Promotion of Colitis in B Cell-Deficient C57BL/6 Mice Infected with Enterotoxigenic Bacteroides fragilis
Abstract
:1. Introduction
2. Results
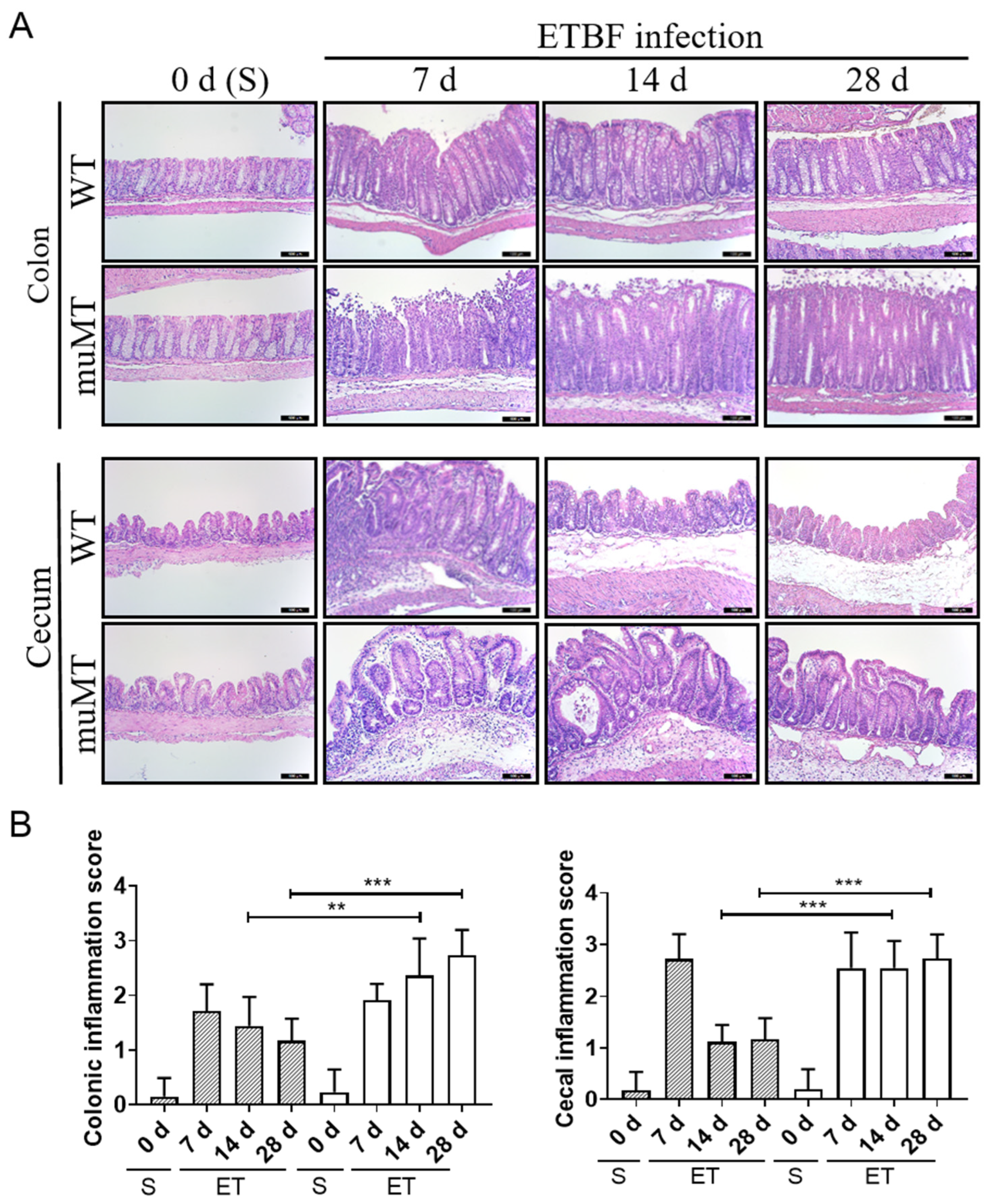
2.1. ETBF Infection Increases Indirect Parameters of Colon Inflammation in B-Cell-Deficient Mice
2.2. Persistent Inflammation in Large Intestines of ETBF-Infected B-Cell-Deficient Mice
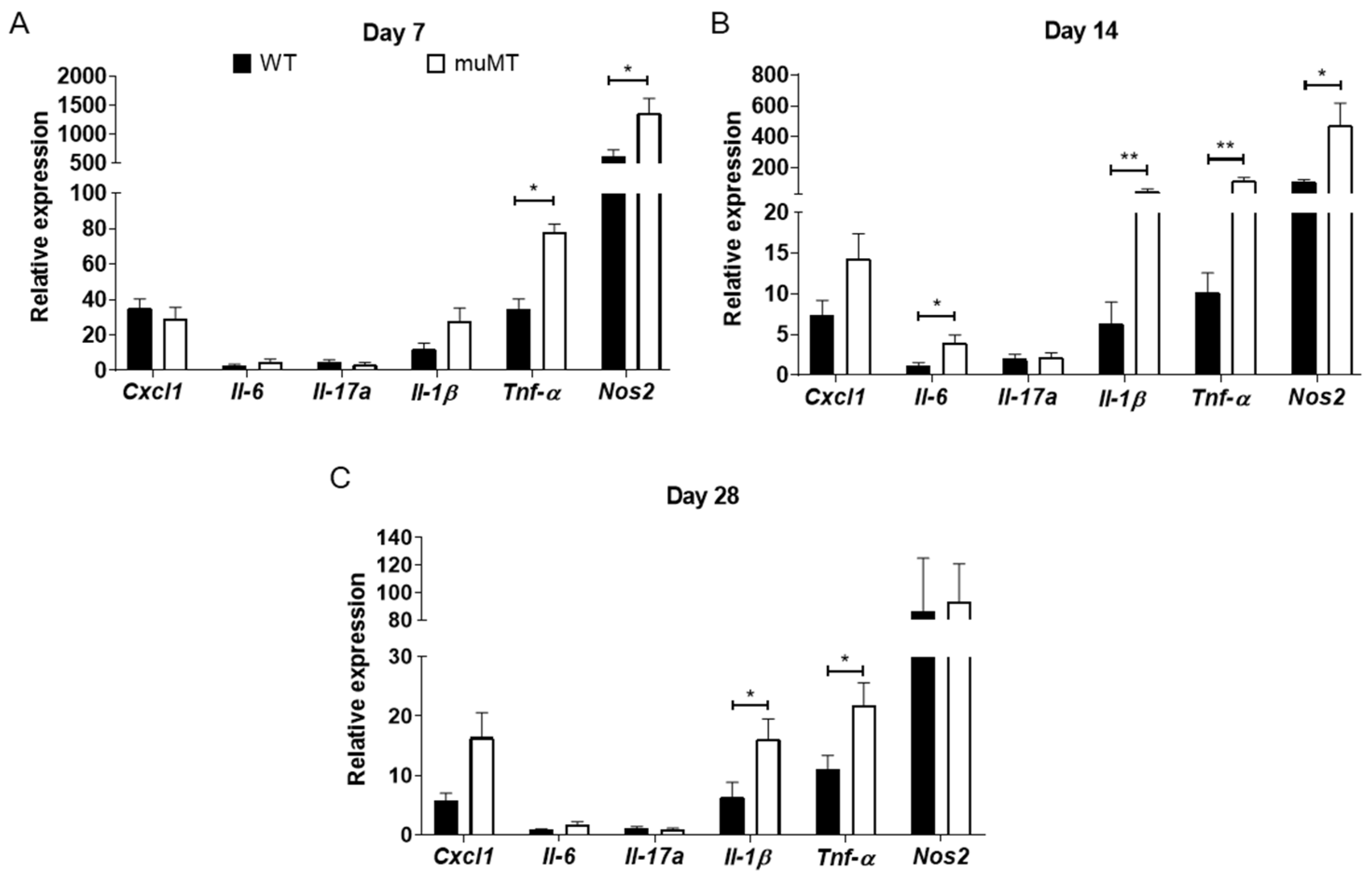
2.3. Elevated Inflammatory Mediators in Cecum of B-Cell-Deficient Mice
2.4. ETBF Does Not Induce Inflammation in the Small Intestine
2.5. Th17/γδ T and Treg Response in ETBF-Infected B-Cell-Deficient Mice
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Mouse Experiments
4.3. Hematoxylin and Eosin (H&E) Staining
4.4. Histological Evaluation of Inflammation in the Colon and Ileum
4.5. Bacterial Colonization
4.6. RNA Extraction
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Flow Cytometry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Mehrabian, P.; Boyajian, S.; Wu, W.L.; Selicha, J.; Vonderfecht, S.; Mazmanian, S.K. The protective role of Bacteroides fragilis in a murine model of colitis-associated colorectal cancer. mSphere 2018, 3, e00587-18. [Google Scholar] [CrossRef] [PubMed]
- Basset, C.; Holton, J.; Bazeos, A.; Vaira, D.; Bloom, S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig. Dis. Sci. 2004, 49, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Prindiville, T.P.; Sheikh, R.A.; Cohen, S.H.; Tang, Y.J.; Cantrell, M.C.; Silva, J., Jr. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg. Infect. Dis. 2000, 6, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Haghi, F.; Goli, E.; Mirzaei, B.; Zeighami, H. The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer 2019, 19, 879. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Enterotoxigenic Bacteroides fragilis: A possible etiological candidate for bacterially-induced colorectal precancerous and cancerous lesions. Front. Cell. Infect. Microbiol. 2020, 9, 449. [Google Scholar] [CrossRef]
- Yim, S.; Gwon, S.Y.; Hwang, S.; Kim, N.H.; Jung, B.D.; Rhee, K.J. Enterotoxigenic Bacteroides fragilis causes lethal colitis in Mongolian gerbils. Anaerobe 2013, 21, 64–66. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Geis, A.L.; Fan, H.; Wu, X.; Wu, S.; Huso, D.L.; Wolfe, J.L.; Sears, C.L.; Pardoll, D.M.; Housseau, F. Regulatory T-cell response to enterotoxigenic Bacteroides fragilis colonization triggers IL17-dependent colon carcinogenesis. Cancer Discov. 2015, 5, 1098–1109. [Google Scholar] [CrossRef]
