New Perspectives in the Management of Chronic Hand Eczema: Lessons from Pathogenesis
Abstract
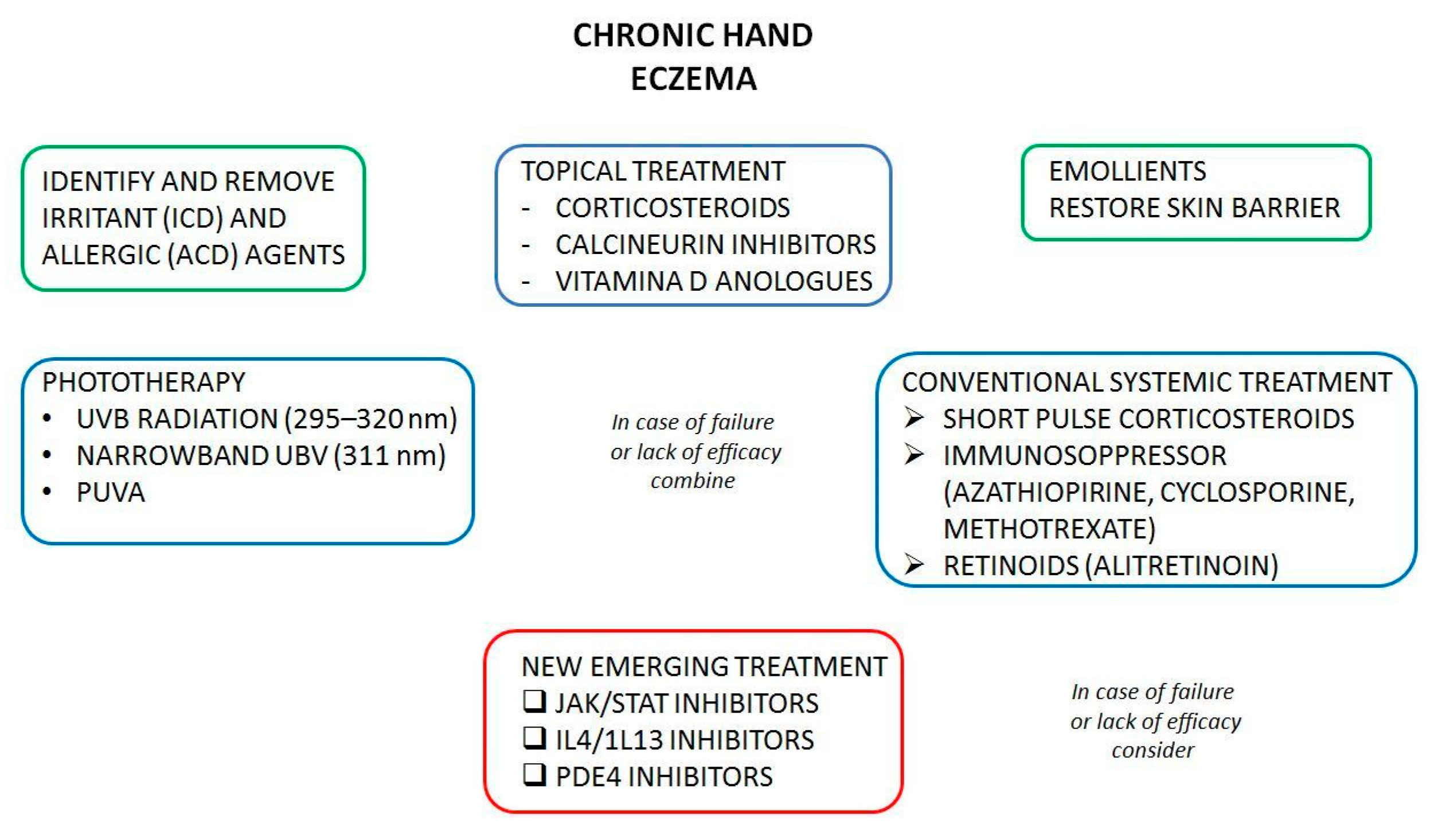
:1. Introduction
2. Material and Methods
3. Results
3.1. Classic and New Models of Contact Dermatitis
3.2. Conventional Treatments
3.3. New Treatments
3.3.1. JAK-STAT Pathway
Delgocitinib
Ruxolitinib
Gusacitinib
Upadacitinib
Baricitinib
Abrocitinib
3.3.2. Phosphodiesterase 4 and Cyclic Adenosine Monophosphate in Skin Inflammation
Apremilast
Roflumilast
Crisaborole
3.3.3. IL-4/13 Axis
Dupilumab
Tralokinumab
3.3.4. Chemo-Attractant Cytokines in Contact Dermatitis
AFX5931
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agner, T.; Elsner, P. Hand eczema: Epidemiology, prognosis and prevention. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–12. [Google Scholar] [CrossRef]
- Armstrong, A.; Hahn-Pedersen, J.; Bartlett, C.; Glanville, J.; Thyssen, J.P. Economic Burden of Chronic Hand Eczema: A Review. Am. J. Clin. Dermatol. 2022, 23, 287–300. [Google Scholar] [CrossRef]
- Errichetti, E.; Stinco, G. Dermoscopy in differential diagnosis of palmar psoriasis and chronic hand eczema. J. Dermatol. 2016, 43, 423–425. [Google Scholar] [CrossRef]
- Behroozy, A.; Keegel, T.G. Wet-work Exposure: A Main Risk Factor for Occupational Hand Dermatitis. Saf. Health Work 2014, 5, 175–180. [Google Scholar] [CrossRef]
- Suzuki, N.M.; Hafner, M.d.F.S.; Lazzarini, R.; Duarte, I.A.G.; Veasey, J.V. Patch tests and hand eczema: Retrospective study in 173 patients and literature review. An. Bras. Dermatol. 2023, 98, 339–346. [Google Scholar] [CrossRef]
- Johansen, J.D.; Bonefeld, C.M.; Schwensen, J.F.B.; Thyssen, J.P.; Uter, W. Novel insights into contact dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1162–1171. [Google Scholar] [CrossRef]
- Bakker, D.; de Bruin-Weller, M.; Drylewicz, J.; van Wijk, F.; Thijs, J. Biomarkers in atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1163–1168. [Google Scholar] [CrossRef]
- Blair, H.A.; Scott, L.J. Alitretinoin: A Review in Severe Chronic Hand Eczema. Drugs 2016, 76, 1271–1279. [Google Scholar] [CrossRef]
- Malekpour, M.; Etebari, A.; Hezarosi, M.; Anissian, A.; Karimi, F. Mouse Model of Irritant Contact Dermatitis. Iran. J. Pharm. Res. 2022, 21, e130881. [Google Scholar] [CrossRef]
- Koppes, S.A.; Engebretsen, K.A.; Agner, T.; Angelova-Fischer, I.; Berents, T.; Brandner, J.; Brans, R.; Clausen, M.; Hummler, E.; Jakasa, I.; et al. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermat. 2017, 77, 1–16. [Google Scholar] [CrossRef]
