Icariin Improves Stress Resistance and Extends Lifespan in Caenorhabditis elegans through hsf-1 and daf-2-Driven Hormesis
Abstract
:1. Introduction
2. Results
2.1. Icariin Prolongs the Lifespan and Improves the Fitness of Wild-Type C. elegans in Comparison to daf-2 Loss-of-Function Mutant
2.2. Icariin Ameliorates Healthspan in C. elegans via Enhanced Response to Stressors
2.3. Icariin Decreases the Lipid Droplet Accumulation
2.4. Icariin Alters the Transcriptional Regulation of Evolutionarily Conserved Genes Involved in Stress Resistance and Lifespan Regulation
2.5. Icariin Regulates DAF-16 Subcellular Localization
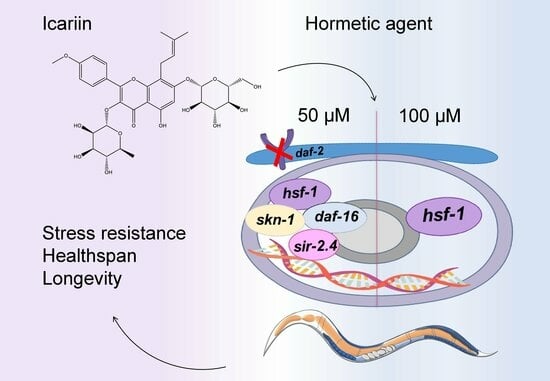
2.6. Model Mechanism Representing the Hormetic Effect of Icariin in C. elegans
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Caenorhabditis Elegans Maintenance and Treatment
4.3. Fecundity Assay
4.4. Locomotion Assay
4.5. Lifespan Measurement
4.6. Chemotaxis
4.7. Nile Red Triglyceride Staining
4.8. Heat Stress
4.9. Oxidative Stress
4.10. Gene Expression Analysis through RT-qPCR
4.11. Detection of DAF-16 Nuclear Translocation
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonkowski, M.S.; Sinclair, D.A. Slowing aging by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Anderson, R.M. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell 2022, 185, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Di Tano, M.; Mattson, M.P.; Guidi, N. Intermittent and periodic fasting, longevity and disease. Nat. Aging 2021, 1, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Mavromatis, L.A.; Rosoff, D.B.; Bell, A.S.; Jung, J.; Wagner, J.; Lohoff, F.W. Multi-omic underpinnings of epigenetic aging and human longevity. Nat. Commun. 2023, 14, 2236. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the aging process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric restriction mimetics against age-associated disease: Targets, mechanisms, and therapeutic potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Kern, C.C.; Srivastava, S.; Ezcurra, M.; Hsiung, K.C.; Hui, N.; Townsend, S.; Maczik, D.; Zhang, B.; Tse, V.; Konstantellos, V.; et al. C. elegans ageing is accelerated by a self-destructive reproductive programme. Nat. Commun. 2023, 14, 4381. [Google Scholar] [CrossRef] [PubMed]
- Son, H.G.; Altintas, O.; Kim, E.J.E.; Kwon, S.; Lee, S.V. Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell 2019, 18, e12853. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Q.; Song, Y.; He, Z.; Zhang, H.; Song, M.; Zhang, X.; Dai, Y.; Karalay, O.; Dieterich, C.; et al. Ageing induces tissue-specific transcriptomic changes in Caenorhabditis elegans. EMBO J. 2022, 19, e109633. [Google Scholar] [CrossRef] [PubMed]
- El-Shitany, N.A.; Eid, B.G. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signalling pathways. Biomed. Pharmacother. 2019, 120, 109567. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, Y. Icariin, an anti-atherosclerotic drug from Chinese medicinal herb Horny goat weed. Front. Pharmacol. 2017, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Li, L.R.; Sethi, G.; Zhang, X.; Liu, C.L.; Huang, Y.; Liu, Q.; Ren, B.X.; Tang, F.R. The neuroprotective effects of icariin on ageing, various neurological, neuropsychiatric disorders, and brain injury induced by radiation exposure. Aging 2022, 14, 1562–1588. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Huang, J.H.; Zhang, S.Q.; Wu, B.; Kapahi, P.; Zhang, X.M.; Shen, Z.Y. Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans. PLoS ONE 2011, 6, e28835. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Cai, W.J.; Huang, J.H.; Wu, B.; Xia, S.J.; Chen, X.L.; Zhang, X.M.; Shen, Z.Y. Icariin, a natural flavonol glycoside, extends healthspan in mice. Exp. Gerontol. 