Metabolic Pathway Engineering Improves Dendrobine Production in Dendrobium catenatum
Abstract
:1. Introduction
2. Results
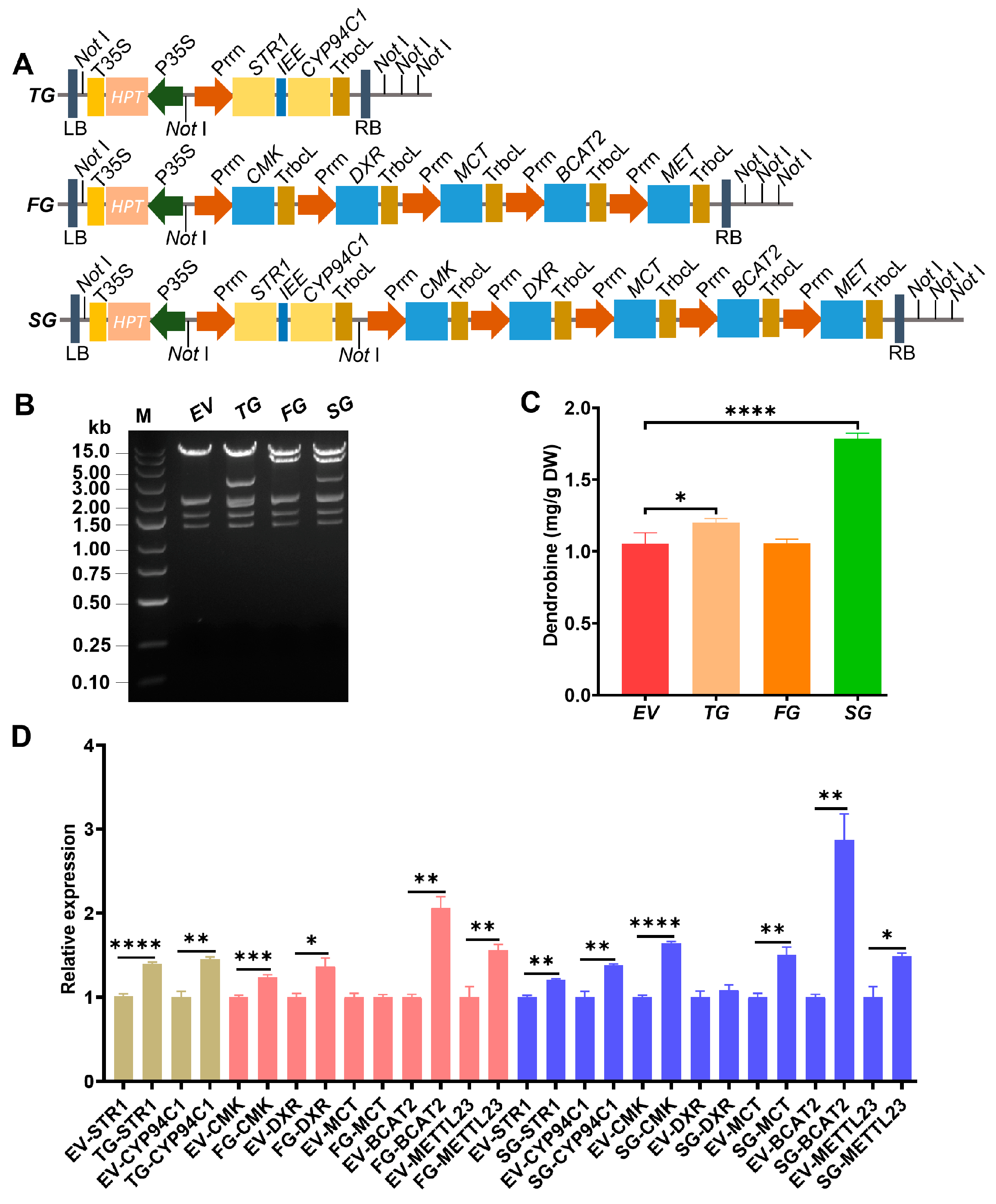
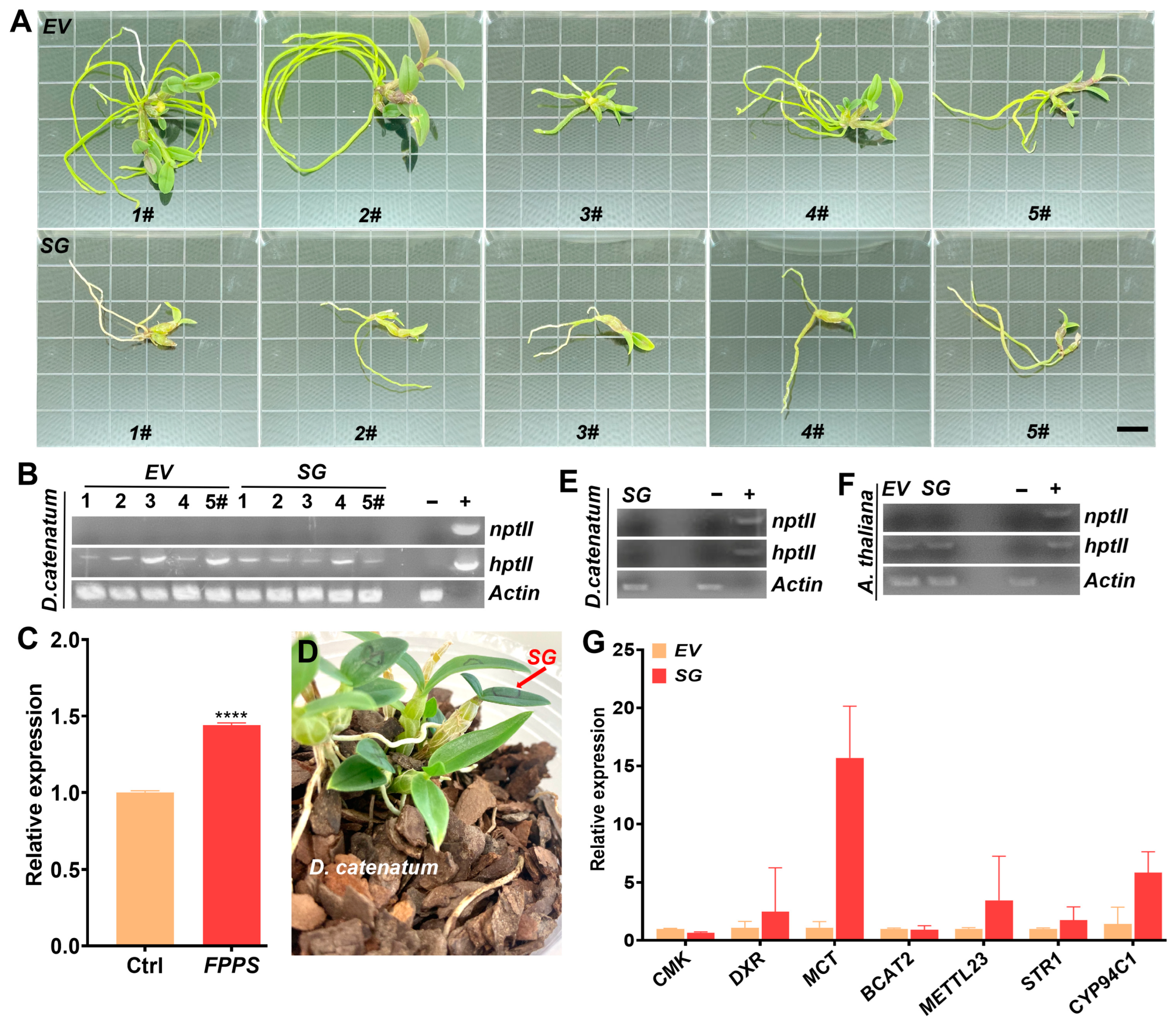
2.1. Reconstitution of Multigene for Improved Dendrobine Synthesis
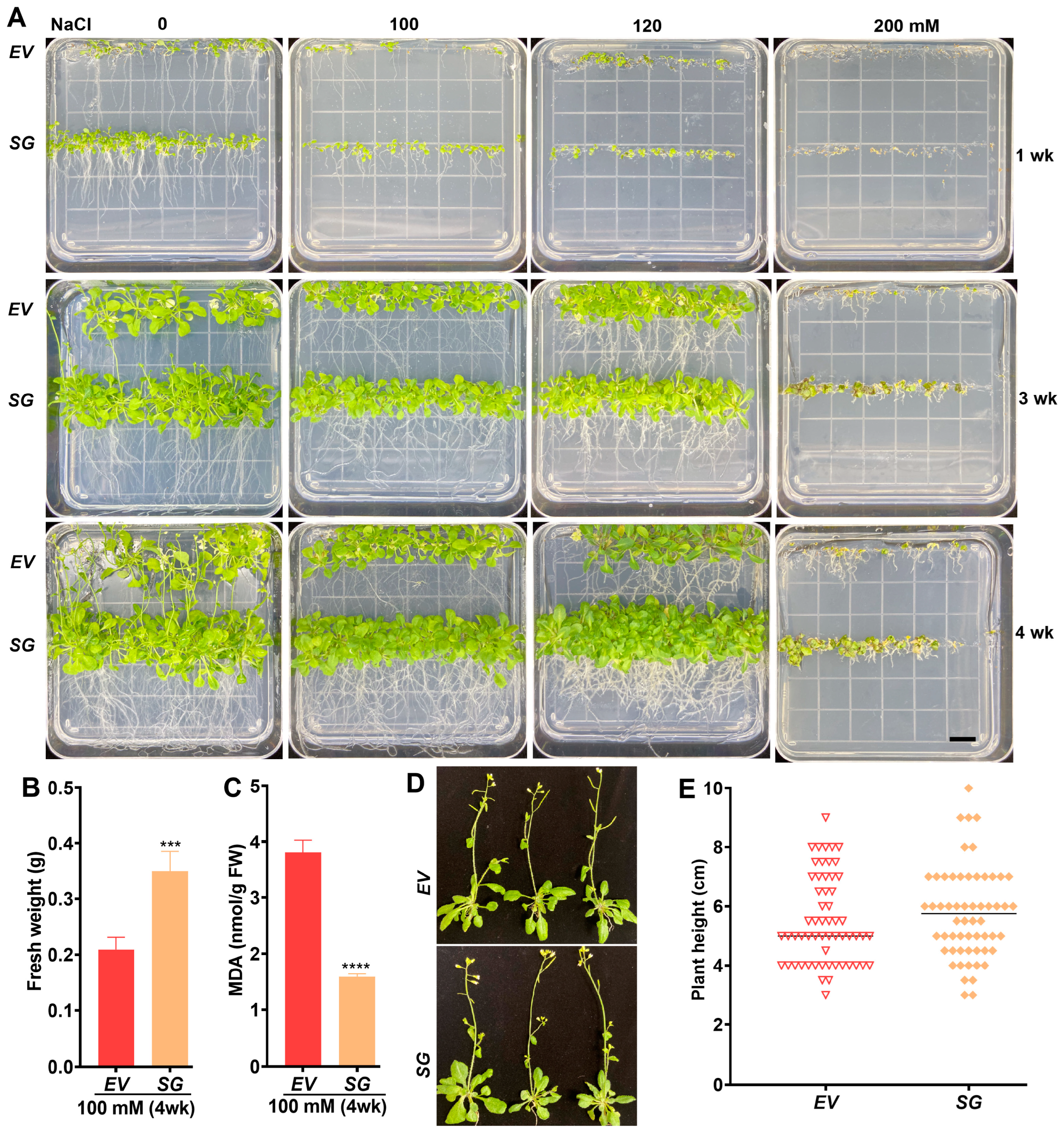
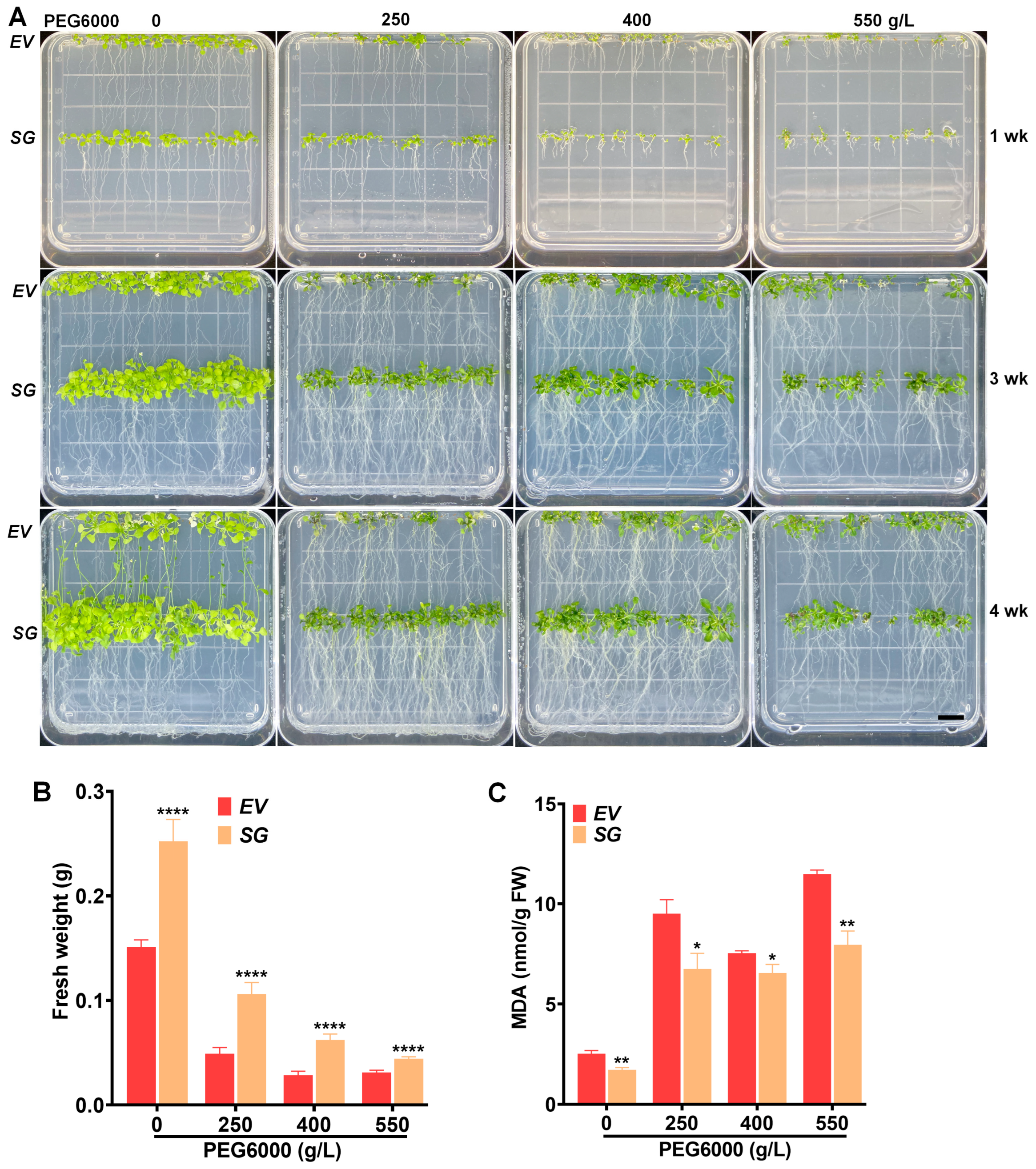
2.2. Overexpression of SG-Multigene Confers Salt and Drought Tolerance to Transgenic Arabidopsis
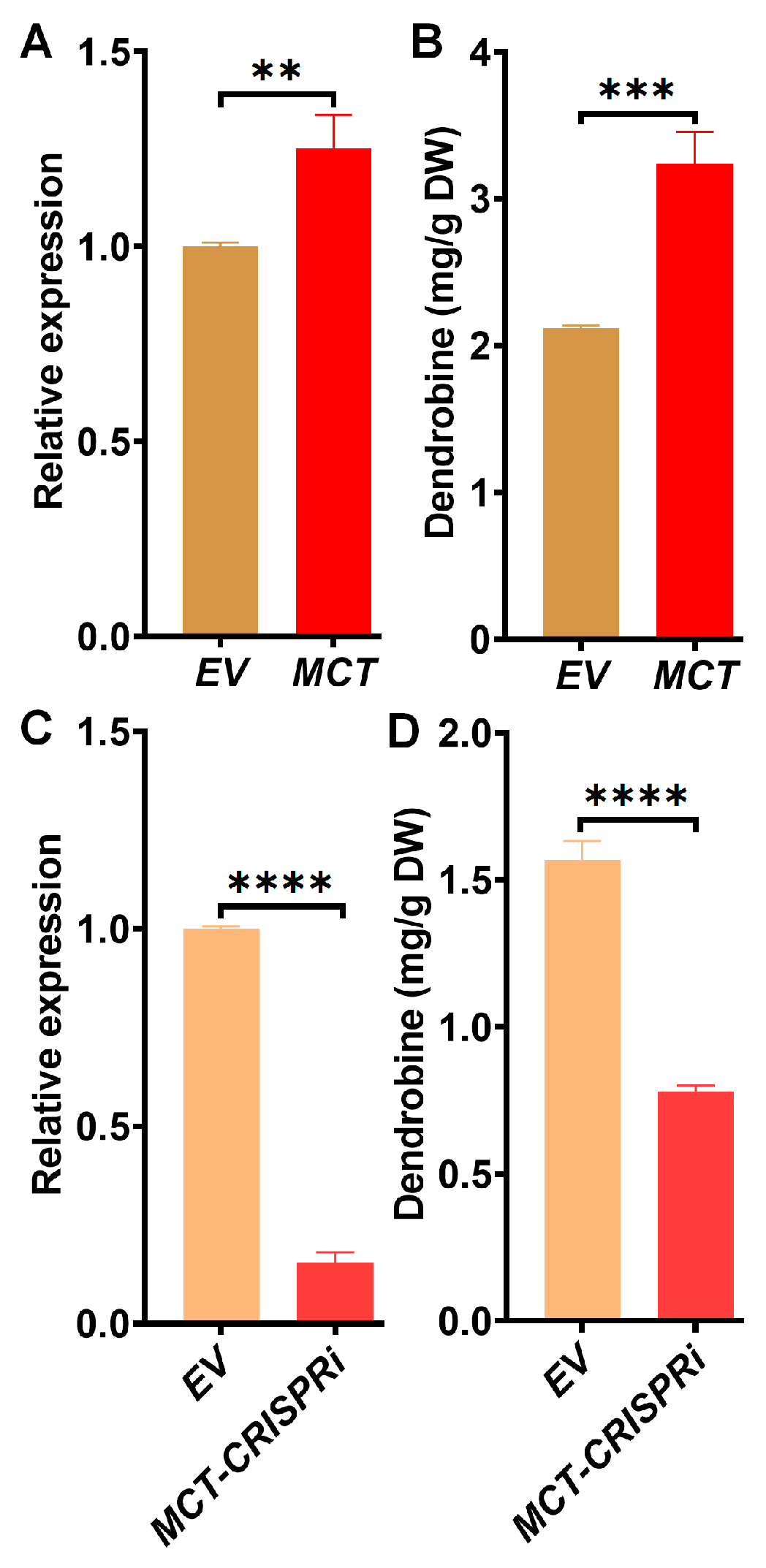
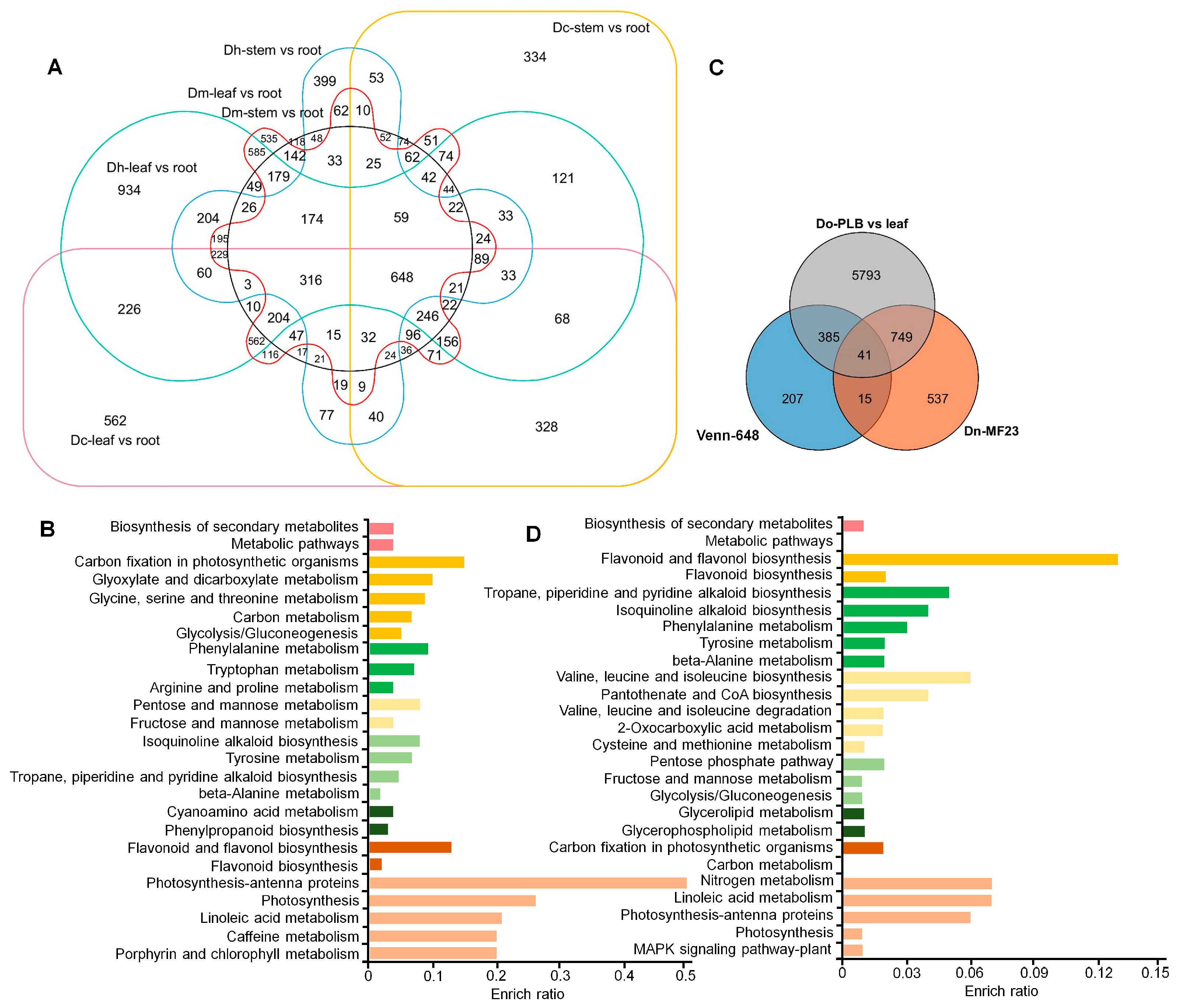
2.3. Identification of Downstream and Regulatory Genes Related to Dendrobine Synthesis
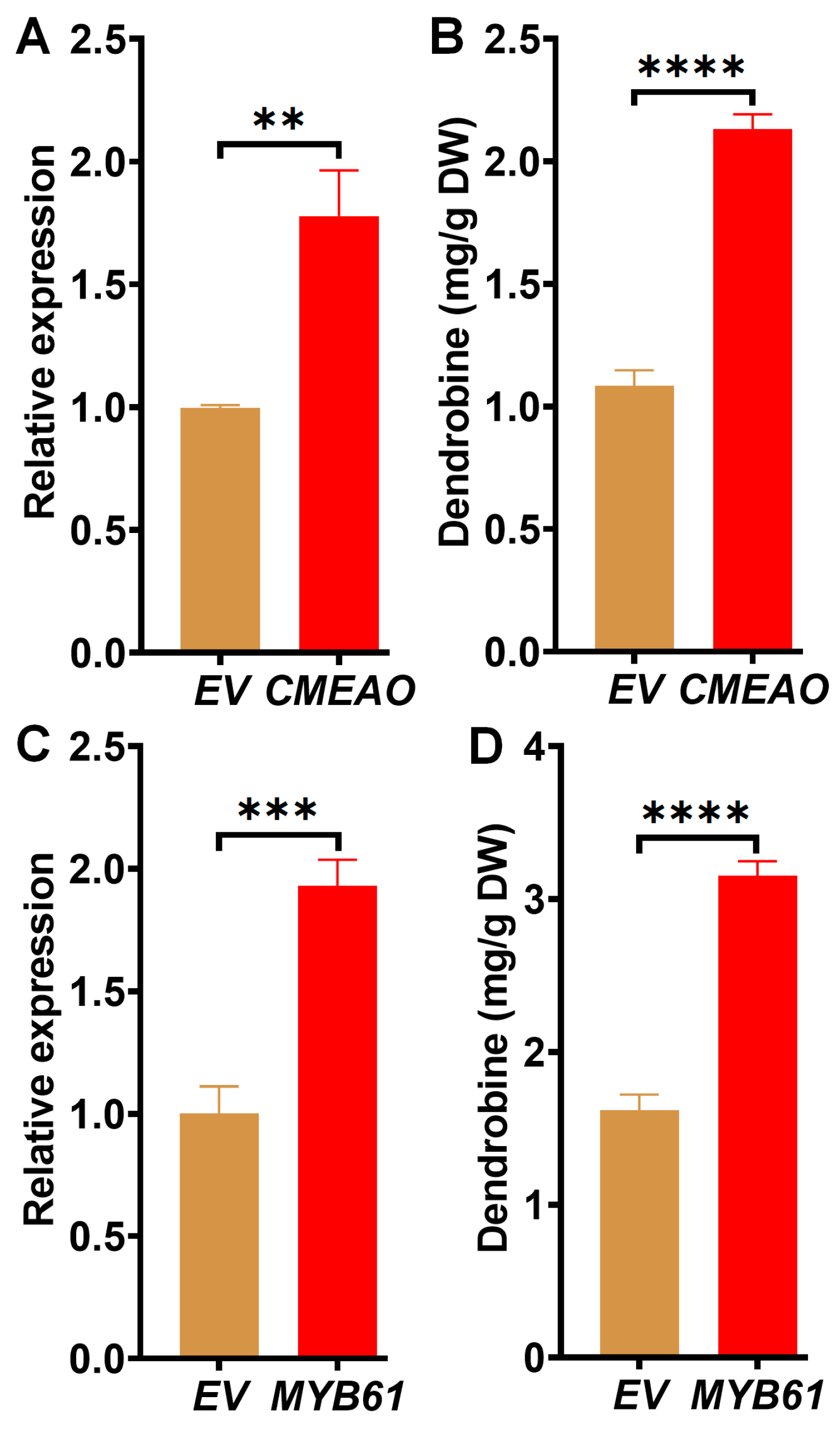
2.4. Characterization of Novel Genes Associated with Dendrobine Accumulation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Reagents
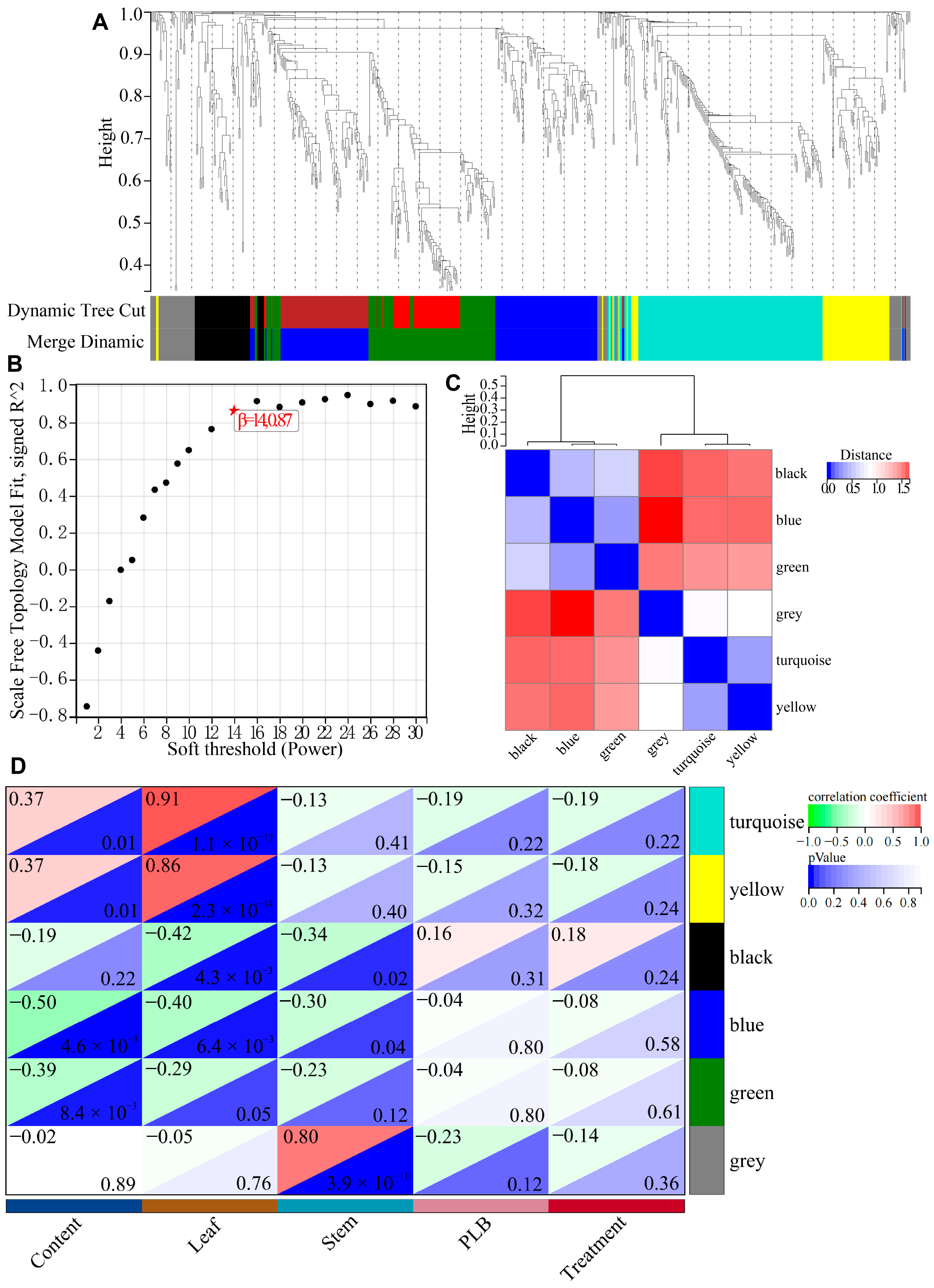
4.2. WGCNA and Modular Characterization
4.3. Vector Construction
4.4. Genetic Transformation
4.5. Metabolic Profiling
4.6. qRT-PCR Validation of Gene Expression
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Shen, Q.; Lv, P.; Sun, C. Comparative metabolomic analyses of Dendrobium officinale Kimura et Migo responding to UV-B radiation reveal variations in the metabolisms associated with its bioactive ingredients. PeerJ 2020, 8, e9107. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhu, F.; Fan, Y.; Li, C.; Zhang, B.; Zhu, S.; Hou, Z.; Wang, M.; Yang, J.; Xue, Q.; et al. The chromosome-level reference genome assembly for dendrobium officinale and its utility of functional genomics research and molecular breeding study. Acta Pharm. Sin. B. 2021, 11, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Y.; Adejobi, O.I.; Wang, Y.; Liu, A. Research advances in multi-omics on the traditional Chinese herb Dendrobium officinale. Front. Plant Sci. 2021, 12, 808228. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Zeng, D.; Yu, Z.; Teixeira da Silva, J.A.; Duan, J.; He, C.; Zhang, J. Transcriptomic and metabolomic analyses reveal the main metabolites in Dendrobium officinale leaves during the harvesting period. Plant Physiol. Biochem. 2022, 190, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.K.; Stanly, C.; Muniandy, A.; Ramanathan, S.; Murugaiyah, V.; Chew, B.L.; Subramaniam, S. Protocorm-like bodies (PLBs) of Dendrobium Sabin Blue: A novel source for in vitro production of dendrobine and anthocyanin. In Vitro Cell Dev. Biol. 2021, 57, 874–882. [Google Scholar] [CrossRef]
- Cao, H.; Ji, Y.L.; Li, S.H.; Lu, L.; Tian, M.; Yang, W.; Li, H. Extensive metabolic profiles of leaves stems from the medicinal plant Dendrobium officinale Kimura et Migo. Metabolites 2019, 9, 215. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Lyu, P.; Chen, L.; Shen, C.; Sun, C. Comparative transcriptomic analysis reveal the regulation mechanism underlying MeJA-induced accumulation of alkaloids in Dendrobium officinale. J. Plant Res. 2019, 132, 419–429. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, Z.P.; Zhang, X.F.; Si, J.P. Effects of various processing methods on the metabolic profile and antioxidant activity of Dendrobium catenatum Lindley leaves. Metabolites 2021, 11, 351. [Google Scholar] [CrossRef]
- Duan, H.; Er-bu, A.; Dongzhi, Z.; Xie, H.; Ye, B.; He, J. Alkaloids from Dendrobium and their biosynthetic pathway, biological activity and total synthesis. Phytomedicine 2022, 102, 154132. [Google Scholar] [CrossRef]
- Feng, Y.; Jia, B.; Feng, Q.; Zhang, Y.; Chen, Y.; Meng, J. Dendrobine attenuates gestational diabetes mellitus in mice by inhibiting Th17 cells. Basic. Clin. Pharmacol. Toxicol. 2021, 128, 379–385. [Google Scholar] [CrossRef]
- Gong, D.Y.; Chen, X.Y.; Guo, S.X.; Wang, B.C.; Li, B. Recent advances and new insights in biosynthesis of dendrobine and sesquiterpenes. Appl. Microbiol. Biotechnol. 2021, 105, 6597–6606. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, L.; Qian, X.; Jin, H.; Shu, F.; Sarsaiya, S.; Jin, L.; Chen, J. Transcriptome analysis of dendrobine biosynthesis in Trichoderma longibrachiatum MD33. Front. Microbiol. 2022, 13, 890733. [Google Scholar] [CrossRef]
