The Bmtret1 Gene Family and Its Potential Role in Response to BmNPV Stress in Bombyx mori
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Identification and Phylogenetic Analysis of the Bmtret1s in B. mori
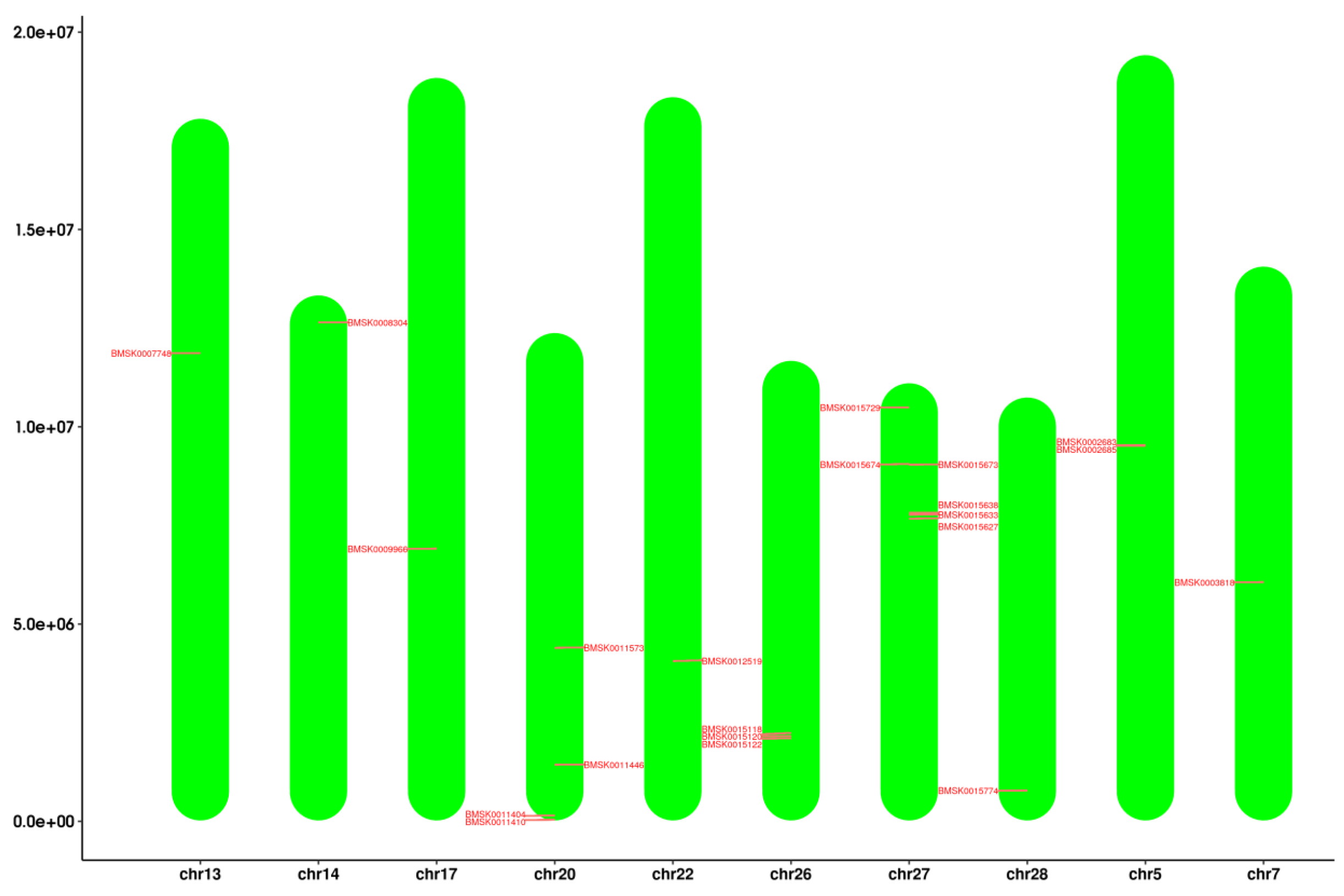
