Effects of Secondary Metabolites of Rice on Brown Planthopper and Its Symbionts
Abstract
:1. Introduction
2. Results
2.1. Results of the Effect of Secondary Metabolites on the Brown Planthopper
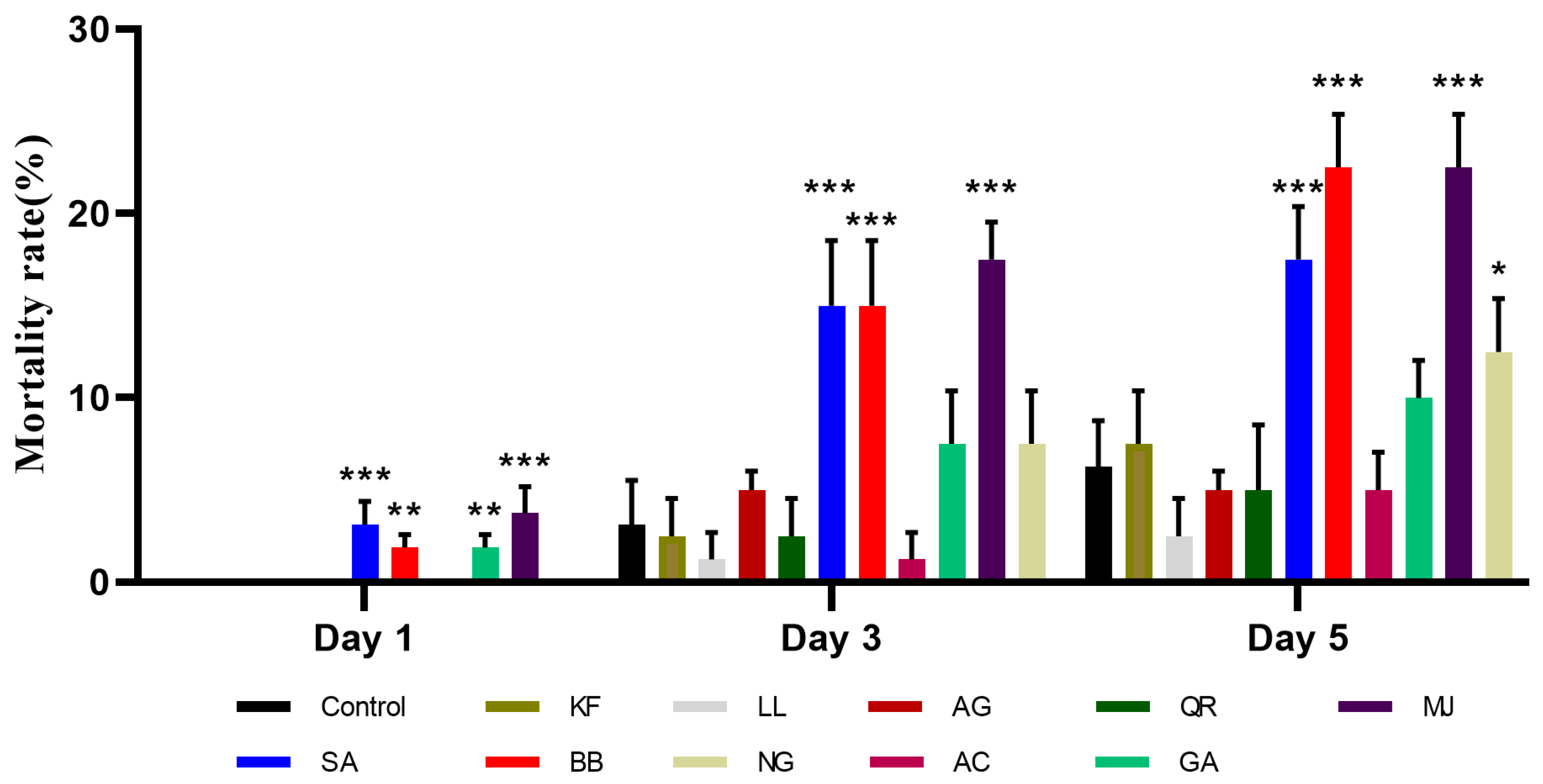
2.1.1. Mortality Rate
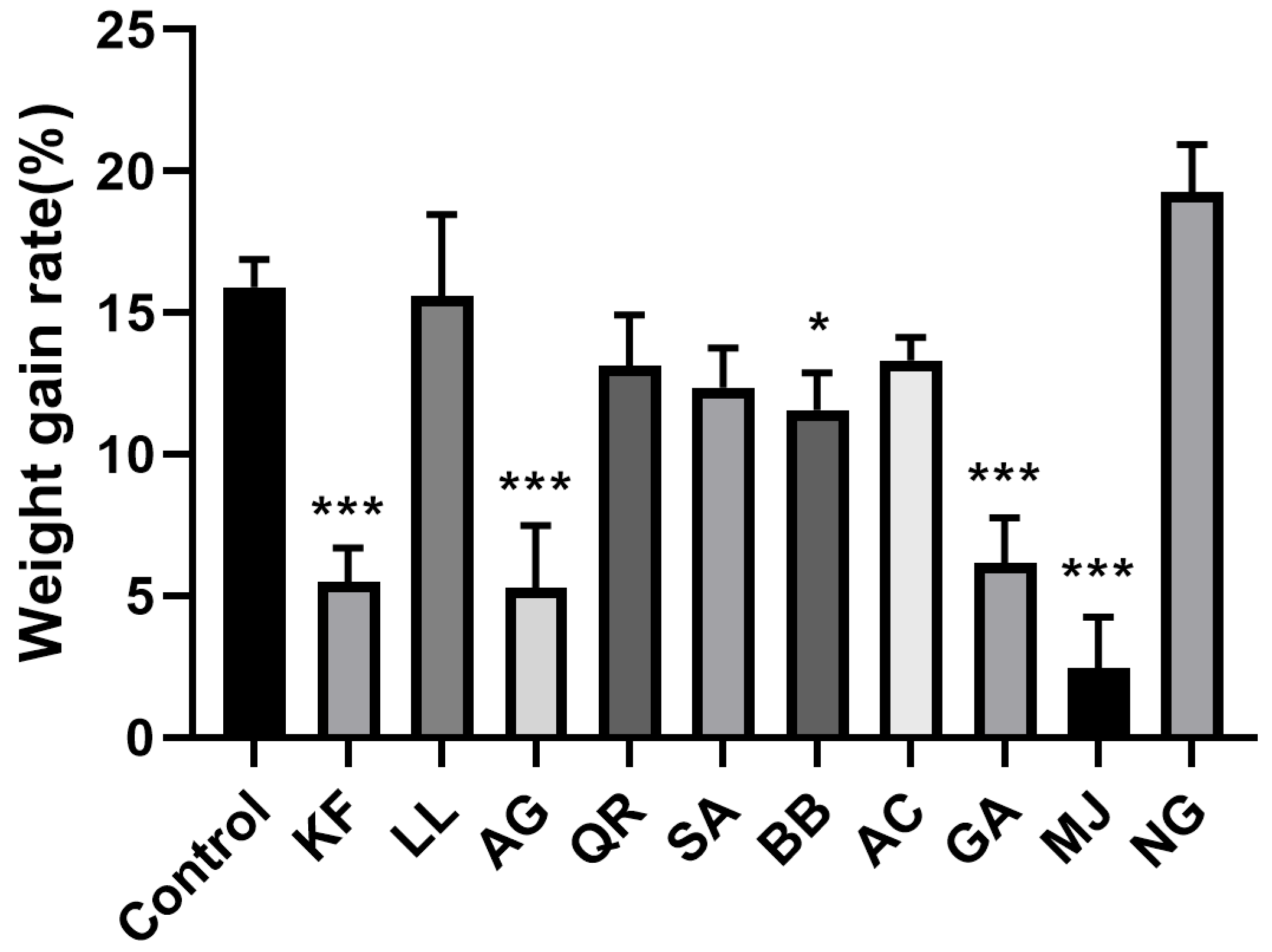
2.1.2. Weight Gain Rate
2.1.3. Fecundity
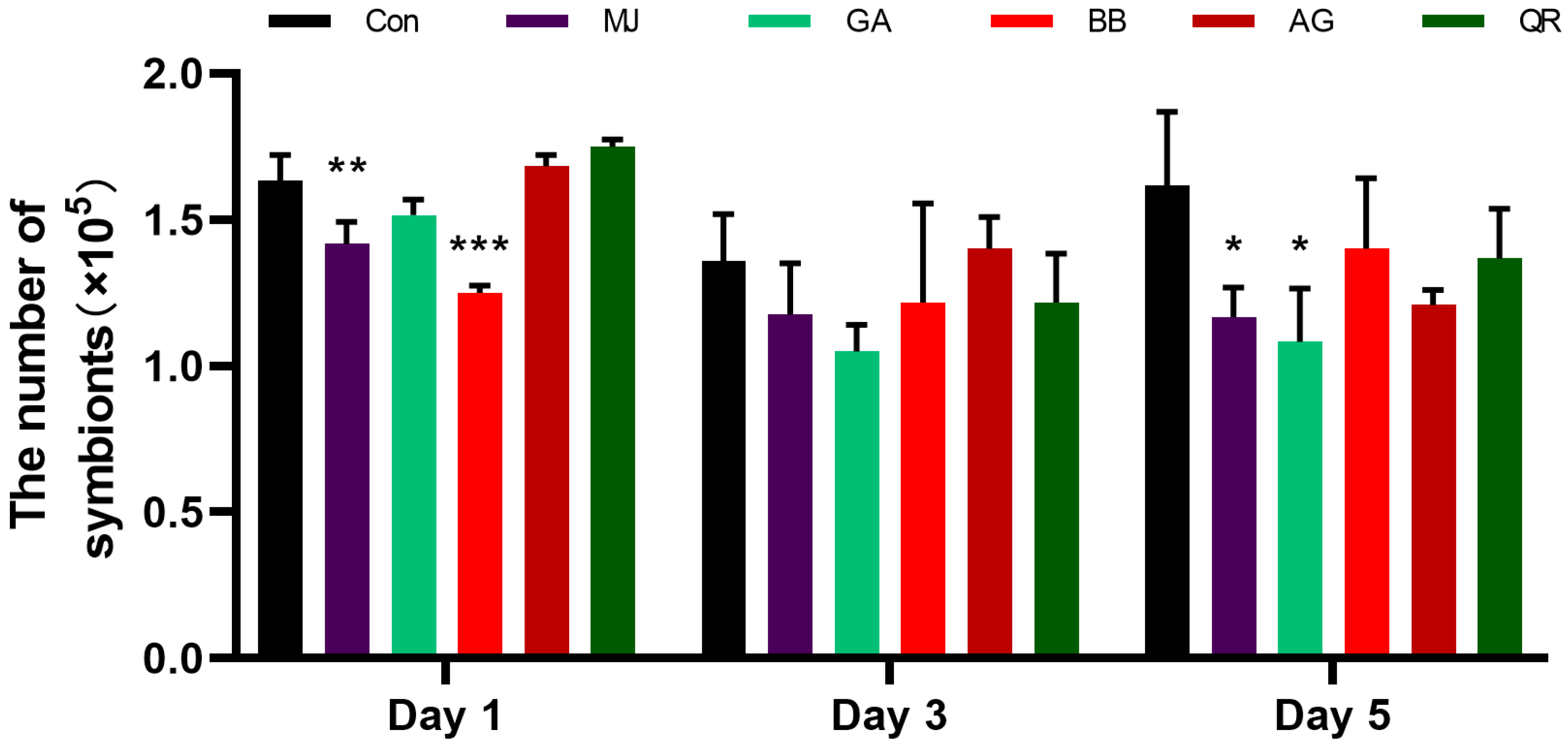
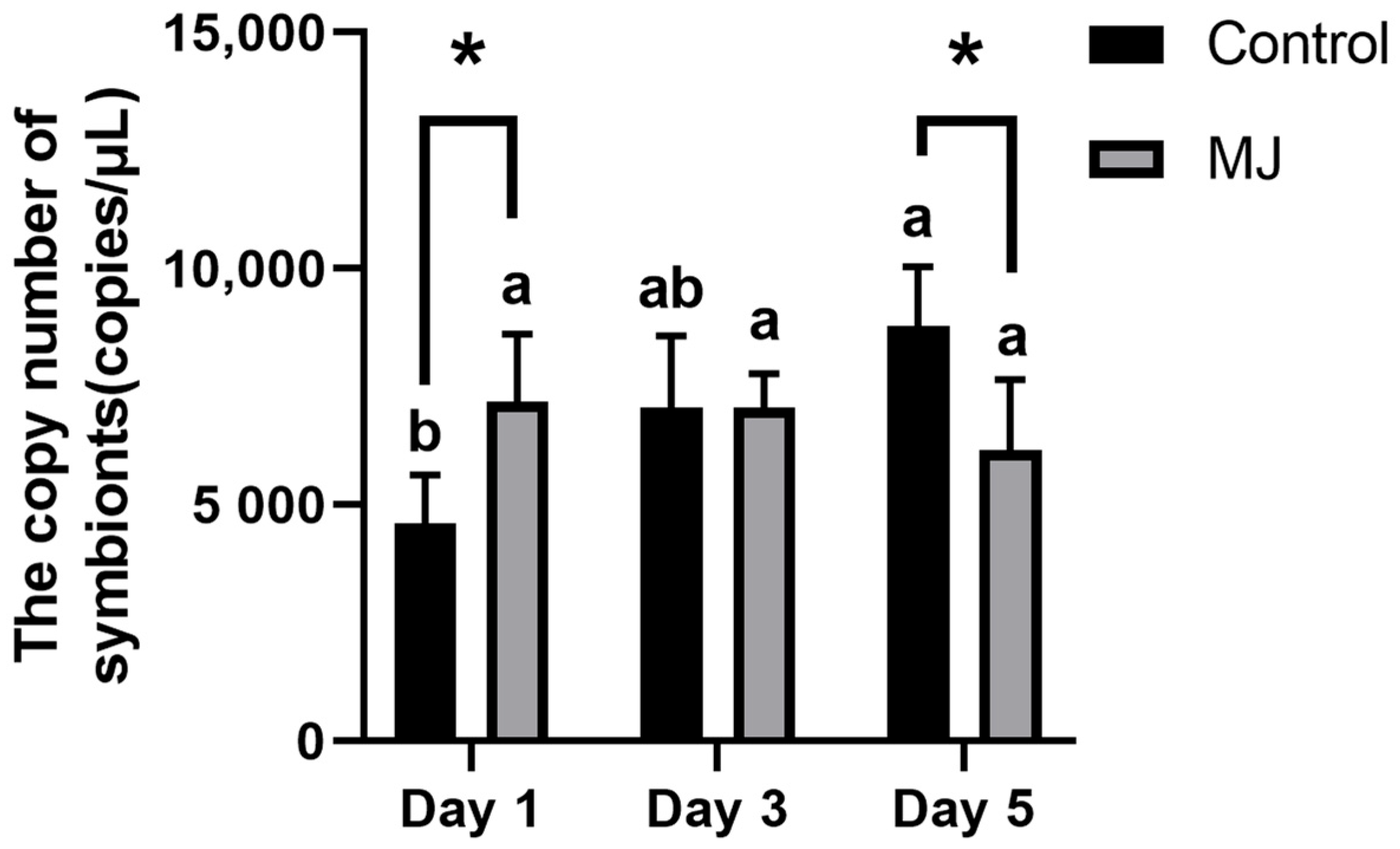
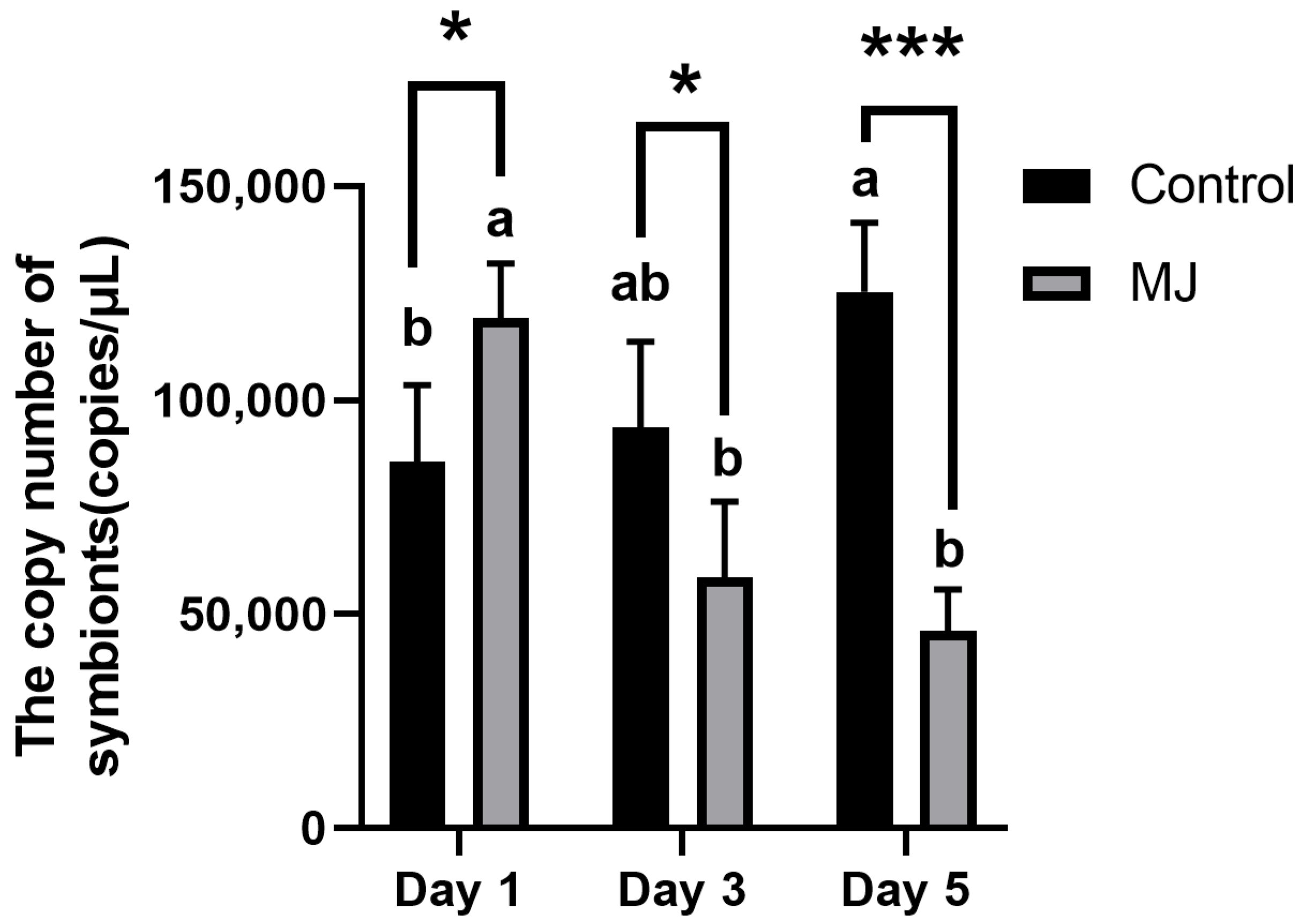
2.1.4. The Number of Symbionts
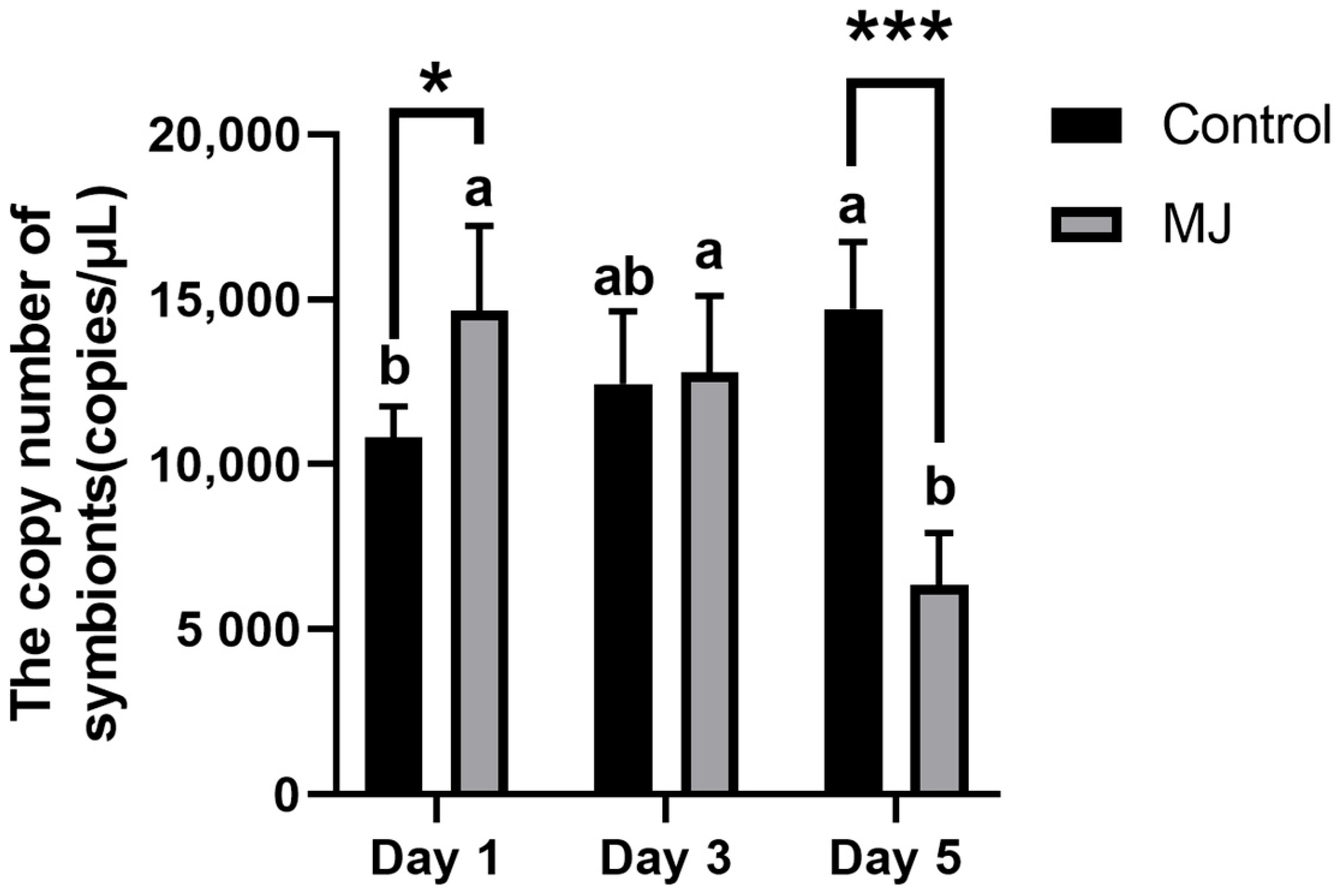
2.2. The Symbionts of Ascomycetes Symbionts Were Quantitatively Detected by Real-Time Quantitative Polymerase Chain Reaction
3. Discussion
4. Materials and Methods
4.1. Principal Reagent, Instruments, and Materials
4.1.1. Experimental Reagent
4.1.2. Reagent for Artificial Feeding Solution
4.1.3. Instruments and Materials
4.2. Test Insect
4.3. Preparation of Artificial Feeding Liquid
4.4. Preparation of Artificial Feeding Solution Containing Secondary Metabolites
4.5. The Brown Planthopper Was Fed with Artificial Feeding Solution
4.6. Bioassay of the Brown Planthopper under Secondary Metabolite Stress
4.6.1. Mortality
4.6.2. Weight Gain Rate
4.6.3. Fecundity
4.6.4. The Quantity of Symbionts
4.6.5. Data Statistics and Analysis
4.7. Effect of Methyl Jasmonate on Symbionts in Nilaparvata Lugens
4.7.1. Sample Collection of the Brown Planthopper under Methyl Jasmonate Stress
4.7.2. Acquisition of Intestinal Tract, Fat Body, and Hemolymph from the Brown Planthopper
4.7.3. Extraction of Symbiotic DNA
4.7.4. Quantification of the Symbionts by Real-Time Quantitative Polymerase Chain Reaction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Wu, W.; Chen, H.; Liu, Q.; Jia, D.; Mao, Q.; Chen, Q.; Wu, Z.; Wei, T. An insect cell line derived from the small brown planthopper supports replication of rice stripe virus, a tenuivirus. J. Gen. Virol. 2013, 94, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, Z.; Wang, J.; Liu, Y.; Wang, J. Cloning and characterization of two genes coding for the histone acetyltransferases, Elp3 and Mof, in brown planthopper (BPH), Nilaparvata lugens (Stål). Gene 2013, 513, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhu, L.; He, G. Towards understanding of molecular interactions between rice and the brown planthopper. Mol. Plant 2013, 6, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.G.; Baldwin, I.T. Silencing of a Germin-like Gene in Nicotiana attenuata Improves Performance of Native Herbivores. Plant Physiol. 