- Chung, L.; Thiele Orberg, E.; Geis, A.L.; Chan, J.L.; Fu, K.; DeStefano Shields, C.E.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host. Microbe 2018, 23, 203–214. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, C.G.; Jo, M.; Park, C.O.; Gwon, S.Y.; Hwang, S.; Yi, H.C.; Lee, S.Y.; Eom, Y.B.; Karim, B.; et al. Enterotoxigenic Bacteroides fragilis infection exacerbates tumorigenesis in AOM/DSS mouse model. Int. J. Med. Sci. 2020, 17, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Gwon, S.Y.; Kim, M.S.; Lee, S.; Rhee, K.J. Bacteroides fragilis toxin induces IL-8 secretion in HT29/C1 cells through disruption of E-cadherin junctions. Immune Netw. 2013, 13, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 2009, 77, 1708–1718. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Zhang, M.; Franco, A.; Sears, C.L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and γ-secretase-dependent E-cadherin cleavage. J. Cell Sci. 2007, 120, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Morin, P.J.; Maouyo, D.; Sears, C.L. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 2003, 124, 392–400. [Google Scholar] [CrossRef]
- Wu, S.; Powell, J.; Mathioudakis, N.; Kane, S.; Fernandez, E.; Sears, C.L. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-κB pathway. Infect. Immun. 2004, 72, 5832–5839. [Google Scholar] [CrossRef] [PubMed]
- Geis, A.L.; Housseau, F. Procarcinogenic regulatory T cells in microbial-induced colon cancer. Oncoimmunology 2016, 5, e1118601. [Google Scholar] [CrossRef]
- Nanton, M.R.; Way, S.S.; Shlomchik, M.J.; McSorley, S.J. B cells are essential for protective immunity against Salmonella independent of antibody secretion. J. Immunol. 2012, 189, 5503–5507. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Symonds, E.L.; Riedel, C.U.; O’Mahony, D.; Lapthorne, S.; O’Mahony, L.; Shanahan, F. Involvement of T helper type 17 and regulatory T cell activity in Citrobacter rodentium invasion and inflammatory damage. Clin. Exp. Immunol. 2009, 157, 148–154. [Google Scholar] [CrossRef]