2015, 69, 226–235. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, T.; Wu, J.; Kalionis, B.; Zhang, C.; Yuan, D.; Huang, J.; Cai, W.; Fang, H.; Xia, S. Icariin intervenes in cardiac inflammaging through upregulation of SIRT6 enzyme activity and inhibition of the NF-kappa B pathway. Biomed. Res. Int. 2015, 2015, 895976. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, F.; Li, H.; He, Y.; Zhang, G.; Huang, N.; Guo, M.; Li, X. Long-term icariin treatment ameliorates cognitive deficits via CD4+ T cell-mediated immuno-inflammatory responses in APP/PS1 mice. Clin. Interv. Aging 2019, 14, 817–826. [Google Scholar] [CrossRef]
- Amanat, S.; Shal, B.; Seo, E.K.; Ali, H.; Khan, S. Icariin attenuates cyclophosphamide-induced cystitis via down-regulation of NF-κB and up-regulation of Nrf-2/HO-1 signalling pathways in mice model. Int. Immunopharmacol. 2022, 106, 108604. [Google Scholar] [CrossRef]
- Yellurkar, M.L.; Singh, V.; Sai Prasanna, V.; Das, P.; Nanjappan, S.; Velayutham, R.; Arumugam, S. Evaluation of a natural compound extracted from Dolichandrone atrovirens as a novel antioxidant agent using Caenorhabditis elegans. PLoS ONE 2021, 16, e0257702. [Google Scholar] [CrossRef]
- Singh, J.; Aballay, A. Intestinal infection regulates behavior and learning via neuroendocrine signalling. eLife 2019, 8, e50033. [Google Scholar] [CrossRef]
- De-Souza, E.A.; Thompson, M.A.; Taylor, R.C. Olfactory chemosensation extends lifespan through TGF-β signalling and UPR activation. Nat. Aging 2023, 3, 938–947. [Google Scholar] [CrossRef]
- Scharf, F.; Pohl, B.M.; Egan, Z.; Kocsisova, K.; Kornfeld, K. Reproductive aging in Caenorhabditis elegans: From molecules to ecology. Front. Cell Dev. Biol. 2021, 9, 718522. [Google Scholar] [CrossRef]
- Spanoudakis, E.; Tavernarakis, N. Age-associated anatomical and physiological alterations in Caenorhabditis elegans. Mech. Ageing Dev. 2023, 213, 111827. [Google Scholar] [CrossRef]
- Bansal, A.; Zhu, L.J.; Yen, K.; Tissenbaum, H.A. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. USA 2015, 112, E277–E286. [Google Scholar] [CrossRef]
- Bao, K.; Liu, W.; Song, Z.; Feng, J.; Mao, Z.; Bao, L.; Sun, T.; Hu, Z.; Li, J. Crotamiton derivative JM03 extends lifespan and improves oxidative and hypertonic stress resistance in Caenorhabditis elegans via inhibiting OSM-9. eLife 2022, 11, e72410. [Google Scholar] [CrossRef]
- Rasulova, M.; Zecic, A.; Moreno, J.M.M.; Vandemeulebroucke, L.; Dhondt, I.; Braeckmann, B.P. Elevated trehalose levels in C. elegans daf-2 mutants increase stress resistance, not lifespan. Metabolites 2021, 11, 105. [Google Scholar] [CrossRef]
- Senchuk, M.M.; Dues, D.J.; Van Raamsdonk, J.M. Measuring oxidative stress in Caenorhabditis elegans: Paraquat and juglone sensitivity assays. Bio-protocol 2017, 7, e2086. [Google Scholar] [CrossRef]
- Statzer, C.; Reichert, P.; Dual, J.; Ewald, C.Y. Longevity interventions temporally scale healthspan in Caenorhabditis elegans. iScience 2022, 25, 103983. [Google Scholar] [CrossRef] [PubMed]
- Hermeling, J.C.; Herholz, M.; Baumann, L.; Cores, E.C.; Zecic, A.; Hoppe, T.; Riemer, J.; Trifunovic, A. Mitochondria-originated redox signalling regulates KLF-1 to promote longevity in Caenorhabditis elegans. Redox Biol. 2022, 58, 102533. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Kim, E.; Park, Y. Cafestol increases fat oxidation and energy expenditure in Caenorhabditis elegans via DAF-12-dependent pathway. Food Chem. 2020, 307, 125537. [Google Scholar] [CrossRef] [PubMed]