- Lu, A.; Cao, L.; Tan, D.; Qin, L.; Lu, Y.; Zhao, Y.; Qian, Y.; Bai, C.; Yang, J.; Ling, H.; et al. UPLC-Q/TOF-MS coupled with multivariate analysis for comparative analysis of metabolomic in Dendrobium nobile from different growth altitudes. Arab. J. Chem. 2022, 15, 104208. [Google Scholar] [CrossRef]
- Song, C.; Ma, J.; Li, G.; Pan, H.; Zhu, Y.; Jin, Q.; Cai, Y.; Han, B. Natural composition and biosynthetic pathways of alkaloids in medicinal Dendrobium species. Front. Plant Sci. 2022, 13, 850949. [Google Scholar] [CrossRef]
- Gong, D.; Wu, B.; Qin, H.; Fu, D.; Guo, S.; Wang, B.; Li, B. Functional characterization of a farnesyl diphosphate synthase from Dendrobium nobile Lindl. AMB Express 2022, 12, 129. [Google Scholar] [CrossRef]
- Jiao, C.; Song, C.; Zheng, S.; Zhu, Y.; Jin, Q.; Cai, Y.; Lin, Y. Metabolic profiling of Dendrobium officinale in response to precursors and methyl jasmonate. Int. J. Mol. Sci. 2018, 19, 728. [Google Scholar] [CrossRef]
- Xin, M.; Zhang, E.; Li, N.; Huang, Z.; Su, Y.; Huang, M.; He, Q. Effects of different drying methods on polysaccharides and dendrobine from Dendrobium candidum. J. South. Agric. 2013, 44, 1347–1350. [Google Scholar]
