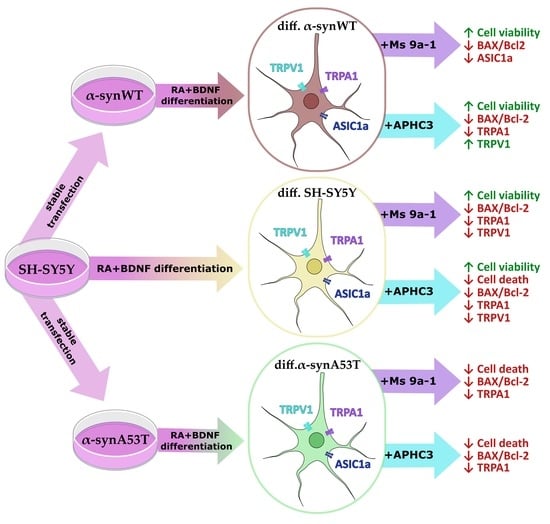
Modulation of TRPV1 and TRPA1 Channels Function by Sea Anemones’ Peptides Enhances the Viability of SH-SY5Y Cell Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
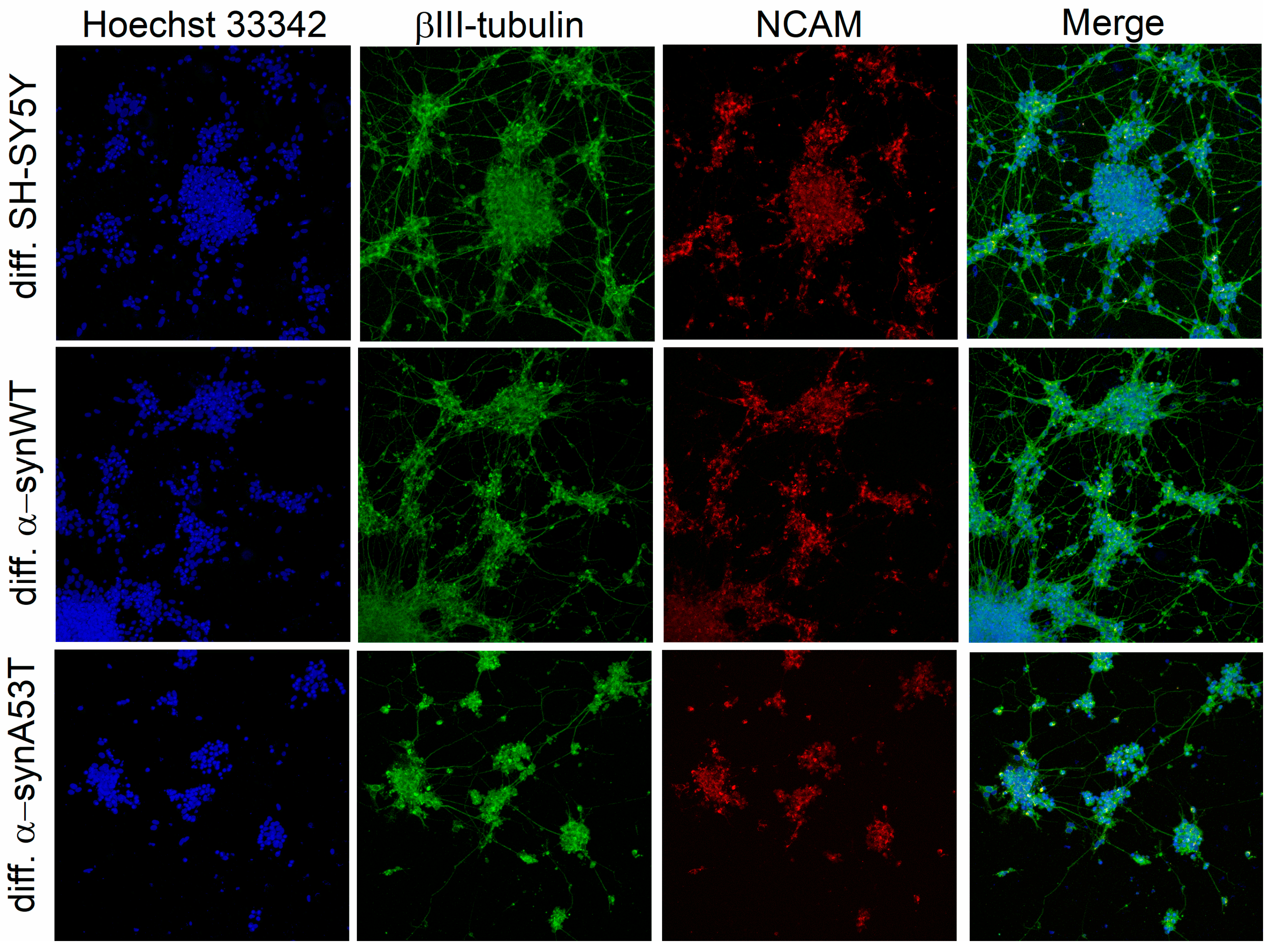
2.1. Analysis of Neuronal Differentiation with RA + BDNF of Overexpressing Alpha-Synuclein SH-SY5Y Cells
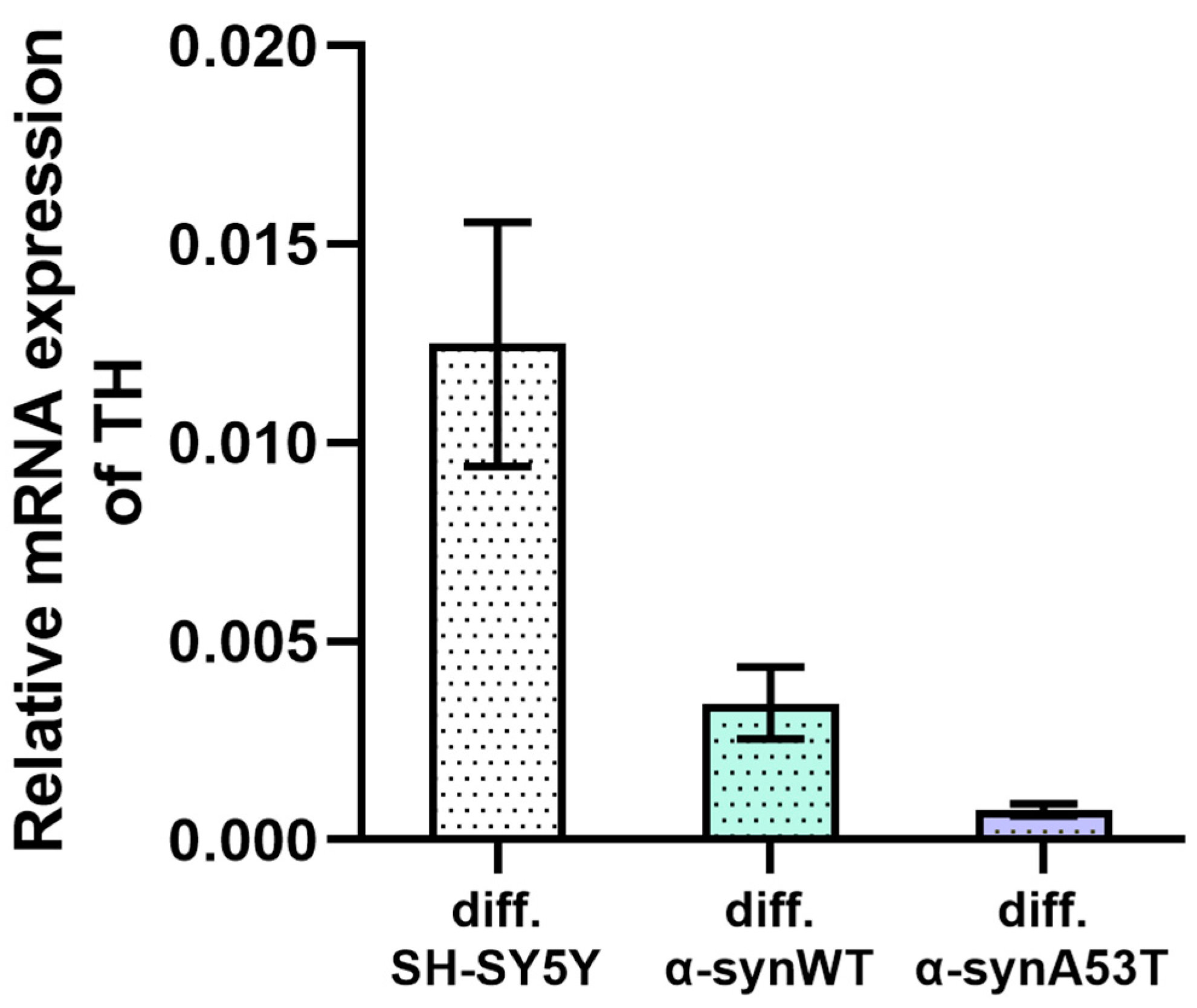
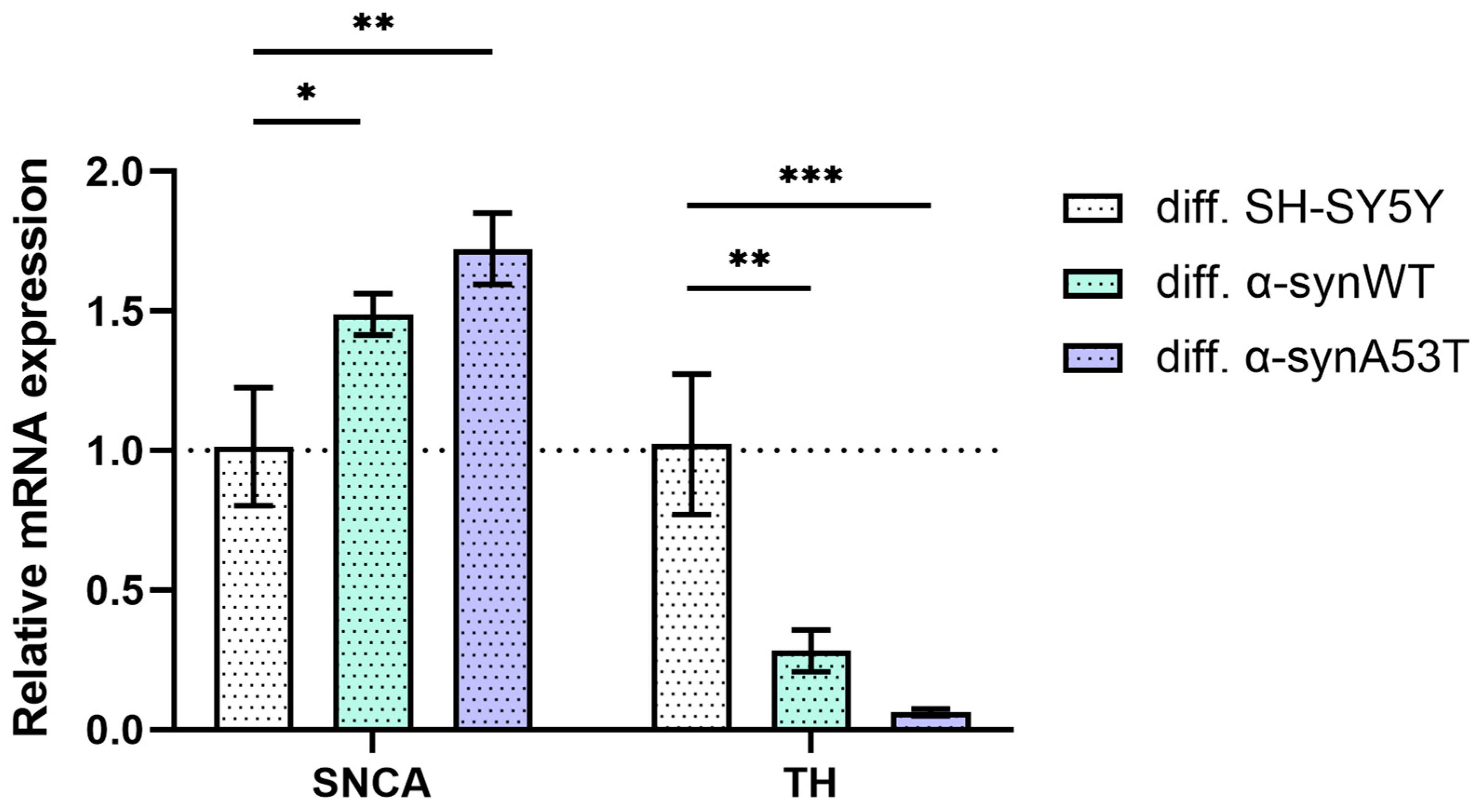
2.2. Quantitative mRNA Difference in Alpha-Synuclein (SNCA) and Tyrosine Hydroxylase (TH) in Overexpressing Alpha-Synuclein SH-SY5Y after Differentiation with RA + BDNF
2.3. Quantitative mRNA Difference in TRPA1, TRPV1, and ASIC1a in Overexpressing Alpha-Synuclein SH-SY5Y Cells after Differentiation with RA + BDNF
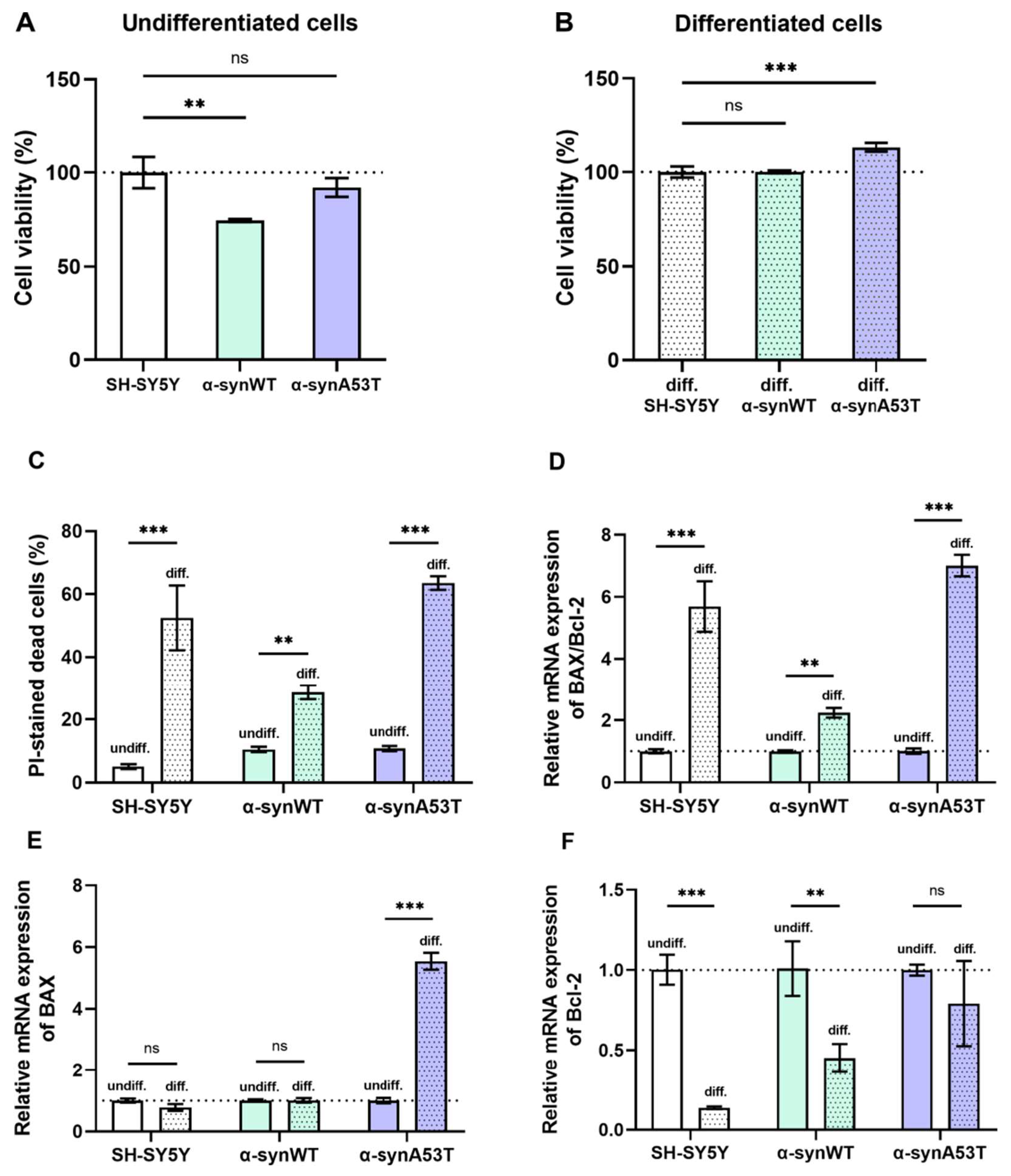
2.4. Viability, Cell Death, and Apoptosis of Overexpressing Alpha-Synuclein SH-SY5Y Cells after Differentiation with RA + BDNF
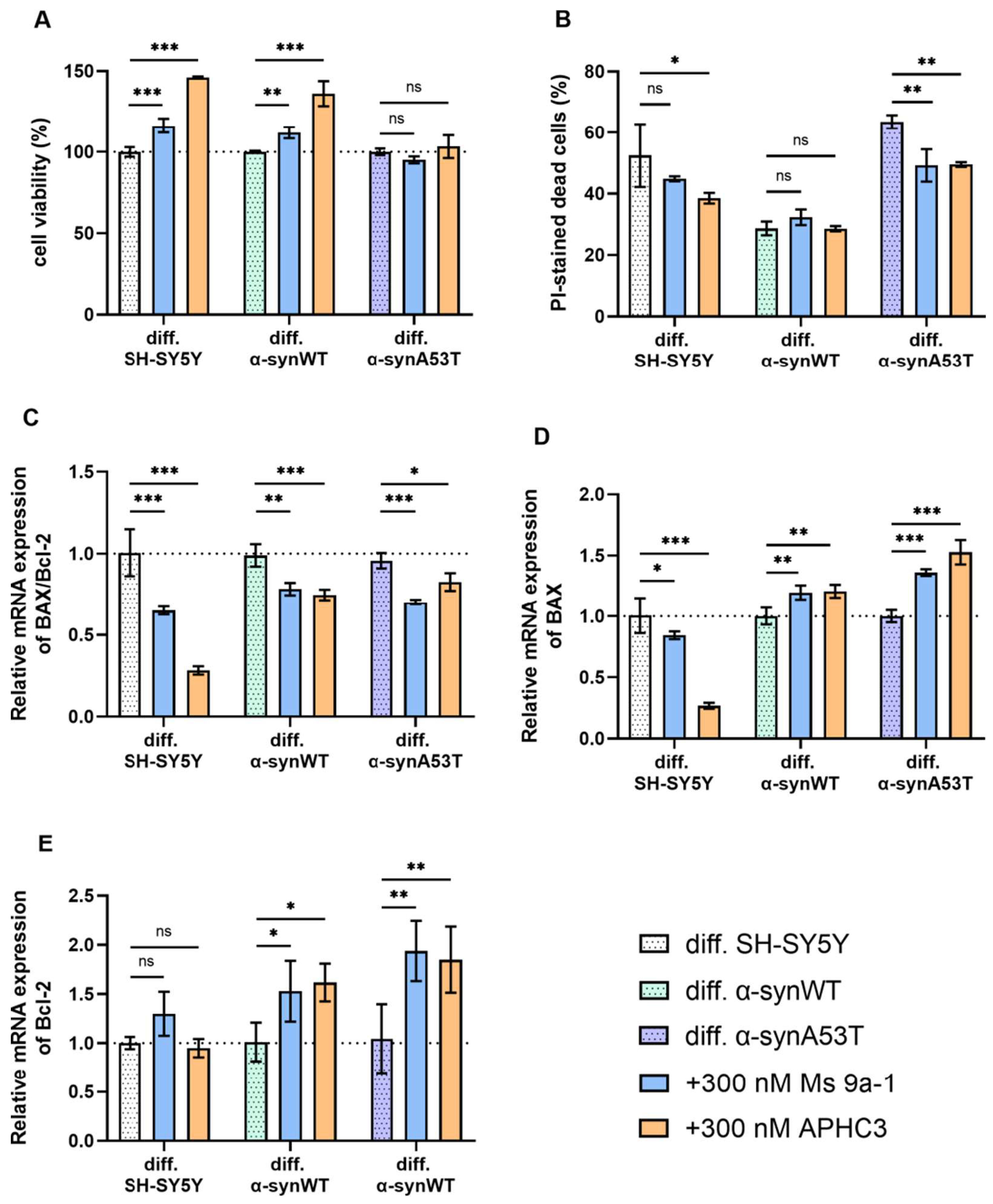
2.5. Ms 9a-1 and APHC3 Peptides Ameliorate Cell Viability and Apoptosis Resistance of Neuron-like Cells
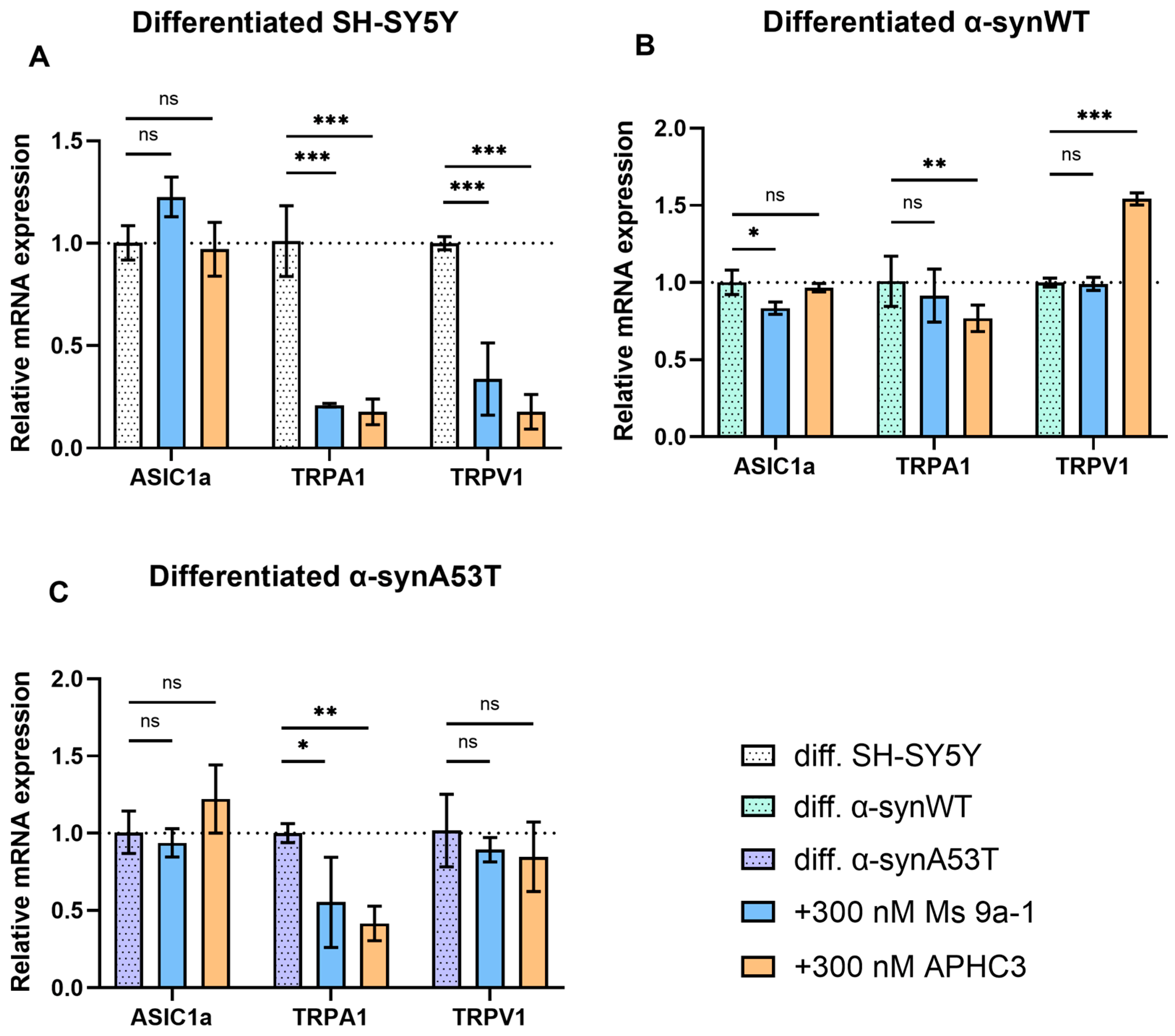
2.6. Quantitative mRNA Difference in TRPA1, TRPV1, and ASIC1a in Differentiated Overexpressing Alpha-Synuclein SH-SY5Y after Ms 9a-1 or APHC3 Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation
4.2. Cell Counting Kit-8 (CCK-8) Cell Viability Assay
4.3. Propidium Iodide (PI) Staining Cell Death Assay
4.4. Immunofluorescence Imaging
4.5. RNA Extraction and cDNA Synthesis
4.6. Quantitative PCR
4.7. Data Presentation and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lindvall, O.; Kokaia, Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson’s disease. Trends Pharmacol. Sci. 2009, 30, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Macroautophagy in sporadic and the genetic form of Parkinson’s disease with the A53T α-synuclein mutation. Transl. Neurodegener. 2012, 1, 2. [Google Scholar] [CrossRef]