- Matai, L.; Sarkar, G.C.; Chamoli, M.; Malik, Y.; Kumar, S.S.; Rautela, U.; Jana, N.R.; Chakraborty, K.; Mukhopadhyay, A. Dietary restriction improves proteostasis and increases life span through endoplasmic reticulum hormesis. Proc. Natl. Acad. Sci. USA 2019, 116, 17383–17392. [Google Scholar] [CrossRef] [PubMed]
- Yerevanian, A.; Murphy, L.M.; Emans, S.; Zhou, Y.; Ahsan, F.M.; Baker, D.; Li, S.; Adedoja, A.; Cedillo, L.; Stuhr, N.L.; et al. Riboflavin depletion promotes longevity and metabolic hormesis in Caenorhabditis elegans. Aging Cell 2022, 21, e13718. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, A.; Salveridou, E.; Brinkmann, V.; Shaik, A.; Meznel, R.; Kalyanasundaram, S.; Nygard, S.; Nilsen, H.; Ventura, N. Mitochondria hormesis delays aging and associated diseases in Caenorhabditis elegans impacting on key ferroptosis players. iScience 2023, 26, 106448. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, M.V.; Guvatova, Z.G.; Zemskaya, N.V.; Koval, L.A.; Schegoleva, E.V.; Gorbunova, A.A.; Golubev, D.A.; Pakshina, N.R.; Ulyasheva, N.S.; Solovev, I.A.; et al. Molecular mechanisms of exceptional lifespan increase of Drosophila melanogaster with different genotypes after combinations of pro-longevity interventions. Commun. Biol. 2022, 5, 566. [Google Scholar] [CrossRef]
- Rodriguez-Colman, M.J.; Dansen, T.B.; Burgering, B.M.T. FOXO transcription factors as mediators of stress adaptation. Nat. Rev. Mol. Cell Biol. 2024, 25, 46–64. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Zhang, W.-H.; Zhang, P.; Li, Q.; Sun, Y.; Wang, J.-W.; Zhang, S.O.; Cai, T.; Zhan, C.; Dong, M.-Q. Intestine-specific removal of DAF-2 nearly doubles lifespan in Caenorhabditis elegans with little fitness cost. Nat. Commun. 2022, 13, 6339. [Google Scholar] [CrossRef]
- Kumsta, C.; Hansen, M. Hormetic heat shock and HSF-1 overexpression improve C. elegans survival and proteostasis by inducing autophagy. Autophagy 2017, 13, 1076–1077. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.F.; Goyala, A.; Statzer, C.; Beckett, C.W.; Tyshkovskiy, A.; Gladyshev, V.N.; Ewald, C.Y.; de Magalhaes, J.P. Rilmenidine extends lifespan and healthspan in Caenorhabditis elegans via a nischarin I1-imidazoline receptor. Aging Cell 2023, 22, e13774. [Google Scholar] [CrossRef]
- Qi, Z.; Ji, H.; Le, M.; Li, H.; Wieland, A.; Bauer, S.; Liu, L.; Wink, M.; Herr, I. Sulforaphane promotes C. elegans longevity and healthspan via DAF-16/DAF-2 insulin/IGF-1 signaling. Aging 2021, 13, 1649–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, B.; Marcu, I.; Athar, F.; Wang, H.; Galimov, E.R.; Chapman, H.; Gems, D. Mutation of daf-2 extends lifespan via tissue-specific effectors that suppress distinct life-limiting pathologies. Aging Cell 2021, 20, e13324. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Quipildor, G.F.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K.; et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 2018, 9, 2394. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. Role of IGF-1 system in the modulation of longevity: Controversies and new insights from a centenarians’ perspective. Front. Endocrinol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Ben-Avraham, D.; Govindaraju, D.R.; Budagov, T.; Fradin, D.; Durda, P.; Liu, B.; Ott, S.; Gutman, D.; Sharvit, L.; Kaplan, R.; et al. The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature. Sci. Adv. 2017, 3, e1602025. [Google Scholar] [CrossRef]
- Huffman, D.M.; Quipildor, G.F.; Mao, K.; Zhang, X.; Wan, J.; Apontes, P.; Cohen, P.; Barzilai, N. Central insulin-like growth factor-1 (IGF-1) restores whole-body insulin action in a model of age-related insulin resistance and IGF-1 decline. Aging Cell 2016, 15, 181–186. [Google Scholar] [CrossRef]