- Zhang, G.Q.; Xu, Q.; Bian, C.; Tsai, W.C.; Yeh, C.M.; Liu, K.W.; Yoshida, K.; Zhang, L.S.; Chang, S.B.; Chen, F.; et al. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, M.; Cui, H.; Li, J.; Wang, M. Transcriptomic landscape of medicinal Dendrobium reveals genes associated with the biosynthesis of bioactive components. Front. Plant Sci. 2020, 11, 391. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, M.; Zhang, X.; Zhang, Z.; Li, S.; Li, J.; Cui, H.; Li, W.; Liu, Y.; Wang, Y.; et al. Phytohormone-triggered transcriptional changes revealed β-glucosidase as a key player for polysaccharide metabolism in Dendrobium officinale. Prog. Biochem. Biophys. 2022, 49, 1751–1762. [Google Scholar]
- Wang, Z.; Zhao, M.; Zhang, X.; Deng, X.; Li, J.; Wang, M. Genome-wide identification and characterization of active ingredients related β-glucosidases in Dendrobium catenatum. BMC Genom. 2022, 23, 612. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, Y.; Zhao, R.; Yan, S.; Zhang, X.; Zhao, T.; Chun, Z. Genome-wide researches and applications on Dendrobium. Planta 2018, 248, 769–784. [Google Scholar] [CrossRef]
- Li, Q.; Ding, G.; Li, B.; Guo, S.X. Transcriptome analysis of genes involved in dendrobine biosynthesis in Dendrobium nobile Lindl. infected with mycorrhizal fungus MF23 (Mycena sp.). Sci. Rep. 2017, 7, 316. [Google Scholar] [CrossRef]