- Virgens, A.R.; Goes, H.F.O.; de Carvalho, G.C.; Pietrobon, A.J.; Branco, A.C.C.C.; Ramos, Y.A.L.; Pereira, N.V.; Orfali, R.L.; Aoki, V.; da Silva, L.F.F.; et al. Perivascular clusters of Th2 cells and M2 macrophages in allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone. Exp. Dermatol. 2022, 31, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Kader, H.; Kerstan, A.; Hetta, H.F.; Serfling, E.; Goebeler, M.; Muhammad, K. Intricate Relationship Between Adaptive and Innate Immune System in Allergic Contact Dermatitis. Yale J. Biol. Med. 2020, 93, 699–709. [Google Scholar] [PubMed]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: A Review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Yu, Q.; Zhu, B.; Yuan, F.; Zhang, J.; Peng, L.; Lin, W.; Chen, M. Topical 0.05% clobetasol cream in the treatment of chronic hand eczema: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e24418. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Fonacier, L.S. Adverse Effects of Nonsystemic Steroids (Inhaled, Intranasal, and Cutaneous): A Review of the Literature and Suggested Monitoring Tool. Curr. Allergy Asthma Rep. 2016, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Dubin, C.; Del Duca, E.; Guttman-Yassky, E. Drugs for the Treatment of Chronic Hand Eczema: Successes and Key Challenges. Ther. Clin. Risk Manag. 2020, 17, 1319–1332. [Google Scholar] [CrossRef]
- Jensen, L.; Stensgaard, A.; Andersen, K.E. Psoralen plus ultraviolet A (PUVA) soaks and UVB TL01 treatment for chronic hand dermatoses. Dermatol. Rep. 2012, 4, e3. [Google Scholar] [CrossRef]
- Gooderham, M.J. Alitretinoin: An Update of Real-World Evidence in The Management of Chronic Hand Dermatitis. Ski. Ther. Lett. 2018, 23, 1–4. [Google Scholar]
- Al-Dhubaibi, M.S.; Settin, A.A. The effectiveness of alitretinoin for the treatment of chronic hand eczema: A meta-analysis. Int. J. Health Sci. 2018, 12, 67–76. [Google Scholar]
- Cheng, J.; Facheris, P.; Ungar, B.; Guttman-Yassky, E. Current emerging and investigational drugs for the treatment of chronic hand eczema. Expert Opin. Investig. Drugs 2022, 31, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Miot, H.A.; Criado, P.R.; de Castro, C.C.S.; Ianhez, M.; Talhari, C.; Ramos, P.M. JAK-STAT pathway inhibitors in dermatology. An. Bras. Dermatol. 2023, 98, 656–677. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.; Cornman, H.; Kambala, A.; Kwatra, S.G. A Review on the Safety of Using JAK Inhibitors in Dermatology: Clinical and Laboratory Monitoring. Dermatol. Ther. 2023, 13, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Rajasimhan, S.; Pamuk, O.; Katz, J.D. Safety of Janus Kinase Inhibitors in Older Patients: A Focus on the Thromboembolic Risk. Drugs Aging 2020, 37, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Proof of Concept, Twice Daily Applications of LEO 124249 Ointment in the Treatment of Chronic Hand Eczema. Available online: https://ClinicalTrials.gov/show/NCT02664805 (accessed on 21 December 2020).
- Phase 2b Dose-Ranging Trial to Evaluate Delgocitinib Cream 1, 3, 8, and 20 mg/g Compared to Delgocitinib Cream Vehicle over a 16-Week Treatment Period in Adult Subjects with Chronic Hand Eczema. Available online: https://ClinicalTrials.gov/show/NCT03683719 (accessed on 21 December 2020).