- Silberger, D.J.; Zindl, C.L.; Weaver, C.T. Citrobacter rodentium: A model enteropathogen for understanding the interplay of innate and adaptive components of type 3 immunity. Mucosal Immunol. 2017, 10, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.W.; Keeney, K.M.; Crepin, V.F.; Rathinam, V.A.; Fitzgerald, K.A.; Finlay, B.B.; Frankel, G. Citrobacter rodentium: Infection, inflammation and the microbiota. Nat. Rev. Microbiol. 2014, 12, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Goulas, T.; Arolas, J.L.; Gomis-Rüth, F.X. Structure, function and latency regulation of a bacterial enterotoxin potentially derived from a mammalian adamalysin/ADAM xenolog. Proc. Natl. Acad. Sci. USA 2011, 108, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Buendia, A.J.; Del Rio, L.; Ortega, N.; Sanchez, J.; Gallego, M.C.; Caro, M.R.; Navarro, J.A.; Cuello, F.; Salinas, J. B-cell-deficient mice show an exacerbated inflammatory response in a model of Chlamydophila abortus infection. Infect. Immun. 2002, 70, 6911–6918. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Clare, S.; Ghaem-Maghami, M.; Uren, T.K.; Rankin, J.; Huett, A.; Goldin, R.; Lewis, D.J.; MacDonald, T.T.; Strugnell, R.A.; et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 2003, 71, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, S.; Rouse, B.T. Interplay of regulatory T cell and Th17 cells during infectious diseases in humans and animals. Front. Immunol. 2017, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.L.; Garrett, W.S. Microbes, microbiota, and colon cancer. Cell Host. Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pages, F.; et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in patients with colorectal cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef]
- Roche, A.M.; Richard, A.L.; Rahkola, J.T.; Janoff, E.N.; Weiser, J.N. Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 2015, 8, 176–185. [Google Scholar] [CrossRef]
- Tontanahal, A.; Sperandio, V.; Kovbasnjuk, O.; Loos, S.; Kristoffersson, A.C.; Karpman, D.; Arvidsson, I. IgG binds Escherichia coli serine protease EspP and protects mice from E. coli O157:H7 infection. Front. Immunol. 2022, 13, 807959. [Google Scholar] [CrossRef]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, C.; Magri, G.; Cerutti, A. Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 2014, 260, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O.; Slack, E. IgA and the intestinal microbiota: The importance of being specific. Mucosal Immunol. 2020, 13, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Nart, P.; Holden, N.; McAteer, S.P.; Wang, D.; Flockhart, A.F.; Naylor, S.W.; Low, J.C.; Gally, D.L.; Huntley, J.F. Mucosal antibody responses of colonized cattle to Escherichia coli O157-secreted proteins, flagellin, outer membrane proteins and lipopolysaccharide. FEMS Immunol. Med. Microbiol. 