- Fuentes, P.; Zhou, F.; Erban, A.; Karcher, D.; Kopka, J.; Bock, R. A new synthetic biology approach allows transfer of an entire metabolic pathway from a medicinal plant to a biomass crop. eLife 2016, 5, e13664. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Kang, Y.; Wang, P.; Li, W.; Yu, W.; Wang, J.; Wang, J.; Song, X.; Jiang, X. The Dendrobium catenatum DcCIPK24 increases drought and salt tolerance of transgenic Arabidopsis. Ind. Crops Prod. 2022, 187, 115375. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Z.; Zeng, D.; Si, C.; Zhao, C.; Wang, H.; Li, C.; He, C.; Duan, J. Transcriptome and metabolome reveal salt-stress responses of leaf tissues from Dendrobium officinale. Biomolecules 2021, 11, 736. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, M.; Jia, Z.; Song Xe Liang, Y.; Zhang, J. Analysis of Dendrobium huoshanense transcriptome unveils putative genes associated with active ingredients synthesis. BMC Genom. 2018, 19, 978. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, W.; Liu, Y.; Meng, X.; Su, X.; Cao, M.; Wu, L.; Yu, N.; Xing, S.; Peng, D. Putative genes in alkaloid biosynthesis identified in Dendrobium officinale by correlating the contents of major bioactive metabolites with genes expression between protocorm-like bodies and leaves. BMC Genom. 2021, 22, 579. [Google Scholar] [CrossRef]
