introductory review. Br. J. Pharmacol. 2023, 180, 2697–2720. [Google Scholar] [CrossRef] [PubMed]
- Khvorova, A. Oligonucleotide Therapeutics—A New Class of Cholesterol-Lowering Drugs. N. Engl. J. Med. 2017, 376, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Charlton, F.; Rye, K.-A.; Piper, D.E. Molecular basis of PCSK9 function. Atherosclerosis 2009, 203, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Soffer, D.; Stoekenbroek, R.; Plakogiannis, R. Small interfering ribonucleic acid for cholesterol lowering—Inclisiran: Inclisiran for cholesterol lowering. J. Clin. Lipidol. 2022, 16, 574–582. [Google Scholar] [CrossRef]
- Fitzgerald, K.; Frank-Kamenetsky, M.; Shulga-Morskaya, S.; Liebow, A.; Bettencourt, B.R.; Sutherland, J.E.; Hutabarat, R.M.; Clausen, V.A.; Karsten, V.; Cehelsky, J.; et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: A randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 2014, 383, 60–68. [Google Scholar] [CrossRef]
- Wright, R.S.; Collins, M.G.; Stoekenbroek, R.M.; Robson, R.; Wijngaard, P.L.J.; Landmesser, U.; Leiter, L.A.; Kastelein, J.J.P.; Ray, K.K.; Kallend, D. Effects of Renal Impairment on the Pharmacokinetics, Efficacy, and Safety of Inclisiran: An Analysis of the ORION-7 and ORION-1 Studies. Mayo Clin. Proc. 2020, 95, 77–89. [Google Scholar] [CrossRef]
- Kallend, D.; Stoekenbroek, R.; He, Y.; Smith, P.F.; Wijngaard, P. Pharmacokinetics and pharmacodynamics of inclisiran, a small interfering RNA therapy, in patients with hepatic impairment. J. Clin. Lipidol. 2022, 16, 208–219. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Naz, A.; Qamar Masood, M.; Shah, R. Meta-Analysis of Inclisiran for the Treatment of Hypercholesterolemia. Am. J. Cardiol. 2020, 134, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Asbeutah, A.A.A.; Asbeutah, S.A.; Abu-Assi, M.A. A Meta-Analysis of Cardiovascular Outcomes in Patients with Hypercholesterolemia Treated with Inclisiran. Am. J. Cardiol. 2020, 128, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Inclisiran: How Widely and When Should We Use It? Curr. Atheroscler. Rep. 2022, 24, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.; Watford, M.; Egolum, U.O.; Ombengi, D.N.; Ling, H.; Cates, D.W. Inclisiran: A First-in-Class siRNA Therapy for Lowering Low-Density Lipoprotein Cholesterol. Ann. Pharmacother. 2023, 57, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Allevi, M.; Sarnari, S.; Giulietti, F.; Spannella, F.; Di Pentima, C.; Sarzani, R. Painful and recurring injection site reaction to alirocumab and evolocumab in a young woman with familial hypercholesterolemia and effective therapeutic alternative based on inclisiran: A case report. Front. Cardiovasc. Med. 2023, 10, 1181720. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.S.K.; Kiese, S.; Fischer, S.; Pappenberger, A.; Grauschopf, U.; Mahler, H.-C. The degradation of polysorbates 20 and 80 and its potential impact on the stability of biotherapeutics. Pharm. Res. 2011, 28, 1194–1210. [Google Scholar] [CrossRef]
- Moussa, E.M.; Panchal, J.P.; Moorthy, B.S.; Blum, J.S.; Joubert, M.K.; Narhi, L.O.; Topp, E.M. Immunogenicity of Therapeutic Protein Aggregates. J. Pharm. Sci. 2016, 105, 417–430. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009, 301, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; Kerr, K.F.; Pechlivanis, S.; Budoff, M.J.; Harris, T.B.; et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 2013, 368, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhu, Y.; Chen, Z.; Yan, R.; Liu, J.; He, Z.; Zhang, L.; Zhang, F.; Yan, S. Impact of alirocumab/evolocumab on lipoprotein (a) concentrations in patients with familial hypercholesterolaemia: A systematic review and meta-analysis of randomized controlled trials. Endokrynol. Pol. 2023, 74, 234–242. [Google Scholar] [CrossRef]