2006, 140, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.B.; Wu, S.R.; Shen, L.B.; Zhang, S.Z.; Yang, B.P. A review on plant resistance to insect pests. Chin. J. Trop. Agric. 2014, 34, 61–68, 89. [Google Scholar]
- Harborne, J.B. Secondary plant products: Encyclopedia of plant physiology, New Series Volume 8. Phytochemistry 1980, 19, 2803–2804. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Maag, D.; Erb, M.; Kollner, T.G.; Gershenzon, J. Defensive weapons and defense signals in plants: Some metabolites serve both roles. BioEssays 2015, 37, 167–174. [Google Scholar] [CrossRef]
- Zhao, Y. Protective Effect of Kaempferol on Acute Lung Injury Induced by Lipopolysaccharide in Mice. Master’s Thesis, Northeast Agricultural University, Haerbin, China, 2013. [Google Scholar]
- Zhang, J.F.; Sun, J.Z.; Wu, Z.B.; Liu, J.L. Identification of cotton varieties resistant to cannme spider mite and exploration of resistance mechanism. Acta Phytophylacica Sin. 1993, 20, 155–161. [Google Scholar]
- Wang, Y.J.; Zou, C.S.; Wang, R.X.; Lin, L.N. Insecticidal activity analysis of three plant secondary metabolites on Lymantria dispar. J. Beijing For. Univ. 2017, 39, 75–81. [Google Scholar]
- Fan, N.N.; Wang, J.Y.; Wan, N.F.; Jiang, J.X. Effects of plant secondary metabolites on the growth, development and detoxification enzyme activity, of Spodoptera exigua. Chin. J. Appl. Entomol. 2022, 59, 165–171. [Google Scholar]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Sudjaroen, Y.; Haubner, R.; Wurtele, G.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Changbumrung, S.; Bartsch, H.; Owen, R.W. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem. Toxicol. 2005, 43, 1673–1682. [Google Scholar] [CrossRef]
- Deng, L.L.; Chen, S.H.; Lv, G.Y.; Yu, J.J.; Yan, M.Q. Compare Luteolin Content in Different Parts of Peanut. Zhejiang Chin. Med. Univ. 2013, 37, 591–594, 605. [Google Scholar]
- Ding, A.F.; Wang, H.F.; Yang, X.R.; Wang, Z.C.; Shen, H.J.; Zhang, Z.M. Study on the luteolin content of different cultivars and types of peanut hulls. China Oils Fats 2005, 30, 51–54. [Google Scholar]