2008, 52, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tsvetikova, S.A.; Koshel, E.I. Microbiota and cancer: Host cellular mechanisms activated by gut microbial metabolites. Int. J. Med. Microbiol. 2020, 310, 151425. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Mori, T. Antiproliferative effects of short-chain fatty acids on human colorectal cancer cells via gene expression inhibition. Anticancer Res. 2019, 39, 4659–4666. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Q.; Dorfman, R.G.; Huang, X.; Fan, T.; Zhang, H.; Zhang, J.; Yu, C. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016, 16, 84. [Google Scholar] [CrossRef]
- Phillips, J.A.; Romball, C.G.; Hobbs, M.V.; Ernst, D.N.; Shultz, L.; Weigle, W.O. CD4+ T cell activation and tolerance induction in B cell knockout mice. J. Exp. Med. 1996, 183, 1339–1344. [Google Scholar] [CrossRef]
- Tay, C.; Kanellakis, P.; Hosseini, H.; Cao, A.; Toh, B.H.; Bobik, A.; Kyaw, T. B Cell and CD4 T cell interactions promote development of atherosclerosis. Front. Immunol. 2019, 10, 3046. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.C.; Gonzaga, L.M.; Mather, J.M.; Messer, R.J.; Hasenkrug, K.J. B cell requirement for robust regulatory T cell responses to friend retrovirus infection. mBio 2017, 8, e01122-17. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.B.; Flach, C.F.; Czerkinsky, C.; Holmgren, J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: Powerful induction by antigen coupled to cholera toxin B subunit. J. Immunol. 2008, 181, 8278–8287. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.S.; Prod’homme, T.; Patarroyo, J.C.; Molnarfi, N.; Karnezis, T.; Lehmann-Horn, K.; Danilenko, D.M.; Eastham-Anderson, J.; Slavin, A.J.; Linington, C.; et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann. Neurol. 2010, 68, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Basu, S.; Williams, C.B.; Salzman, N.H.; Dittel, B.N. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J. Immunol. 2012, 188, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Hamaguchi, Y.; Venturi, G.M.; Steeber, D.A.; St Clair, E.W.; Tedder, T.F. B cell depletion delays collagen-induced arthritis in mice: Arthritis induction requires synergy between humoral and cell-mediated immunity. J. Immunol. 2007, 179, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, J.D.; Yanaba, K.; Venturi, G.M.; Wang, Y.; Tisch, R.M.; Poe, J.C.; Tedder, T.F. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 20878–20883. [Google Scholar] [CrossRef]