- Hardy, J.; Niman, S.; Goldfaden, R.F.; Ashchi, M.; Bisharat, M.; Huston, J.; Hartmann, H.; Choksi, R. A Review of the Clinical Pharmacology of Pelacarsen: A Lipoprotein(a)-Lowering Agent. Am. J. Cardiovasc. Drugs 2022, 22, 47–54. [Google Scholar] [CrossRef]
- Koren, M.J.; Moriarty, P.M.; Baum, S.J.; Neutel, J.; Hernandez-Illas, M.; Weintraub, H.S.; Florio, M.; Kassahun, H.; Melquist, S.; Varrieur, T.; et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat. Med. 2022, 28, 96–103. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- Tsimikas, S.; Clopton, P.; Brilakis, E.S.; Marcovina, S.M.; Khera, A.; Miller, E.R.; de Lemos, J.A.; Witztum, J.L. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: Results from the Dallas Heart Study. Circulation 2009, 119, 1711–1719. [Google Scholar] [CrossRef]
- Nissen, S.E.; Linnebjerg, H.; Shen, X.; Wolski, K.; Ma, X.; Lim, S.; Michael, L.F.; Ruotolo, G.; Gribble, G.; Navar, A.M.; et al. Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a): A Randomized Dose-Ascending Clinical Trial. JAMA 2023, 330, 2075. [Google Scholar] [CrossRef]
- Kraaijenhof, J.M.; Stroes, E.S.G. Apolipoprotein C-III Inhibition—Killing Two Birds with One Stone? NEJM Evid. 2023, 2, EVIDe2300239. [Google Scholar] [CrossRef]
- Gaudet, D.; Clifton, P.; Sullivan, D.; Baker, J.; Schwabe, C.; Thackwray, S.; Scott, R.; Hamilton, J.; Given, B.; Melquist, S.; et al. RNA Interference Therapy Targeting Apolipoprotein C-III in Hypertriglyceridemia. NEJM Evid. 2023, 2, EVIDoa2200325. [Google Scholar] [CrossRef]
- Khetarpal, S.A.; Wang, M.; Khera, A.V. Volanesorsen, Familial Chylomicronemia Syndrome, and Thrombocytopenia. N. Engl. J. Med. 2019, 381, 2582–2584. [Google Scholar] [CrossRef] [PubMed]
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A.; Hawkins, P.N.; Fontana, M. Cardiac amyloidosis. Clin. Med. 2018, 18, s30–s35. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Pagura, L.; Porcari, A.; Cameli, M.; Vergaro, G.; Musumeci, B.; Biagini, E.; Canepa, M.; Crotti, L.; Imazio, M.; et al. Unmasking the prevalence of amyloid cardiomyopathy in the real world: Results from Phase 2 of the AC-TIVE study, an Italian nationwide survey. Eur. J. Heart Fail. 2022, 24, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Fontana, M.; Gillmore, J.D. Patisiran for the Treatment of Transthyretin-mediated Amyloidosis with Cardiomyopathy. Heart Int. 2023, 17, 27–35. [Google Scholar] [CrossRef]
- Ericzon, B.-G.; Wilczek, H.E.; Larsson, M.; Wijayatunga, P.; Stangou, A.; Pena, J.R.; Furtado, E.; Barroso, E.; Daniel, J.; Samuel, D.; et al. Liver Transplantation for Hereditary Transthyretin Amyloidosis: After 20 Years Still the Best Therapeutic Alternative? Transplantation 2015, 99, 1847–1854. [Google Scholar] [CrossRef]
- Tomasoni, D.; Bonfioli, G.B.; Aimo, A.; Adamo, M.; Canepa, M.; Inciardi, R.M.; Lombardi, C.M.; Nardi, M.; Pagnesi, M.; Riccardi, M.; et al. Treating amyloid transthyretin cardiomyopathy: Lessons learned from clinical trials. Front. Cardiovasc. Med. 2023, 10, 1154594. [Google Scholar] [CrossRef]
- Kristen, A.V.; Ajroud-Driss, S.; Conceição, I.; Gorevic, P.; Kyriakides, T.; Obici, L. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegener. Dis. Manag. 2019, 9, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Adams, D.; Silva, A.; Lozeron, P.; Hawkins, P.N.; Mant, T.; Perez, J.; Chiesa, J.; Warrington, S.; Tranter, E.; et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013, 369, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A Review of Patisiran (ONPATTRO®) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Cullis, P.R.; Van Der Meel, R. Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Adams, D.; Conceição, I.; Waddington-Cruz, M.; Schmidt, H.H.; Buades, J.; Campistol, J.; Berk, J.L.; Polydefkis, M.; Wang, J.J.; et al. A phase II, open-label, extension study of long-term patisiran treatment in patients with hereditary transthyretin-mediated (hATTR) amyloidosis. Orphanet J. Rare Dis. 2020, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Adams, D.; Kristen, A.; Grogan, M.; González-Duarte, A.; Maurer, M.S.; Merlini, G.; Damy, T.; Slama, M.S.; Brannagan, T.H., 3rd; et al. Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients with Hereditary Transthyretin-Mediated Amyloidosis. Circulation 2019, 139, 431–443. [Google Scholar] [CrossRef]
- Di Stefano, V.; Fava, A.; Gentile, L.; Guaraldi, P.; Leonardi, L.; Poli, L.; Tagliapietra, M.; Vastola, M.; Fanara, S.; Ferrero, B.; et al. Italian Real-Life Experience of Patients with Hereditary Transthyretin Amyloidosis Treated with Patisiran. Pharmacogenomics Pers. Med. 2022, 15, 499–514. [Google Scholar] [CrossRef]
- Adams, D.; Polydefkis, M.; González-Duarte, A.; Wixner, J.; Kristen, A.V.; Schmidt, H.H.; Berk, J.L.; Losada López, I.A.; Dispenzieri, A.; Quan, D.; et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. 2021, 20, 49–59. [Google Scholar] [CrossRef]
- Zhang, X.; Goel, V.; Attarwala, H.; Sweetser, M.T.; Clausen, V.A.; Robbie, G.J. Patisiran Pharmacokinetics, Pharmacodynamics, and Exposure-Response Analyses in the Phase 3 APOLLO Trial in Patients with Hereditary Transthyretin-Mediated (hATTR) Amyloidosis. J. Clin. Pharmacol. 2020, 60, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Fontana, M.; Berk, J.; Gustafsson, F.; Simoes, M.; Grogan, M.; Fernandes, F.; Gottlieb, R.L.; Kubanek, M.; Poulsen, S.; et al. Primary results from APOLLO-B, a phase 3 study of patisiran in patients with trans-thyretin-mediated amyloidosis with cardiomyopathy. J. Card. Fail. 2023, 29, 550. [Google Scholar] [CrossRef]
- Maurer, M.S.; Kale, P.; Fontana, M.; Berk, J.L.; Grogan, M.; Gustafsson, F.; Hung, R.R.; Gottlieb, R.L.; Damy, T.; González-Duarte, A.; et al. Patisiran Treatment in Patients with Transthyretin Cardiac Amyloidosis. N. Engl. J. Med. 2023, 389, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Gentile, L.; Di Stefano, V.; Di Bella, G.; Minutoli, F.; Toscano, A.; Brighina, F.; Vita, G.; Mazzeo, A. Use of Drugs for ATTRv Amyloidosis in the Real World: How Therapy Is Changing Survival in a Non-Endemic Area. Brain Sci. 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Thomas, E.; Alonge, P.; Giustino, V.; Pillitteri, G.; Leale, I.; Torrente, A.; Pignolo, A.; Norata, D.; Iacono, S.; et al. Patisiran Enhances Muscle Mass after Nine Months of Treatment in ATTRv Amyloidosis: A Study with Bioelectrical Impedance Analysis and Handgrip Strength. Biomedicines 2022, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Tournev, I.L.; Taylor, M.S.; Coelho, T.; Planté-Bordeneuve, V.; Berk, J.L.; González-Duarte, A.; Gillmore, J.D.; Low, S.-C.; Sekijima, Y.; et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: A randomized clinical trial. Amyloid Int. J. Exp. Clin. Investig. Off. J. Int. Soc. Amyloidosis 2023, 30, 18–26. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Sarzani, R.; Laureti, G.; Gezzi, A.; Spannella, F.; Giulietti, F. Single-pill fixed-dose drug combinations to reduce blood pressure: The right pill for the right patient. Ther. Adv. Chronic Dis. 2022, 13, 20406223221102750. [Google Scholar] [CrossRef]