- De Campos, M.P.; Cechinel Filho, V.; Da Silva, R.Z.; Yunes, R.A.; Zacchino, S.; Juarez, S.; Bella Cruz, R.C.; Bella Cruz, A. Evaluation of antifungal activity of Piper solmsianum C. DC. var. solmsianum (Piperaceae). Biol. Pharm. Bull. 2005, 28, 1527–1530. [Google Scholar] [CrossRef]
- Sartori, M.R.K.; Pretto, J.B.; Cruz, A.B.; Bresciani, L.F.V.; Yunes, R.A.; Sortino, M.; Zacchino, S.A.; Cechinel Filho, V. Antifungal activity of fractions and two pure compounds of flowers from Wedelia paludosa (Acmela brasiliensis) (Asteraceae). Pharmazie 2003, 58, 567–569. [Google Scholar]
- DeLucca, A.J.; Palmgren, M.S.; Daigle, D.J. Depression of aflatoxin production by flavonoid-type compounds from peanut shells. Phytopathology 1987, 77, 1560–1563. [Google Scholar] [CrossRef]
- Ashida, M.; Kinoshita, K.; Braey, P.T. Studies on phenoloxidase activation in mosquito Aedes aegypti L. Eur. J. Biochem. 1990, 188, 507–515. [Google Scholar] [CrossRef]
- Li, J.; Christensen, B.M. Involvement of L-tyrosine and phenol oxidase in the tanning of Aedes aegypti eggs. Insect Biochem. Mol. Biol. 1993, 23, 739–748. [Google Scholar] [CrossRef]
- Gao, X.X.; Luo, W.C.; Xie, G.Y.; Xue, C.B. Effects of apigenin, vanillic acid, and kojic acid on the phenoloxidase activity in Spodoptera exigua Hubner. J. Plant Resour. Environ. 2003, 12, 16–19. [Google Scholar]
- Shaver, T.N.; Lukefahr, M.J. Effect of flavonoid pigments and gossypol on growth and development of the bollworm, tobacco budworm, and pink bollworm. J. Econ. Entomol. 1969, 62, 643–646. [Google Scholar] [CrossRef]
- Chan, B.G.; Waiss, A.C.; Binder, R.G.; Elliger, C.A. Inhibition of Lepidopterous larval growth by cotton constituents. Entomol Exp. Appl. 1978, 24, 294–300. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Peterson, S.S. Effects of plant phenols on the performance of southern armyworm larvae. Oecologia 1988, 75, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.G.; Jing, H.M.; Zheng, H.L.; Zhao, Z.Q. Progress in plant resistance induced by salicylic acid. Life Sci. Res. 2001, 5, 185–189. [Google Scholar]
- Nwanade, F.C. Acaricidal Activity of Plant Extracts and Essential Oil and Their Isolated Compounds against Haemaphysalis longicornis. Ph.D. Thesis, Hebei Normal University, Shijiazhuang, China, 2022. [Google Scholar]
- Luo, J.Y.; Cui, J.J.; Wang, C.Y.; Xin, H.J.; Zhang, S.; Lü, L.M. Relationship between the contents of protein, soluble sugar and anthocyanidins in cotton leaf and their resistance to Apolygus lucorum Meyer-Dür. J. Northwest A F Univ. 2011, 39, 75–89. [Google Scholar]