- More, S.V.; Kumar, H.; Kim, I.S.; Song, S.-Y.; Choi, D.-K. Cellular and Molecular Mediators of Neuroinflammation in the Pathogenesis of Parkinson’s Disease. Mediat. Inflamm. 2013, 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Savitt, J.M.; Dawson, V.L.; Dawson, T.M. Diagnosis and treatment of Parkinson disease: Molecules to medicine. J. Clin. Investig. 2006, 116, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Brakedal, B.; Toker, L.; Haugarvoll, K.; Tzoulis, C. A nationwide study of the incidence, prevalence and mortality of Parkinson’s disease in the Norwegian population. NPJ Park. Dis. 2022, 8, 19. [Google Scholar] [CrossRef]
- Sawamura, S.; Shirakawa, H.; Nakagawa, T.; Mori, Y.; Kaneko, S. TRP Channels in the Brain: What Are They There For? In Neurobiology of TRP Channels; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 295–322. [Google Scholar]
- Zhao, R.; Tsang, S.Y. Versatile Roles of Intracellularly Located TRPV1 Channel. J. Cell. Physiol. 2017, 232, 1957–1965. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Nabissi, M. TRP channels: New potential therapeutic approaches in CNS neuropathies. CNS Neurol. Disord. Drug Targets 2013, 12, 274–293. [Google Scholar] [CrossRef]
- Li, M.; Zhu, M.; Xu, Q.; Ding, F.; Tian, Y.; Zhang, M. Sensation of TRPV1 via 5-hydroxytryptamine signaling modulates pain hypersensitivity in a 6-hydroxydopamine induced mice model of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2020, 521, 868–873. [Google Scholar] [CrossRef]
- Hellenthal, K.E.M.; Brabenec, L.; Gross, E.R.; Wagner, N.M. TRP Channels as Sensors of Aldehyde and Oxidative Stress. Biomolecules 2021, 11, 1401. [Google Scholar] [CrossRef]