- Yu, S.; Ellis, J.S.; Dunn, R.; Kehry, M.R.; Braley-Mullen, H. Transient depletion of B cells in young mice results in activation of regulatory T cells that inhibit development of autoimmune disease in adults. Int. Immunol. 2012, 24, 233–242. [Google Scholar] [CrossRef]
- Thiele Orberg, E.; Fan, H.; Tam, A.J.; Dejea, C.M.; Destefano Shields, C.E.; Wu, S.; Chung, L.; Finard, B.B.; Wu, X.; Fathi, P.; et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017, 10, 421–433. [Google Scholar] [CrossRef]
- Ermund, A.; Schutte, A.; Johansson, M.E.; Gustafsson, J.K.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G341–G347. [Google Scholar] [CrossRef]
- Salzman, N.H. Paneth cell defensins and the regulation of the microbiome: Detente at mucosal surfaces. Gut Microbes 2010, 1, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Slavinskaya, Z.; Merrill, A.R.; Kaufmann, S.H. Human α-defensins neutralize toxins of the mono-ADP-ribosyltransferase family. Biochem. J. 2006, 399, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Gajendran, N.; Mittrucker, H.W.; Weiwad, M.; Song, Y.H.; Hurwitz, R.; Wilmanns, M.; Fischer, G.; Kaufmann, S.H. Human α-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl. Acad. Sci. USA 2005, 102, 4830–4835. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, M.; Hwang, S.; Lee, C.-G.; Hong, J.-E.; Kang, D.-H.; Yoo, S.-H.; Kim, W.-S.; Yoo, J.-Y.; Rhee, K.-J. Promotion of Colitis in B Cell-Deficient C57BL/6 Mice Infected with Enterotoxigenic Bacteroides fragilis. Int. J. Mol. Sci. 2024, 25, 364. https://doi.org/10.3390/ijms25010364
Jo M, Hwang S, Lee C-G, Hong J-E, Kang D-H, Yoo S-H, Kim W-S, Yoo J-Y, Rhee K-J. Promotion of Colitis in B Cell-Deficient C57BL/6 Mice Infected with Enterotoxigenic Bacteroides fragilis. International Journal of Molecular Sciences. 2024; 25(1):364. https://doi.org/10.3390/ijms25010364
Chicago/Turabian StyleJo, Minjeong, Soonjae Hwang, Chang-Gun Lee, Ju-Eun Hong, Da-Hye Kang, Sang-Hyeon Yoo, Woo-Seung Kim, Jung-Yoon Yoo, and Ki-Jong Rhee. 2024. "Promotion of Colitis in B Cell-Deficient C57BL/6 Mice Infected with Enterotoxigenic Bacteroides fragilis" International Journal of Molecular Sciences 25, no. 1: 364. https://doi.org/10.3390/ijms25010364