- Kim, S.H.; Jun, C.D.; Suk, K.; Choi, B.J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.Y.; Kim, D.K.; Shin, T.Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef]
- Giftson, J.S.; Jayanthi, S.; Nalini, N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine-induced rat colon carcinogenesis. Investig. New Drugs 2009, 28, 251–259. [Google Scholar] [CrossRef]
- Coruh, S.; Ercisli, S. Interactions between galling insects and plant total phenolic contents in Rosa canina L. genotypes. Sci. Res. Essays 2010, 5, 1935–1937. [Google Scholar]
- Huang, M.Y.; Li, X.F. Effects of plant secondary metabolite on detoxification enzyme activity of Spodoptera litura. Genom. Appl. Biol. 2018, 37, 3495–3502. [Google Scholar]
- Chen, J.L.; Ni, H.X.; Sun, J.R.; Cheng, D.F. Effects of major secondary chemicals of wheat plants on enzyme activity in Sitobion avenae. Acta Entomol. Sin. 2003, 46, 144–149. [Google Scholar]
- Xie, X. Extraction and Analysis of Phenolic Acids in Poplar Branches and Their Effect on Saperda populnea L. Master’s Thesis, Northeast Forestry University, Haerbin, China, 2010. [Google Scholar]
- Veronési, C.; Pouénat, M.L.; Rickauer, M.; Esquerré, T.M.T. Regulation of tobacco lipoxygenase by methyl jasmonate and fatty acids. C. R. Acad. Sci. Paris Sci. Vie/Life Sci. 1999, 332, 491–497. [Google Scholar] [CrossRef]
- Roda, A.L.; Oldham, N.J.; Svatos, A.; Baldwin, I.T. Allometric analysis of the induced flavonols on the surface of wild tobacco (Nicotiana attenuata). Phytochemistry 2003, 62, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Compagnon, V.; Crouch, N.P.; Prerre, B.S.; Luca, V.D. Jasmonate-induced epoxidation of tabersonine by a cytochrome P-450 in hairy root cultures of Catharanthus roseus. Phytochemistry 2003, 64, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D. Study on the Phenoloxidase of the Diamondback Moth Plutella Xylostella (L.) (Lepidoptera Plutellidae) and Its Inhibitors. Master’s Thesis, Shandong Agricultural University, Taian, China, 2005. [Google Scholar]
- Noda, H.; Omura, T. Purification of yeast-like symbiotes of planthoppers. J. Invertebr. Pathol. 1992, 59, 104–105. [Google Scholar] [CrossRef]
- Tang, M.; Lv, L.; Jing, S.; Zhu, L.; He, G. Bacterial symbionts of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Appl. Environ. Microb. 2010, 76, 1740–1745. [Google Scholar] [CrossRef]