- Sarzani, R.; Giulietti, F.; Filipponi, A.; Marziali, S.; Ristori, L.; Buscarini, S.; Garbuglia, C.; Biondini, S.; Allevi, M.; Spannella, F. The Number of Pills, Rather Than the Type of Renin-Angiotensin System Inhibitor, Predicts Ambulatory Blood Pressure Control in Essential Hypertensives on Triple Therapy: A Real-Life Cross-Sectional Study. Adv. Ther. 2021, 38, 4013–4025. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Radauceanu, A.; Boivin, J.-M.; Bernaud, C.; Fay, R.; Zannad, F.; the CIC General Practitioners Investigators’ Group. Differential time effect profiles of amlodipine, as compared to valsartan, revealed by ambulatory blood pressure monitoring, self blood pressure measurements and dose omission protocol. Fundam. Clin. Pharmacol. 2004, 18, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Driver, M.; Ouyang, D.; Botting, P.; Ji, H.; Rashid, M.A.; Blyler, C.A.; Bello, N.A.; Rader, F.; Niiranen, T.J.; et al. Variability independent of mean blood pressure as a real-world measure of cardiovascular risk. EClinicalMedicine 2022, 48, 101442. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, T.; Niimura, F.; Shimizu, A.; Pastan, I.; Saito, A.; Kobori, H.; Nishiyama, A.; Ichikawa, I. Liver angiotensinogen is the primary source of renal angiotensin II. J. Am. Soc. Nephrol. 2012, 23, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Uijl, E.; Mirabito Colafella, K.M.; Sun, Y.; Ren, L.; van Veghel, R.; Garrelds, I.M.; de Vries, R.; Poglitsch, M.; Zlatev, I.; Kim, J.B.; et al. Strong and Sustained Antihypertensive Effect of Small Interfering RNA Targeting Liver Angiotensinogen. Hypertension 2019, 73, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Mullick, A.E.; Yeh, S.T.; Graham, M.J.; Engelhardt, J.A.; Prakash, T.P.; Crooke, R.M. Blood Pressure Lowering and Safety Improvements with Liver Angiotensinogen Inhibition in Models of Hypertension and Kidney Injury. Hypertension 2017, 70, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Webb, D.J.; Taubel, J.; Casey, S.; Cheng, Y.; Robbie, G.J.; Foster, D.; Huang, S.A.; Rhyee, S.; Sweetser, M.T.; et al. Zilebesiran, an RNA Interference Therapeutic Agent for Hypertension. N. Engl. J. Med. 2023, 389, 228–238. [Google Scholar] [CrossRef] [PubMed]
- van Harmelen, V.; Elizalde, M.; Ariapart, P.; Bergstedt-Lindqvist, S.; Reynisdottir, S.; Hoffstedt, J.; Lundkvist, I.; Bringman, S.; Arner, P. The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 673–678. [Google Scholar] [CrossRef]
- Sarzani, R.; Bordicchia, M.; Marcucci, P.; Minardi, D.; Muzzonigro, G.; Dessì-Fulgheri, P.; Rappelli, A. Angiotensinogen promoter variants influence gene expression in human kidney and visceral adipose tissue. J. Hum. Hypertens. 2010, 24, 213–219. [Google Scholar] [CrossRef][Green Version]
- Taubel, J.; Desai, A.S.; Lasko, M.; Zee, T.; Stiglitz, D.; Rhyee, S.; Zappe, D.H.; Bakris, G.L. Abstract 116: Safety And Tolerability Of Zilebesiran, An RNA Interference Therapeutic Targeting Hepatic Angiotensinogen Synthesis, In Obese Patients with Hypertension. Hypertension 2023, 80 (Suppl. S1), A116. Available online: https://www.ahajournals.org/doi/10.1161/hyp.80.suppl_1.116 (accessed on 18 December 2023). [CrossRef]
- Uijl, E.; Ye, D.; Ren, L.; Mirabito Colafella, K.M.; van Veghel, R.; Garrelds, I.M.; Lu, H.S.; Daugherty, A.; Hoorn, E.J.; Nioi, P.; et al. Conventional Vasopressor and Vasopressor-Sparing Strategies to Counteract the Blood Pressure-Lowering Effect of Small Interfering RNA Targeting Angiotensinogen. J. Am. Heart Assoc. 2022, 11, e026426. [Google Scholar] [CrossRef]
- Ahn, I.; Kang, C.S.; Han, J. Where should siRNAs go: Applicable organs for siRNA drugs. Exp. Mol. Med. 2023, 55, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, M.; Corteville, D.; Szabo, G.; Swaminathan, M.; Lamy, A.; Lehner, L.J.; Brown, C.D.; Noiseux, N.; Atta, M.G.; Squiers, E.C.; et al. Teprasiran, a Small Interfering RNA, for the Prevention of Acute Kidney Injury in High-Risk Patients Undergoing Cardiac Surgery: A Randomized Clinical Study. Circulation 2021, 144, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Martínez, T.; González, M.V.; Roehl, I.; Wright, N.; Pañeda, C.; Jiménez, A.I. In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol. Ther. 2014, 22, 81–91. [Google Scholar] [CrossRef]