- Colafigli, G.; Scalzulli, E.; Pepe, S.; Di Prima, A.; Efficace, F.; Martelli, M.; Foà, R.; Breccia, M. The advantages and risks of ruxolitinib for the treatment of polycythemia vera. Expert Rev. Hematol. 2020, 13, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Breccia, M.; Mazzoni, C.; Auteri, G.; Elli, E.M.; Trawinska, M.M.; Polverelli, N.; Tiribelli, M.; Benevolo, G.; Iurlo, A.; et al. Ruxolitinib in cytopenic myelofibrosis: Response, toxicity, drug discontinuation, and outcome. Cancer 2023, 129, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Yao, W.; Shepard, S.; Covington, M.; Lee, J.; Lofland, J.; Naim, A.; Sheth, T.; Parikh, B.; Yeleswaram, S. Developing a JAK Inhibitor for Targeted Local Delivery: Ruxolitinib Cream. Pharmaceutics 2021, 13, 1044. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Leung, D.Y.; Forman, S.B.; Venturanza, M.E.; Sun, K.; Kuligowski, M.E.; et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J. Am. Acad. Dermatol. 2021, 85, 863–872. [Google Scholar] [CrossRef]
- Smith, H.; Moy, A.; De Benedetto, A. 384 Ruxolitinib 1.5% cream efficacy data for moderate-to-severe chronic hand dermatitis: Open-label trial 4-weeks interim analysis. Br. J. Dermatol. 2023, 188, ljad162.012. [Google Scholar] [CrossRef]
- Armario-Hita, J.; Galán-Gutiérrez, M.; Dodero-Anillo, J.; Carrascosa, J.; Ruiz-Villaverde, R. Updated Review on Treatment of Atopic Dermatitis. J. Investig. Allergol. Clin. Immunol. 2023, 33, 158–167. [Google Scholar] [CrossRef]
- Jimenez, P.A.; Sofen, H.L.; Bissonnette, R.; Lee, M.; Fowler, J.; Zammit, D.J.; Chen, Y.; Rao, N.; Denis, L.; Gupta, S. Oral spleen tyrosine kinase/Janus Kinase inhibitor gusacitinib for the treatment of chronic hand eczema: Results of a randomized phase 2 study. J. Am. Acad. Dermatol. 2023, 89, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Teixeira, H.D.; Simpson, E.L.; Papp, K.A.; Pangan, A.L.; Blauvelt, A.; Thaçi, D.; Chu, C.-Y.; Hong, H.C.-H.; Katoh, N.; et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): Results from two replicate double-blind, randomised controlled phase 3 trials. Lancet 2021, 397, 2151–2168, Erratum in Lancet 2021, 397, 2150. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, E.; Loman, L.; Han, H.L.; Romeijn, G.L.E.; Politiek, K.; Schuttelaar, M.L.A. Experiences from daily practice of upadacitinib treatment on atopic dermatitis with a focus on hand eczema: Results from the BioDay registry. Contact Dermat. 2023, 88, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Papp, K.A.; Blauvelt, A.; Chu, C.-Y.; Hong, H.C.-H.; Katoh, N.; Calimlim, B.M.; Thyssen, J.P.; Chiou, A.S.; Bissonnette, R.; et al. Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis: Analysis of Follow-up Data From the Measure Up 1 and Measure Up 2 Randomized Clinical Trials. JAMA Dermatol. 2022, 158, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.; Carrascosa, J.M.; Torres, T. Baricitinib for the treatment of atopic dermatitis. J. Dermatol. Treat. 2022, 33, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.; Guttman-Yassky, E.; Torres, T. Baricitinib for the Treatment of Alopecia Areata. Drugs 2023, 83, 761–770. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, H.; Shin, M.K. Nine cases of chronic hand and foot eczema treated with baricitinib. Australas. J. Dermatol. 2023, 64, 408–412. [Google Scholar] [CrossRef]