- Fan, H.W.; Noda, H.; Xie, H.Q.; Suetsugu, Y.; Zhu, Q.H.; Zhang, C.X. Genomic analysis of an ascomycete fungus from the rice planthopper reveals how it adapts to an endosymbiotic lifestyle. Genome Biol. Evol. 2015, 7, 2623–2634. [Google Scholar] [CrossRef]
- Crotti, E.; Balloi, A.; Hamdi, C.; Sansonno, L.; Marzorati, M.; Gonella, E.; Favia, G.; Cherif, A.; Bandi, C.; Alma, A.; et al. Microbial symbionts: A resource for the management of insect-related problems. Microb. Biotechnol. 2012, 5, 307–317. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Masson, F.; Lemaitre, B. Growing ungrowable bacteria: Overview and perspectives on insect symbiont culturability. Microbiol. Mol. Biol. Rev. 2020, 84, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xu, X.R.; Hong, K.; Yi, Y. Distribution of bacterial symbionts in brown planthopper. Guizhou Agric. Sci. 2014, 42, 89–94. [Google Scholar]
- Nasu, S. Studies on some leafhoppers and planthoppers which transmit virus disease of rice plant in Japan. Bull. Kyushu Agric. Exp. Stn. 1963, 8, 153. [Google Scholar]
- He, L.M.; Shentu, X.P.; Li, D.T.; Sun, F.; Yu, X.P. Effects of fungicide/insecticide mixtures on population growth of brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Chin. J. Biol. Control 2018, 34, 280–286. [Google Scholar]
- Wang, T.Z.; Wang, Z.L.; Zhu, H.F.; Wang, Z.Y.; Yu, X.P. Analysis of the gut microbial diversity of the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) by high-throughput sequencing. Acta Entomol. Sin. 2019, 62, 323–333. [Google Scholar]
- Noda, H.; Nakashima, N.; Koizumi, M. Phylogenetic position of yeast-like symbiotes or rice planthopper based on partial 18S rDNA sequences. Insect Biochem. Mol. Biol. 1995, 25, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Zheng, X.S.; Yang, Y.J.; Wang, X.; Fu, Q.; Ye, G.Y.; Lü, Z.X. PCR-DGGE analysis of the bacterial community in different populations of rice brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Chin. J. Rice Sci. 2014, 28, 217–222. [Google Scholar]
- Ju, J.F.; Bing, X.L.; Zhao, D.S.; Guo, Y.; Xi, Z.Y.; Hoffmann, A.A.; Zhang, K.J.; Huang, H.J.; Gong, J.T.; Zhang, X.; et al. Wolbachia supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2020, 14, 676–687. [Google Scholar] [CrossRef]