- Cho, W.G.; Albuquerque, R.J.C.; Kleinman, M.E.; Tarallo, V.; Greco, A.; Nozaki, M.; Green, M.G.; Baffi, J.Z.; Ambati, B.K.; De Falco, M.; et al. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc. Natl. Acad. Sci. USA 2009, 106, 7137–7142. [Google Scholar] [CrossRef]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
| Trial | Subjects and Sample Size (n) | Objectives | Results | Phase | |
|---|---|---|---|---|---|
| ORION-9 | Subjects with HeFH and elevated LDL-C (482) | To evaluate the effect of inclisiran 300 mg compared to placebo in LDL-C lowering | A 39.7% (95% CI, −43.7 to −35.7) LDL-C lowering at day 510 with inclisiran | Phase III | |
| ORION-10 | Subjects with established ASCVD (1561) | To evaluate the effect of inclisiran 284 mg compared to placebo in LDL-C lowering | A 52.3% (95% CI, 48.8 to 55.7) LDL-C lowering with inclisiran | Phase III | |
| Inclisiran | ORION-11 | Subjects with ASCVD equivalent risk (1617) | To assess efficacy and safety of inclisiran 284 mg compared to placebo in LDL-C lowering | A 49.2% (95% CI, 46.6 to 53.1) LDL-C lowering with inclisiran | Phase III |
| HPS-4/TIMI 65/ORION-4 | Subjects with established ASCVD (16124) | To evaluate if inclisiran 300 mg reduces the risk of MACE | Expected in July 2026 | Phase III | |
| VICTORION-2P | Subjects with established ASCVD (16500) | To evaluate if inclisiran 300 mg reduces the risk of MACE | Expected in October 2027 | Phase III | |
| NCT03626662 | Subjects with elevated plasma Lp(a) (80) | To assess safety, tolerability, pharmacokinetics, and pharmacodynamic effects of olpasiran | A safe and persisting 71–97% reduction in Lp(a) concentration | Phase I | |
| Olpasiran | OCEAN(a)-DOSE | Subjects with established ASCVD and a serum Lp(a) concentration of more than 150 nmol/L (~70 mg/dL) (281) | To evaluate the percent change in the Lp(a) concentration from baseline to week 36 with four different doses of olpasiran | Serum Lp(a) levels significantly reduced in a dose-dependent manner at 36 weeks | Phase II |
| OCEAN(a)-Outcomes (NCT05581303) | Subjects with established ASCVD and elevated plasma Lp(a) (6000) | To evaluate if olpasiran reduces the risk of coronary heart disease death, myocardial infarction, or urgent coronary revascularization | Expected in December 2026 | Phase III | |
| Lepodisiran | NCT04914546 | Subjects without CV disease and with a serum Lp(a) concentration of 75 nmol/L or greater (or ≥30 mg/dL) | To assess the safety, tolerability, pharmacokinetics, and pharmacodynamic effects of lepodisiran | A safe and persisting 41–94% reduction in Lp(a) concentration | Phase I |
| ARO-APOC3 | NCT03783377 | Healthy subjects and subjects with hypertriglyceridemia (112) | To assess the safety, tolerability, pharmacokinetics, and pharmacodynamic effects of ARO-APOC3 | ARO-APOC3 associated with few adverse events and reduced serum levels of APOC3 and triglycerides | Phase I |
| NCT01961921 | Subjects with vATTR-polyneuropathy (27) | To assess safety, effects on serum TTR levels, and clinical parameters (mNIS + 7 and multiple disease-relevant measures) of patisiran treatment (0.3 mg/kg intravenously every 3 weeks) | No drug-related adverse events leading to treatment discontinuation, sustained reduction in mean transthyretin levels, a mean 6.95-point improvement in mNIS + 7 from baseline | Phase II | |