- AM, M.; MR, S. Plasma membrane Ca-ATPases in the nervous system during development and ageing. World J. Biol. Chem. 2010, 1, 229. [Google Scholar]
- Nikolaev, M.V.; Dorofeeva, N.A.; Komarova, M.S.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Grishin, E.V.; Tikhonov, D.B.; Kozlov, S.A. TRPV1 activation power can switch an action mode for its polypeptide ligands. PLoS ONE 2017, 12, e0177077. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Korolkova, Y.V.; Dyachenko, I.A.; Bondarenko, D.A.; Skobtsov, D.I.; Murashev, A.N.; Kotova, P.D.; Rogachevskaja, O.A.; Kabanova, N.V.; et al. Polypeptide modulators of TRPV1 produce analgesia without hyperthermia. Mar. Drugs 2013, 11, 5100–5115. [Google Scholar] [CrossRef] [PubMed]
- Philyppov, I.B.; Paduraru, O.N.; Andreev, Y.A.; Grishin, E.V.; Shuba, Y.M. Modulation of TRPV1-dependent contractility of normal and diabetic bladder smooth muscle by analgesic toxins from sea anemone Heteractis crispa. Life Sci. 2012, 91, 912–920. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Palikova, Y.A.; Palikov, V.A.; Kazakov, V.A.; Smolskaya, S.V.; Dyachenko, I.A.; Tarasova, N.V.; Andreev, Y.A. Anti-Inflammatory and Analgesic Effects of TRPV1 Polypeptide Modulator APHC3 in Models of Osteo- and Rheumatoid Arthritis. Mar. Drugs 2021, 19, 39. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Mosharova, I.V.; Korolkova, Y.V.; Shelukhina, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Murashev, A.N.; Kozlov, S.A.; Stensvåg, K.; et al. Peptide from Sea Anemone Metridium senile Affects Transient Receptor Potential Ankyrin-repeat 1 (TRPA1) Function and Produces Analgesic Effect. J. Biol. Chem. 2017, 292, 2992. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Lubova, K.I.; Maleeva, E.E.; Palikov, V.A.; Palikova, Y.A.; Dyachenko, I.A.; Andreev, Y.A. Analysis of Structural Determinants of Peptide MS 9a-1 Essential for Potentiating of TRPA1 Channel. Mar. Drugs 2022, 20, 465. [Google Scholar] [CrossRef]