- Rosenberg, F.M.; Loman, L.; Schuttelaar, M.L. Baricitinib treatment of severe chronic hand eczema: Two case reports. Contact Dermat. 2022, 86, 419–421. [Google Scholar] [CrossRef]
- Nezamololama, N.; Fieldhouse, K.; Metzger, K.; Gooderham, M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: A review of abrocitinib, baricitinib, and upadacitinib. Drugs Context 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Bissonnette, R. Efficacy of Abrocitinib and Dupilumab on Chronic Hand Eczema in Patients with Moderate-to-Severe Atopic Dermatitis: Results from the Phase 3 JADE DARE Study. Abstract 1381 Presented at EADV Congress 2022. Available online: https://medfyle.com/articles/05-jade-dare-che-analyses (accessed on 17 November 2023).
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef]
- Wegesser, T.; Coppi, A.; Harper, T.; Paris, M.; Minocherhomji, S. Nonclinical genotoxicity and carcinogenicity profile of apremilast, an oral selective inhibitor of PDE4. Regul. Toxicol. Pharmacol. 2021, 125, 104985. [Google Scholar] [CrossRef] [PubMed]
- Tsentemeidou, A.; Sotiriou, E.; Sideris, N.; Bakirtzi, K.; Papadimitriou, I.; Lallas, A.; Ioannides, D.; Vakirlis, E. Apremilast in psoriasis patients with serious comorbidities: A case series and systematic review of literature. Dermatol. Pract. Concept. 2022, 12, e2022179. [Google Scholar] [CrossRef] [PubMed]
- Maloney, N.J.; Zhao, J.; Tegtmeyer, K.; Lee, E.Y.; Cheng, K. Off-label studies on apremilast in dermatology: A review. J. Dermatol. Treat. 2020, 31, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Triviño, F.J.; Cuenca-Barrales, C.; Vega-Castillo, J.J.; Ruiz-Villaverde, R. Chronic hand eczema and hepatogenic pruritus with good response to apremilast. Dermatol. Ther. 2019, 32, e12879. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.G.; Kircik, L.H.; Moore, A.Y.; Gold, L.S.; Draelos, Z.D.; Gooderham, M.J.; Papp, K.A.; Bagel, J.; Bhatia, N.; Del Rosso, J.Q.; et al. Effect of Roflumilast Cream vs Vehicle Cream on Chronic Plaque Psoriasis: The DERMIS-1 and DERMIS-2 Randomized Clinical Trials. JAMA 2022, 328, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Sideris, N.; Paschou, E.; Bakirtzi, K.; Kiritsi, D.; Papadimitriou, I.; Tsentemeidou, A.; Sotiriou, E.; Vakirlis, E. New and Upcoming Topical Treatments for Atopic Dermatitis: A Review of the Literature. J. Clin. Med. 2022, 11, 4974. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Chronic Hand Eczema with Oral Roflumilast (HERO)—A Randomized Controlled Trial. Available online: https://ichgcp.net/clinical-trials-registry/NCT05682859 (accessed on 18 November 2023).