- Noda, H.; Saito, T. Effects of high temperature on the development of Laodelphax striatellus (Homoptera: Delphacidae) and on its intracellular yeastlike symbiotes. Appl. Entomol. Zool. 1979, 14, 64–75. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, Z.T.; Hu, C.; Lai, F.X. The effects of high temperature on both yeast-like symbionts and amino acid requirements of Nilaparvata lugens. Acta Entomol. Sin. 2001, 44, 534–540. [Google Scholar]
- Xue, J.; Zhou, X.; Zhang, C.X.; Yu, L.L.; Fan, H.W.; Wang, Z.; Xu, H.J.; Xi, Y.; Zhu, Z.R.; Zhou, W.W.; et al. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 2014, 15, 521. [Google Scholar] [CrossRef]
- Yu, H.X. Study on the Mechanism Responsible for the Virulence Variation of the Rice Brown Planthopper, Nilaparvata lugens (Stål). Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2013. [Google Scholar]
- Zhu, H.H. The Mechanism and Effect of Arsenophonus on the Virulence of Metarhizium flavoviride to Nilaparvata lugens. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2017. [Google Scholar]
- Pang, R.; Chen, M.; Yue, L.; Xing, K.; Li, T.C.; Kang, K.; Liang, Z.K.; Yuan, L.Y.; Zhang, W.Q. A distinct strain of Arsenophonus symbiont decreases insecticide resistance in its insect host. PLoS Genet. 2018, 14, e1007725. [Google Scholar] [CrossRef]
- Liu, M.Y.; Hong, G.J.; Li, H.J.; Bing, X.L.; Chen, Y.M.; Jing, X.F.; Gershenzon, J.; Lou, Y.G.; Baldwin, I.T.; Li, R. Sakuranetin protects rice from brown planthopper attack by depleting its beneficial endosymbionts. Proc. Natl. Acad. Sci. USA 2023, 120, e2305007120. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. The Main Type of Application and Prospects of Botanical Pesticides. Sci. Technol. Qinghai Agric. For. 2019, 57–60, 68. [Google Scholar]
- Wu, W.J.; Liu, H.X.; Ji, Z.Q.; Hu, Z.N.; Qi, Z.J. Research and Development on the Botanical Insecticide of 0.2% Celangulins Emulsifiable Concentrate. Agrochemicals 2001, 40, 17–19. [Google Scholar]
- Liu, H.; Wu, W.; Ji, Z. Studies on the Selective Toxicity and Selective Mechanism of Poisonous Ingredient Celangulin V of Celastrus angulatus Against the Insect Pests. Acta Agric. Boreali-Occident. Sin. 1998, 7, 41–44. [Google Scholar]
- Mordue, A.J.; Blackwell, A. Azadirachtin: An update. J. Insect Physiol. 1993, 39, 903–924. [Google Scholar] [CrossRef]