- Maleeva, E.E.; Palikova, Y.A.; Palikov, V.A.; Kazakov, V.A.; Simonova, M.A.; Logashina, Y.A.; Tarasova, N.V.; Dyachenko, I.A.; Andreev, Y.A. Potentiating TRPA1 by Sea Anemone Peptide Ms 9a-1 Reduces Pain and Inflammation in a Model of Osteoarthritis. Mar. Drugs 2023, 21, 617. [Google Scholar] [CrossRef]
- Weng, Y.; Batista-Schepman, P.A.; Barabas, M.E.; Harris, E.Q.; Dinsmore, T.B.; Kossyreva, E.A.; Foshage, A.M.; Wang, M.H.; Schwab, M.J.; Wang, V.M.; et al. Prostaglandin metabolite induces inhibition of TRPA1 and channel-dependent nociception. Mol. Pain 2012, 8, 75. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.E.; Andersson, A.; Bucher, B.; Galzi, J.L.; Sterner, O.; Bevan, S.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Oncü, G.; Alper Yılmaz, M.; Saybas, H. Transformation of SH-SY5Y cell line into neuron-like cells: Investigation of electrophysiological and biomechanical changes. Neurosci. Lett. 2021, 745, 135628. [Google Scholar]
- Ioghen, O.C.; Ceafalan, L.C.; Popescu, B.O. SH-SY5Y Cell Line In Vitro Models for Parkinson Disease Research-Old Practice for New Trends. J. Integr. Neurosci. 2023, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, A.; Pozdyshev, D.; Barinova, K.; Kudryavtseva, S.; Muronetz, V.I. α-Synuclein Overexpression in SH-SY5Y Human Neuroblastoma Cells Leads to the Accumulation of Thioflavin S-positive Aggregates and Impairment of Glycolysis. Biochemistry 2020, 85, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Kalinovskii, A.P.; Osmakov, D.I.; Koshelev, S.G.; Lubova, K.I.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a). Biology 2022, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, 2016, 53193. [Google Scholar]