| Patisiran | APOLLO | Subjects with vATTR-polyneuropathy (225); cardiac subpopulation with a left ventricular wall thickness ≥ 13 mm without history of hypertension or aortic valve disease (126) | To evaluate the effect of patisiran treatment (0.3 mg/kg intravenously every 3 weeks) on neurological symptoms compared to placebo | Least-squares mean (±SE) change from baseline of mNIS + 7 was −6.0 ± 1.7 versus 28.0 ± 2.6 (difference, −34.0 points; p < 0.001) at 18 months; effects also on gait speed and modified BMI; in the cardiac subpopulation, patisiran reduced mean left ventricular wall thickness (least-squares mean difference ± SEM: –0.9 ± 0.4 mm, p = 0.017), interventricular septal wall thickness, posterior wall thickness, and relative wall thickness; increased end-diastolic volume and reduced NT-proBNP. In a post-hoc analysis, patisiran treatment lowered combined all-cause hospitalization and mortality compared with placebo at month 18 | Phase III |
| APOLLO-B | Subjects with both wild-type and vATTR-cardiomyopathy with clinical evidence of HF and an elevated NT-proBNP between 300 ng/L and 8500 ng/L (360) | To evaluate the efficacy of patisiran treatment (0.3 mg/kg intravenously every 3 weeks) compared to placebo in patients with cardiomyopathy: change from baseline in the distance covered on 6MWT, change from baseline in the KCCQ-OS score, differences in death from any cause, CV events, hospitalizations for any cause, and urgent HF visits over 12 months | The decline in the 6MWT was lower in the patisiran group (95% CI, 0.69 to 28.69; p = 0.02); the KCCQ-OS score increased in the patisiran group and declined in the placebo group (least-squares mean difference, 3.7 points; 95% CI, 0.2 to 7.2; p = 0.04). Significant benefits were not observed for secondary endpoints (all-cause hospitalization, urgent HF visits, and death) | Phase III | |
| Vutrisiran | HELIOS-A | Subjects with vATTR-polyneuropathy (164) | To evaluate the effect of vutrisiran 25 mg every 3 months on neurological symptoms compared to patisiran and placebo | Change from baseline in mNIS + 7 at 9 months (p = 3.54 × 10−12); significant improvements versus external placebo in Norfolk Quality of Life-Diabetic Neuropathy, 10-m walk test, mNIS + 7 | Phase III |
| HELIOS-B | Subjects with both wild-type and vATTR-cardiomyopathy (655) | To evaluate the efficacy and safety of vutrisiran 25 mg every 3 months compared to placebo in patients with ATTR amyloidosis and cardiomyopathy. Composite endpoint of all-cause mortality and recurrent CV events | Expected in early 2024 | Phase III | |
| NCT03934307 | Patients with arterial hypertension (107) | To assess safety, pharmacokinetics, and pharmacodynamic effects of single doses of zilebesiran (10, 25, 50, 100, 200, 400, or 800 mg), and the change from baseline in systolic and diastolic BP | Single doses of zilebesiran (≥200 mg) associated with decreases in systolic (>10 mmHg) and diastolic BP (>5 mmHg) by week 8 and sustained to week 24. Mild ISRs | Phase I | |
| Zilebesiran | KARDIA-1 | Subjects with a daytime mean systolic BP ≥135 mmHg and ≤160 mmHg (378) | To evaluate the efficacy of different doses of zilebesiran compared to placebo in patients with hypertension | Change in ambulatory systolic BP: –11.1 mmHg for zilebesiran 150 mg every 6 months vs. –14.5 mmHg for zilebesiran 300 mg every 6 months vs. –4.1 mmHg for zilebesiran 300 mg every 3 months vs. –14.2 mmHg for zilebesiran 600 mg every 6 months (p < 0.05 for each group) | Phase II |
| KARDIA-2 | Subjects with hypertension not adequately controlled by a standard of care antihypertensive medication (672) | To evaluate the efficacy of zilebesiran compared to placebo in patients with hypertension | Expected in early 2024 | Phase II |
| siRNA | Mechanism of Action | Potential Uses and Benefits |
|---|---|---|