- Zhan, X.; Qi, J.; Zhou, B.; Mao, B. Metabolomic and transcriptomic analyses reveal the regulation of pigmentation in the purple variety of Dendrobium officinale. Sci. Rep. 2020, 10, 17700. [Google Scholar] [CrossRef]
- Matías-Hernández, L.; Jiang, W.; Yang, K.; Tang, K.; Brodelius, P.E.; Pelaz, S. AaMYB1 and its orthologue AtMYB61 affect terpene metabolism and trichome development in Artemisia annua and Arabidopsis thaliana. Plant J. 2017, 90, 520–534. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Tuo, D.; Shen, W.; Deng, H.; Zhou, P.; Gao, X. A Nimble Cloning-compatible vector system for high-throughput gene functional analysis in plants. Plant Commun. 2023, 4, 100471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, J.; Tan, J.; Yang, Y.; Li, S.; Gou, Y.; Luo, Y.; Li, T.; Xiao, W.; Xue, Y.; et al. Efficient assembly of long DNA fragments and multiple genes with improved nickase-based cloning and Cre/loxP recombination. Plant Biotechnol. J. 2022, 20, 1983–1995. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.; Niu, Q.; Chua, N. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Zeng, D.; Si, C.; Zhang, M.; Duan, J.; He, C. ERF5 enhances protocorm-like body regeneration via enhancement of STM expression in Dendrobium orchid. J. Integr. Plant Biol. 2023, 65, 2071–2085. [Google Scholar] [CrossRef]