- Zhang, X. The Toxic Symptoms Caused by Toosendanin to the Larvae of Imported Cabbage Worm (Pieris Rapae L.). Acta Univ. Agric. Boreali-Occident. 1993, 21, 27–30. [Google Scholar]
- Wan, W.; Chen, X.H.; Dai, W.H.; Zhao, Y.L.; Duan, L.Q. Effects of Two Plant Essential Oils and Two Monoterpenoids on Contact Toxicity and Enzyme Activities of Paratrioza sinica. J. Northeast For. Univ. 2020, 48, 110–114, 117. [Google Scholar]
- Qian, J.; Cen, X.; Du, S.; Liu, S.B.; Feng, Q.; Lin, Z.W. Oral Toxicity and Antifeedant Activity of Mikania micrantha Extracts on Brontispa longissima (Coleoptera: Chrysomelidae). Chin. Agric. Sci. Bull. 2015, 31, 258–261. [Google Scholar]
- Qian, J.; Tang, W.F.; Lü, Z.J.; Gou, Z.H.; Wu, H.X.; Li, D.X. Biological activity of the extracts from the invasive Mikania micrantha on the aphid Cerataphis lataniae. J. Biosaf. 2015, 24, 238–240. [Google Scholar]
- Rosado, H.C.; Anholeti, M.C.; Santos, M.G.; dos Santos-Mallet, J.R.; Figueiredo, M.R.; Mello, C.B.; Gonzalez, M.S.; Paiva, S.R.; Feder, D. Effects of semi-purified fractions from stems of Clusia hilariana on the development of Dysdercus peruvianus. Rev. Bras. Farmacogn. 2019, 29, 801–806. [Google Scholar] [CrossRef]
- Ma, H.; Xin, C.Y.; Xu, Y.Y.; Wang, D.; Lin, X.Q.; Chen, Z.Z. Effect of salt stress on secondary metabolites of cotton and biological characteristics and detoxification enzyme activity of cotton spider mites. Crop Prot. 2021, 141, 105498. [Google Scholar] [CrossRef]
- Chabaane, Y.; Arce, C.M.; Glauser, G.; Benrey, B. Altered capsaicin levels in domesticated chili pepper varieties affect the interaction between a generalist herbivore and its ectoparasitoid. J. Pest Sci. 2022, 95, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shi, D.X.; Zhang, S.; Du, K.J. Effects of four plant secondary metabolites on the growth and development of Tenebrio molitor and the synergistic effect of Vitamin B1. For. Ecol. Sci. 2021, 36, 49–55. [Google Scholar]
- Chen, H.; Liu, J.; Cui, K.; Lu, Q.; Wang, C.; Wu, H.X.; Yang, Z.X.; Ding, W.F.; Shao, S.X.; Wang, H.Y.; et al. Molecular mechanisms of tannin accumulation in Rhus galls and genes involved in plant-insect interactions. Sci. Rep. 2018, 8, 9841. [Google Scholar] [CrossRef] [PubMed]
- Feeny, P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, Y.T.; Duan, L.Q.; Li, H.P.; Feng, S.J. Effects of four plant phenolics on the growth and development and fecundity of the gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae). Acta Entomol. Sin. 2014, 57, 831–836. [Google Scholar]