- Goranov, B.B.; Campbell Hewson, Q.D.; Pearson, A.D.J.; Redfern, C.P.F. Overexpression of RARγ increases death of SH-SY5Y neuroblastoma cells in response to retinoic acid but not fenretinide. Cell Death Differ. 2005, 13, 676–679. [Google Scholar] [CrossRef]
- Dravid, A.; Raos, B.; Svirskis, D.; O’Carroll, S.J. Optimised techniques for high-throughput screening of differentiated SH-SY5Y cells and application for neurite outgrowth assays. Sci. Rep. 2021, 11, 23935. [Google Scholar] [CrossRef]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.C.; Onisiforou, A.; Zanos, P.; Papanicolaou, E.Z. Unraveling the transcriptomic signatures of Parkinson’s disease and major depression using single-cell and bulk data. Front. Aging Neurosci. 2023, 15, 1273855. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Bramanti, P.; Ruggeri, A.; Donato, L.; Alafaci, C.; Crisafulli, C.; Mucciardi, M.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. CCM3/SERPINI1 bidirectional promoter variants in patients with cerebral cavernous malformations: A molecular and functional study. BMC Med. Genet. 2016, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Yang, Y.; Anantharam, V.; Kanthasamy, A.G. α-Synuclein Negatively Regulates Protein Kinase Cδ Expression to Suppress Apoptosis in Dopaminergic Neurons by Reducing p300 Histone Acetyltransferase Activity. J. Neurosci. 2011, 31, 2035. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Nakashima, A.; Ichinose, H.; Kobayashi, K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural Transm. 2019, 126, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Nam, J.H.; Park, E.S.; Won, S.Y.; Lee, Y.A.; Kim, K.I.; Jeong, J.Y.; Baek, J.Y.; Cho, E.J.; Jin, M.; Chung, Y.C.; et al. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson’s disease via CNTF. Brain 2015, 138, 3610–3622. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jeong, J.Y.; Kim, K.I.; Won, S.Y.; Chung, Y.C.; Nam, J.H.; Cho, E.J.; Ahn, T.B.; Bok, E.; Shin, W.H.; et al. Inhibition of Microglia-Derived Oxidative Stress by Ciliary Neurotrophic Factor Protects Dopamine Neurons In Vivo from MPP+ Neurotoxicity. Int. J. Mol. Sci. 2018, 19, 3543. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Zhang, H.; Wang, T.; Zheng, Q.; Li, Z.; Yuan, J.; Liu, H.; Zhang, H.; Wang, T.; et al. Controlled Activation of TRPV1 Channels on Microglia to Boost Their Autophagy for Clearance of Alpha-Synuclein and Enhance Therapy of Parkinson’s Disease. Adv. Mater. 2022, 34, 2108435. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Baek, J.Y.; Jeong, J.Y.; Nam, J.H.; Park, E.S.; Bok, E.; Shin, W.H.; Chung, Y.C.; Jin, B.K. Delayed Treatment of Capsaicin Produces Partial Motor Recovery by Enhancing Dopamine Function in MPP+-lesioned Rats via Ciliary Neurotrophic Factor. Exp. Neurobiol. 2019, 28, 289. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Sharma, S.S. Transient Receptor Potential Channels as an Emerging Target for the Treatment of Parkinson’s Disease: An Insight Into Role of Pharmacological Interventions. Front. Cell Dev. Biol. 2020, 8, 584513. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, D.Y.; Chung, E.S.; Oh, U.T.; Kim, S.U.; Jin, B.K. Transient Receptor Potential Vanilloid Subtype 1 Mediates Cell Death of Mesencephalic Dopaminergic Neurons In Vivo and In Vitro. J. Neurosci. 2005, 25, 662. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. TRPA1 Channel as a Regulator of Neurogenic Inflammation and Pain: Structure, Function, Role in Pathophysiology, and Therapeutic Potential of Ligands. Biochemistry 2019, 84, 101–118. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Akan, T.; Aydın, Y.; Korkmaz, O.T.; Ulupınar, E.; Saydam, F. The Effects of Carvacrol on Transient Receptor Potential (TRP) Channels in an Animal Model of Parkinson’s Disease. Neurotox. Res. 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Y.; Jiao, Y.; Herr, S.A.; Tang, J.; Rogers, E.; Chen, Z.; Shi, R. Acrolein scavenger dimercaprol offers neuroprotection in an animal model of Parkinson’s disease: Implication of acrolein and TRPA1. Transl. Neurodegener. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Ambaw, A.; Zheng, L.; Tambe, M.A.; Strathearn, K.E.; Acosta, G.; Hubers, S.A.; Liu, F.; Herr, S.A.; Tang, J.; Truong, A.; et al. Acrolein-mediated neuronal cell death and alpha-synuclein aggregation: Implications for Parkinson’s disease. Mol. Cell. Neurosci. 2018, 88, 70–82. [Google Scholar] [CrossRef]
- Acosta, G.; Race, N.; Herr, S.; Fernandez, J.; Tang, J.; Rogers, E.; Shi, R. Acrolein-mediated alpha-synuclein pathology involvement in the early post-injury pathogenesis of mild blast-induced Parkinsonian neurodegeneration. Mol. Cell. Neurosci. 2019, 98, 140–154. [Google Scholar] [CrossRef] [PubMed]

| Diff. α-synWT | Diff. α-synA53T | |
|---|---|---|
| cell viability | ns | ↑ 13% *** |
| SNCA1 1 | ↑ 47% * | ↑ 70% ** |
| TH | ↓ 72% ** | ↓ 94% *** |
| ASIC1a | ns | ns |
| TRPA1 | ↑ 84% * | ↑ 95% * |
| TRPV1 | ns | ↑ 45% * |
| Diff. SH-SY5Y + Ms 9a-1 | Diff. SH-SY5Y + APHC3 | Diff. α-synWT + Ms 9a-1 | Diff. α-synWT + APHC3 | Diff. α-synA53T + Ms 9a-1 | Diff. α-synA53T + APHC3 | |
|---|---|---|---|---|---|---|
| cell viability | ↑ 16.37% *** | ↑ 46.05% *** | ↑ 11.97% ** | ↑ 36.12% *** | ns | ns |