- Lau, C.; Siskos, A.P.; Maitre, L.; Robinson, O.; Athersuch, T.J.; Want, E.J.; Urquiza, J.; Casas, M.; Vafeiadi, M.; Roumeliotaki, T. Determinants of the urinary and serum metabolome in children from six European populations. BMC Med. 2018, 16, 202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zhao, Y.; Yang, Z.; Ming, F.; Li, J.; Kong, D.; Wang, Y.; Chen, P.; Wang, M.; Wang, Z. Metabolic Pathway Engineering Improves Dendrobine Production in Dendrobium catenatum. Int. J. Mol. Sci. 2024, 25, 397. https://doi.org/10.3390/ijms25010397
Zhao M, Zhao Y, Yang Z, Ming F, Li J, Kong D, Wang Y, Chen P, Wang M, Wang Z. Metabolic Pathway Engineering Improves Dendrobine Production in Dendrobium catenatum. International Journal of Molecular Sciences. 2024; 25(1):397. https://doi.org/10.3390/ijms25010397
Chicago/Turabian StyleZhao, Meili, Yanchang Zhao, Zhenyu Yang, Feng Ming, Jian Li, Demin Kong, Yu Wang, Peng Chen, Meina Wang, and Zhicai Wang. 2024. "Metabolic Pathway Engineering Improves Dendrobine Production in Dendrobium catenatum" International Journal of Molecular Sciences 25, no. 1: 397. https://doi.org/10.3390/ijms25010397