- Kahn, J.S.M.; Grossman-Kranseler, J.S.; Zancanaro, P.; Griffiths, D.B.; Dumont, N.; Rosmarin, D. Topical Crisaborole in the Treatment of Atopic Hand Dermatitis: A Retrospective Chart Review. Dermatitis 2021, 32, e141–e143. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E. IL-4 and IL-13: Regulators and Effectors of Wound Repair. Annu. Rev. Immunol. 2023, 41, 229–254. [Google Scholar] [CrossRef]
- Koskeridis, F.; Evangelou, E.; Ntzani, E.E.; Kostikas, K.; Tsabouri, S. Treatment With Dupilumab in Patients With Atopic Dermatitis: Systematic Review and Meta-Analysis. J. Cutan. Med. Surg. 2022, 26, 613–621. [Google Scholar] [CrossRef]
- Olbrich, H.; Sadik, C.D.; Ludwig, R.J.; Thaçi, D.; Boch, K. Dupilumab in Inflammatory Skin Diseases: A Systematic Review. Biomolecules 2023, 13, 634. [Google Scholar] [CrossRef]
- Mickevicius, T.; Pink, A.E.B.; Bhogal, M.B.; O’Brart, D.M.; Robbie, S.J.M. Dupilumab-Induced, Tralokinumab-Induced, and Belantamab Mafodotin–Induced Adverse Ocular Events—Incidence, Etiology, and Management. Cornea 2023, 42, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Balakirski, G.; Burmann, S.-N.; Hofmann, S.C.; Kreuter, A. Paradoxical tralokinumab-induced psoriasis in a patient with atopic dermatitis. J. Dermatol. Treat. 2023, 34, 2258240. [Google Scholar] [CrossRef] [PubMed]
- Lauerma, A.; Werner, P.; Wisgrill, L.; Fyhrquist, N. New Key Players in Irritant Contact Dermatitis: Residential Skin Cells and Neutrophils Drive Inflammation. J. Investig. Dermatol. 2022, 142, 509–512. [Google Scholar] [CrossRef]
- A Study for a Topical Medication versus Placebo in Patients with Hand Dermatitis. Available online: https://ClinicalTrials.gov/show/NCT03703895 (accessed on 21 December 2020).

| Target Pathway or Class | Systemic | Topical |
|---|---|---|
| Corticosteroids | Systemic corticosteroids (prednisone, others) | Topical corticosteroids (clobetasol, others) |
| Vitamin D | Calcipotriol | |
| Retinoids | Alitretinoin | |
| Calcineurin inhibitors | Pimecrolimus Tacrolimus | |
| JAK/STAT | Abrocitinib Baricitinib Gusacitinib | Delgocitinib Ruxolitinib |
| Upadacitinib | ||
| IL-4/IL-13 | Dupilumab (IL-4/IL-13) | |
| Tralokinumab (IL-13) | ||
| PDE4 | Apremilast | Crisaborole |
| Roflumilast | ||
| CCL5/CCL2 | AFX5931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tancredi, V.; Buononato, D.; Caccavale, S.; Di Brizzi, E.V.; Di Caprio, R.; Argenziano, G.; Balato, A. New Perspectives in the Management of Chronic Hand Eczema: Lessons from Pathogenesis. Int. J. Mol. Sci. 2024, 25, 362. https://doi.org/10.3390/ijms25010362
Tancredi V, Buononato D, Caccavale S, Di Brizzi EV, Di Caprio R, Argenziano G, Balato A. New Perspectives in the Management of Chronic Hand Eczema: Lessons from Pathogenesis. International Journal of Molecular Sciences. 2024; 25(1):362. https://doi.org/10.3390/ijms25010362
Chicago/Turabian StyleTancredi, Vittorio, Dario Buononato, Stefano Caccavale, Eugenia Veronica Di Brizzi, Roberta Di Caprio, Giuseppe Argenziano, and Anna Balato. 2024. "New Perspectives in the Management of Chronic Hand Eczema: Lessons from Pathogenesis" International Journal of Molecular Sciences 25, no. 1: 362. https://doi.org/10.3390/ijms25010362