| cell death | ns | ↓ 13.97% * | ns | ns | ↓ 14.35 ** | ↓ 14.06 ** |
| BAX/Bcl-2 | ↓ 35.4% *** | ↓ 72% *** | ↓ 21% ** | ↓ 24.5% *** | ↓ 26.6% *** | ↓ 13.7% * |
| ASIC1a | ns | ns | ↓ 16% * | ns | ns | ns |
| TRPA1 | ↓ 79% *** | ↓ 82.5% *** | ns | ↓ 24% ** | ↓ 44.7% * | ↓ 58% ** |
| TRPV1 | ↓ 57% *** | ↓ 77% *** | ns | ↑ 54% *** | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesova, Y.S.; Stroylova, Y.Y.; Maleeva, E.E.; Moysenovich, A.M.; Pozdyshev, D.V.; Muronetz, V.I.; Andreev, Y.A. Modulation of TRPV1 and TRPA1 Channels Function by Sea Anemones’ Peptides Enhances the Viability of SH-SY5Y Cell Model of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 368. https://doi.org/10.3390/ijms25010368
Kolesova YS, Stroylova YY, Maleeva EE, Moysenovich AM, Pozdyshev DV, Muronetz VI, Andreev YA. Modulation of TRPV1 and TRPA1 Channels Function by Sea Anemones’ Peptides Enhances the Viability of SH-SY5Y Cell Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2024; 25(1):368. https://doi.org/10.3390/ijms25010368
Chicago/Turabian StyleKolesova, Yuliya S., Yulia Y. Stroylova, Ekaterina E. Maleeva, Anastasia M. Moysenovich, Denis V. Pozdyshev, Vladimir I. Muronetz, and Yaroslav A. Andreev. 2024. "Modulation of TRPV1 and TRPA1 Channels Function by Sea Anemones’ Peptides Enhances the Viability of SH-SY5Y Cell Model of Parkinson’s Disease" International Journal of Molecular Sciences 25, no. 1: 368. https://doi.org/10.3390/ijms25010368
APA StyleKolesova, Y. S., Stroylova, Y. Y., Maleeva, E. E., Moysenovich, A. M., Pozdyshev, D. V., Muronetz, V. I., & Andreev, Y. A. (2024). Modulation of TRPV1 and TRPA1 Channels Function by Sea Anemones’ Peptides Enhances the Viability of SH-SY5Y Cell Model of Parkinson’s Disease. International Journal of Molecular Sciences, 25(1), 368. https://doi.org/10.3390/ijms25010368