- Miksanek, J.R.; Tuda, M. Endosymbiont-mediated resistance to entomotoxic nanoparticles and sex-specific responses in a seed beetle. J Pest. Sci. 2023, 96, 1257–1270. [Google Scholar] [CrossRef]
- Ling, B.; Dong, H.X.; Zhang, M.X.; Xu, D.; Wang, J.S. Potential resistance of tricin in rice against brown planthopper Nilaparvata lugens (Stål). Acta Ecol. Sin. 2007, 27, 1300–1306. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Wu, D.D.; Shi, Z.B.; Yang, J.; Zhang, G.C.; Zhang, J. Influence of dietary aconitine and nicotine on the intestine microbiota of two lepidopteran herbivores. Arch. Insect Biochem. Physiol. 2020, 104, e21676. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, Z.; Hu, C.; Lai, F.X.; Sun, Z.X. A chemically defined diet enables continuous rearing of the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Appl. Entomol. Zool. 2001, 36, 111–116. [Google Scholar] [CrossRef]


| Symbiont | Primers | Sequences (5′→3′) | Amplified Product Length |
|---|---|---|---|
| Ascomycetes symbionts | AsF | GTCGTAGTCTTAACCATAA | 145 |
| AsR | CTTCCGTCAATTTCTTTAAG |
| Component Name | Volume (μL) |
|---|---|
| 2 ×SYBR Green Pro Taq HS Premix | 5.0 |
| ddH2O | 3.6 |
| Upstream primer | 0.2 |
| Downstream primer | 0.2 |
| Template DNA | 1.0 |
| Total | 10.0 |
| Reaction Temperature (°C) | Reaction Time | Cycle Number |
|---|---|---|
| 95 | 30 s | 1 |
| 95 | 5 s | 40 |
| 56 | 30 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Lai, C.; Zhang, J.; Sun, F.; Li, D.; Hao, P.; Shentu, X.; Pang, K.; Yu, X. Effects of Secondary Metabolites of Rice on Brown Planthopper and Its Symbionts. Int. J. Mol. Sci. 2024, 25, 386. https://doi.org/10.3390/ijms25010386
Deng Z, Lai C, Zhang J, Sun F, Li D, Hao P, Shentu X, Pang K, Yu X. Effects of Secondary Metabolites of Rice on Brown Planthopper and Its Symbionts. International Journal of Molecular Sciences. 2024; 25(1):386. https://doi.org/10.3390/ijms25010386
Chicago/Turabian StyleDeng, Ziyuan, Chengling Lai, Jun Zhang, Fan Sun, Danting Li, Peiying Hao, Xuping Shentu, Kun Pang, and Xiaoping Yu. 2024. "Effects of Secondary Metabolites of Rice on Brown Planthopper and Its Symbionts" International Journal of Molecular Sciences 25, no. 1: 386. https://doi.org/10.3390/ijms25010386