| Inclisiran | PCSK9 mRNA degradation in the liver and inhibition of PCSK9 synthesis and secretion, leading to increased LDLR expression on the surface of hepatocytes and reduced circulating LDL-C levels | Significant, sustained and safe LDL-C lowering in patients at high and very high CV risk that do not reach their LDL-C goal with traditional lipid-lowering therapy (statins and ezetimibe) and/or are statin-intolerant. Potential reduction in the risk of MACE in patients at high and very high CV risk. Optimal adherence to therapy, with only two injections in a year |
| Olpasiran and Lepodisiran | LPA mRNA degradation in the liver and inhibition of Lp(a) assembly and secretion | Significant, sustained, and safe Lp(a) lowering in patients with high Lp(a) circulating levels. Potential reduction in the risk of ischemic CV disease, ASCVD, and calcific valvular aortic stenosis. Reduction in OxPL-apoB levels, a biomarker strongly associated with ASCVD. Optimal adherence to therapy, with only two or three injections in a year |
| ARO-APOC3 | APOC3 mRNA degradation in the liver and inhibition of APOC3 synthesis and secretion, leading to increased triglycerides clearance and hepatocyte uptake of triglyceride-rich lipoproteins | Significant, sustained, and safe triglycerides lowering in patients with hypertriglyceridemia and chylomicronemia. Potential reduction in the risk of ASCVD and pancreatitis in patients with hypertriglyceridemia and chylomicronemia |
| Patisiran and Vutrisiran | TTR mRNA degradation in the liver and inhibition of TTR synthesis and secretion, thereby preventing its abnormal accumulation in amyloidosis | Significant, sustained, and safe TTR lowering in patients with ATTR amyloidosis. Improvement of neurological symptoms and quality of life in patients with ATTR-polyneuropathy. Improvement of cardiac parameters and symptoms in patients with ATTR-cardiomyopathy. Increase in survival in patients with ATTR amyloidosis. Potential reduction in all-cause mortality and CV events in patients with ATTR-cardiomyopathy. Optimal adherence to therapy, with only four injections in a year (only for vutrisiran) |
| Zilebesiran | AGT mRNA degradation in the liver and inhibition of AGT synthesis and secretion, thereby preventing the production of angiotensin peptides and inhibiting the renin-angiotensin-aldosterone system | Significant, sustained, and safe systolic and diastolic BP lowering in patients with uncontrolled hypertension. Reduction in the risk of CV events in patients with uncontrolled or resistant hypertension. Optimal adherence to therapy, with only two or four injections in a year |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarzani, R.; Spannella, F.; Di Pentima, C.; Giulietti, F.; Landolfo, M.; Allevi, M. Molecular Therapies in Cardiovascular Diseases: Small Interfering RNA in Atherosclerosis, Heart Failure, and Hypertension. Int. J. Mol. Sci. 2024, 25, 328. https://doi.org/10.3390/ijms25010328
Sarzani R, Spannella F, Di Pentima C, Giulietti F, Landolfo M, Allevi M. Molecular Therapies in Cardiovascular Diseases: Small Interfering RNA in Atherosclerosis, Heart Failure, and Hypertension. International Journal of Molecular Sciences. 2024; 25(1):328. https://doi.org/10.3390/ijms25010328
Chicago/Turabian StyleSarzani, Riccardo, Francesco Spannella, Chiara Di Pentima, Federico Giulietti, Matteo Landolfo, and Massimiliano Allevi. 2024. "Molecular Therapies in Cardiovascular Diseases: Small Interfering RNA in Atherosclerosis, Heart Failure, and Hypertension" International Journal of Molecular Sciences 25, no. 1: 328. https://doi.org/10.3390/ijms25010328
APA StyleSarzani, R., Spannella, F., Di Pentima, C., Giulietti, F., Landolfo, M., & Allevi, M. (2024). Molecular Therapies in Cardiovascular Diseases: Small Interfering RNA in Atherosclerosis, Heart Failure, and Hypertension. International Journal of Molecular Sciences, 25(1), 328. https://doi.org/10.3390/ijms25010328