- Milman, S.; Atzmon, G.; Huffman, D.M.; Wan, J.; Crandall, J.P.; Cohen, P.; Barzilai, N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 2014, 13, 769–771. [Google Scholar] [CrossRef]
- He, Y.H.; Lu, X.; Yang, L.Q.; Xu, L.Y.; Kong, Q.P. Association of the insulin-like growth factor binding protein 3 (IGFBP-3) polymorphism with longevity in Chinese nonagenarians and centenarians. Aging 2014, 6, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Luo, H.; Liu, X.; Ma, S.; Lin, H.; Chen, R.; Hao, F.; Zhang, D. Association study of polymorphisms in FOXO3, AKT1 and IGF-2R genes with human longevity in a Han Chinese population. Oncotarget 2016, 7, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Santo, E.E.; Ribel-Madsen, R.; Stroeken, P.J.; de Boer, V.C.J.; Hansen, N.S.; Commandeur, M.; Vaag, A.A.; Versteeg, R.; Paik, J.; Westerhout, E.M. FOXO3A-short is a novel regulator of non-oxidative glucose metabolism associated with human longevity. Aging Cell 2023, 22, e13763. [Google Scholar] [CrossRef] [PubMed]
- Savova, M.S.; Todorova, M.N.; Apostolov, A.G.; Yahubyan, G.T.; Georgiev, M.I. Betulinic acid counteracts the lipid accumulation in Caenorhabditis elegans by modulation of nhr-49 expression. Biomed. Pharmacother. 2022, 156, 113862. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Mari, M.; Ploumi, C.; Princz, A.; Filippidis, G.; Tavernarakis, N. Age-dependent nuclear lipid droplet deposition is a cellular hallmark of aging in Caenorhabditis elegans. Aging Cell 2023, 22, e13788. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, M.; Zhao, D.; Li, X.; Yang, C.; Wang, X. Lysosome activity is modulated by multiple longevity pathways and is important for lifespan extension in C. elegans. eLife 2020, 9, e55745. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, R.; von Gromoff, E.D.; Drepper, F.; Knapp, E.; Warscheid, B.; Baumeister, R.; Qi, W. Reprogramming of the transcriptome after heat stress mediates heat hormesis in Caenorhabditis elegans. Nat. Commun. 2023, 13, 4176. [Google Scholar] [CrossRef]
- Pocock, R.; Hobert, O. Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nat. Neurosci. 2010, 13, 610–614. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Ke, P.-Y.; Wu, C.-Y.; Peng, H.; Young, J.D. Hormetic effects of phytochemicals on health and longevity. Trends Endocrinol. Metab. 2019, 30, 335–346. [Google Scholar] [CrossRef]
- Sohrabi, S.; Mor, D.E.; Kaletsky, R.; Keyes, W.; Murphy, C.T. High-throughput behavioral screen in C. elegans reveals Parkinson’s disease drug candidates. Commun. Biol. 2021, 4, 203. [Google Scholar] [CrossRef]
- Gonzales-Moreno, C.; Fernandez-Hubeid, L.E.; Holgado, A.; Virgolini, M.B. Low-dose N-acetyl cysteine prevents paraquat-induced mortality in Caenorhabditis elegans. MicroPubl Biol. 2023, 2023. [Google Scholar] [CrossRef]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic dose-response induced by phytochemicals: Experimental evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Suo, C.; Gao, Y.; Ding, C.; Sun, T. The function and regulation of heat shock transcription factor in Cryptococcus. Front. Cell. Infect. Microbiol. 2023, 13, 1195968. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Li, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Van Meter, M.; Simon, M.; Tombline, G.; May, A.; Morello, T.D.; Hubbard, B.P.; Bredbenner, K.; Park, R.; Sinclair, D.A.; Bohr, V.A.; et al. JNK phosphorylates SIRT6 to stimulate DNA double-strand break repair in response to oxidative stress by recruiting PARP1 to DNA breaks. Cell Rep. 2016, 16, 2641–2650. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Lin, D.; Guo, W.; Xu, Z.; Wang, L.; Guan, S. Ginsenoside extract from ginseng extends lifespan and health span in Caenorhabditis elegans. Food Funct. 2021, 12, 6793–6808. [Google Scholar]
- Duangjan, C.; Curran, S.P. Oolonghomobisflavans from Camellia sinensis increase Caenorhabditis elegans lifespan and healthspan. GeroScience 2022, 44, 533–545. [Google Scholar] [CrossRef]
- McLachlan, I.G.; Kramer, T.S.; Dua, M.; DiLoreto, E.M.; Gomes, M.A.; Dag, U.; Srinivasan, J.; Flavell, S.W. Diverse states and stimuli tune olfactory receptor expression levels to modulate food-seeking behavior. eLife 2022, 11, e79557. [Google Scholar] [CrossRef]
- Stuhr, N.L.; Nhan, J.D.; Hammerquist, A.M.; Van Camp, B.; Reoyo, D.; Curran, S.P. Rapid lipid quantification in Caenorhabditis elegans by oil red O and Nile red staining. Bio-protocol 2022, 12, e4340. [Google Scholar] [CrossRef]
- Reddy, K.C.; Dror, T.; Underwood, R.S.; Osman, G.A.; Elder, C.R.; Desjardins, C.A.; Cuomo, C.A.; Barkoulas, M.; Troemel, E.R. Antagonistic paralogs control a switch between growth and pathogen resistance in C. elegans. PLoS Pathog. 2019, 15, e1007528. [Google Scholar] [CrossRef]
- Chen, A.L.; Lum, K.M.; Lara-Gonzalez, P.; Ogasawara, D.; Cognetta, A.B.; To, A.; Parsons, W.H.; Simon, G.M.; Desai, A.; Petrascheck, M.; et al. Pharmacological convergence reveals a lipid pathway that regulates C. elegans lifespan. Nat. Chem. Biol. 2019, 15, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Qi, Z.; Schrapel, D.; Le, M.; Luo, Y.; Yan, B.; Gladkich, J.; Schaefer, M.; Liu, L.; Herr, I. Sulforaphane targets TRA-1/GLI upstream of DAF-16/FOXO to promote C. elegans longevity and healthspan. Front. Cell Dev. Biol. 2021, 9, 784999. [Google Scholar] [CrossRef] [PubMed]
- Elaswad, M.T.; Munderloh, C.; Watkins, B.M.; Sharp, K.G.; Breton, E.; Schisa, J.A. Imaging-associated stress causes divergent phase transitions of RNA-binding proteins in the Caenorhabditis elegans germ line. G3 2022, 12, jkac172. [Google Scholar] [CrossRef] [PubMed]






| Gene | Forward | Reverse |
|---|---|---|
| daf-2 | GAGACACGATGCGAGTGAGACG | GGATCAGCGGCTTCTTTCCACC |
| daf-16 | TCCGTCCCCGAACTCAATCGAACC | TGCTGGAACCGATTCGCCAACC |
| sir-2.4 | TTATCGGAGCCGGTGTGAGCAC | GGGACGAGCAACTTGAAAGTCCAC |
| sir-2.1 | TGTGTTTGTTTCGGGTGCATCGG | AGAAGTTGCGGTCACACACGGG |
| skn-1 | TTCCGCGTCGACGAATCTTGCG | AGCTTCCAGTGTCGGCGTTCCA |
| hsf-1 | TCATCGTGGGATTTCTGCGCTG | CCATTCTTGCTCCAGCTCCAGC |
| jnk-1 | TCCTGTGCACATGGAGGAACGA | AGGAGGATGCGAGCAGAGTGTT |
| mtl-1 | TGCAGTGGAGACAAGTGTTGTG | GCTCTGCACAATGACAGTTTGC |
| mdh-1 | GGACCATTCATCGCCACTGTCC | TGTGATCACAAGCGGCCTTAGC |
| iscu-1 | CTCCTGCACAAGTTTGCGTTGC | CCGACGCTTGGATCGTTCTTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, M.N.; Savova, M.S.; Mihaylova, L.V.; Georgiev, M.I. Icariin Improves Stress Resistance and Extends Lifespan in Caenorhabditis elegans through hsf-1 and daf-2-Driven Hormesis. Int. J. Mol. Sci. 2024, 25, 352. https://doi.org/10.3390/ijms25010352
Todorova MN, Savova MS, Mihaylova LV, Georgiev MI. Icariin Improves Stress Resistance and Extends Lifespan in Caenorhabditis elegans through hsf-1 and daf-2-Driven Hormesis. International Journal of Molecular Sciences. 2024; 25(1):352. https://doi.org/10.3390/ijms25010352
Chicago/Turabian StyleTodorova, Monika N., Martina S. Savova, Liliya V. Mihaylova, and Milen I. Georgiev. 2024. "Icariin Improves Stress Resistance and Extends Lifespan in Caenorhabditis elegans through hsf-1 and daf-2-Driven Hormesis" International Journal of Molecular Sciences 25, no. 1: 352. https://doi.org/10.3390/ijms25010352
APA StyleTodorova, M. N., Savova, M. S., Mihaylova, L. V., & Georgiev, M. I. (2024). Icariin Improves Stress Resistance and Extends Lifespan in Caenorhabditis elegans through hsf-1 and daf-2-Driven Hormesis. International Journal of Molecular Sciences, 25(1), 352. https://doi.org/10.3390/ijms25010352