2.2. Chromosomal Localization of Bmtret1s
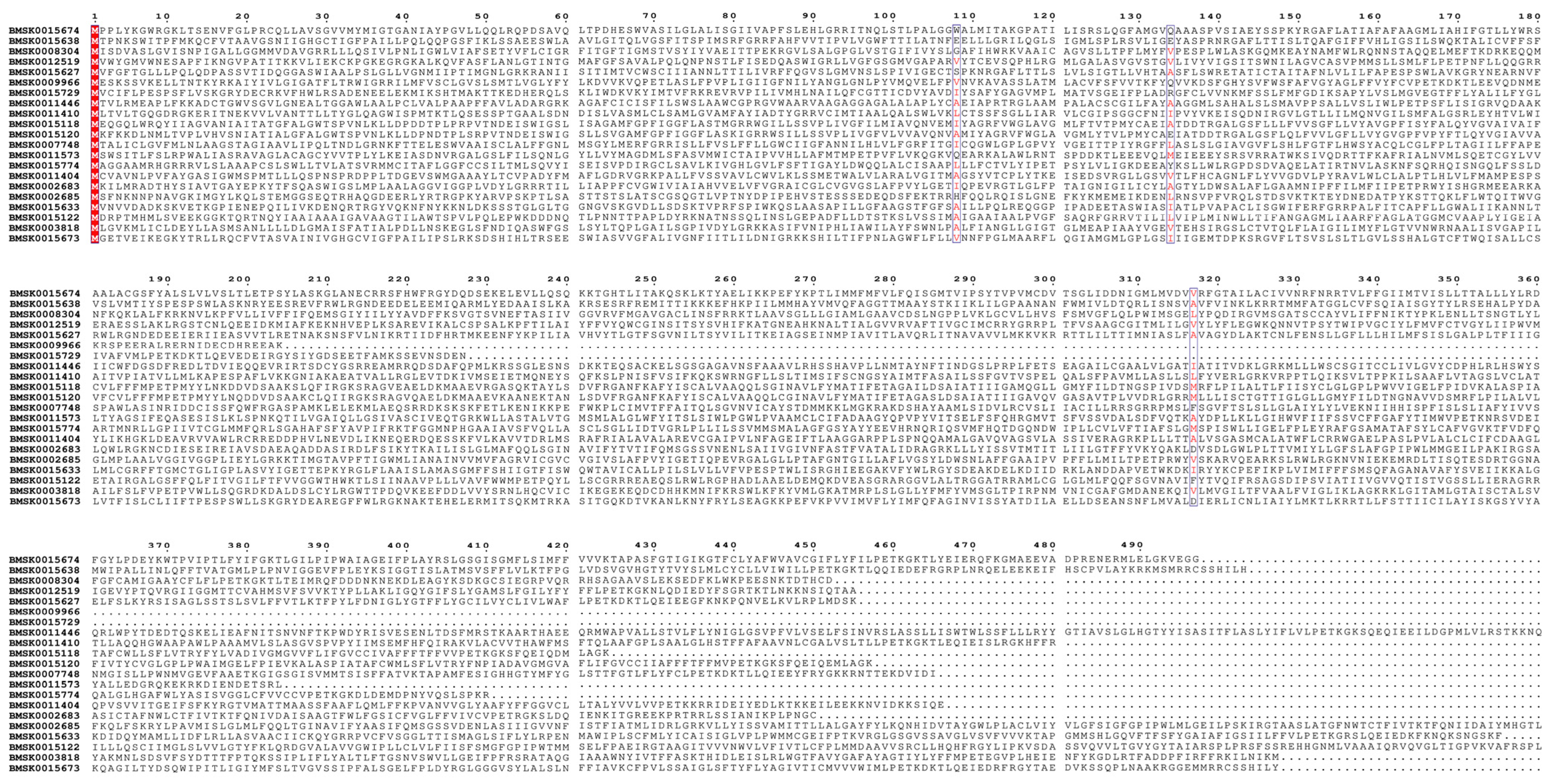
2.3. Sequence Analysis of the Bmtret1s
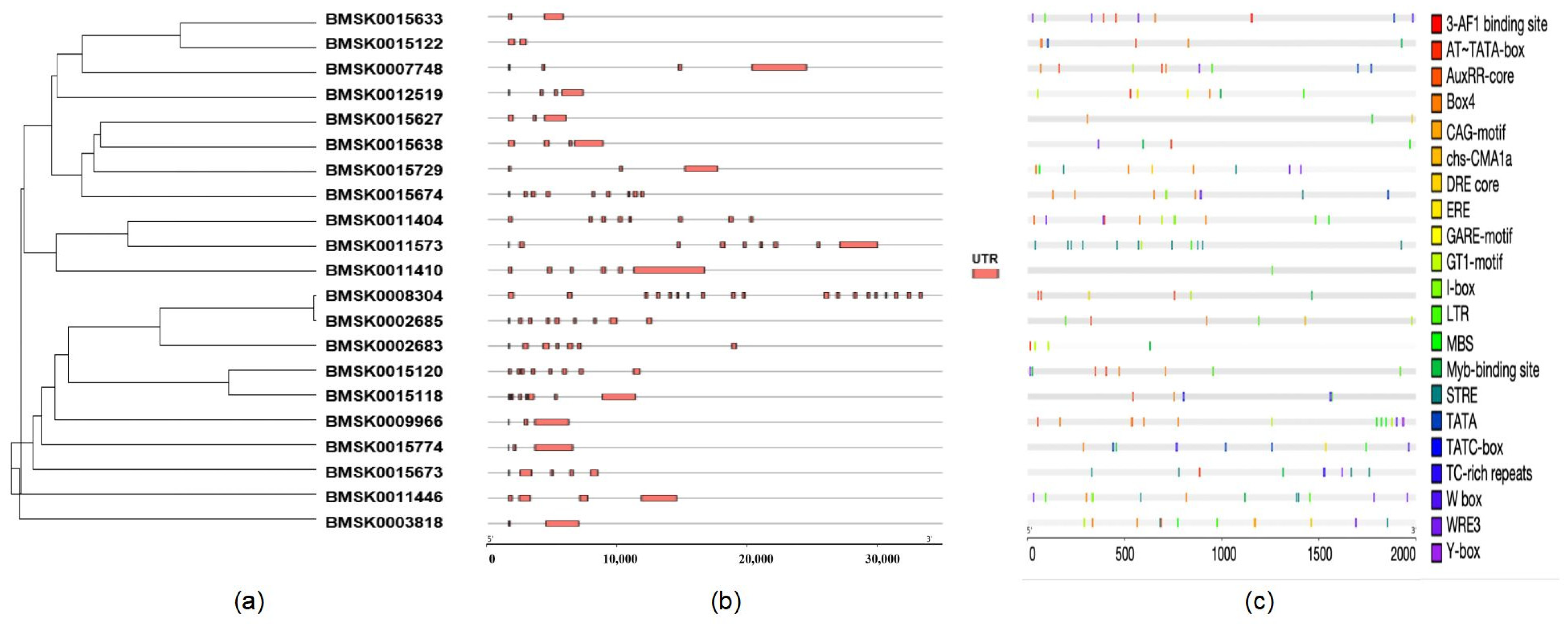
2.4. Gene Organization and Promoter Analysis of Bmtret1s
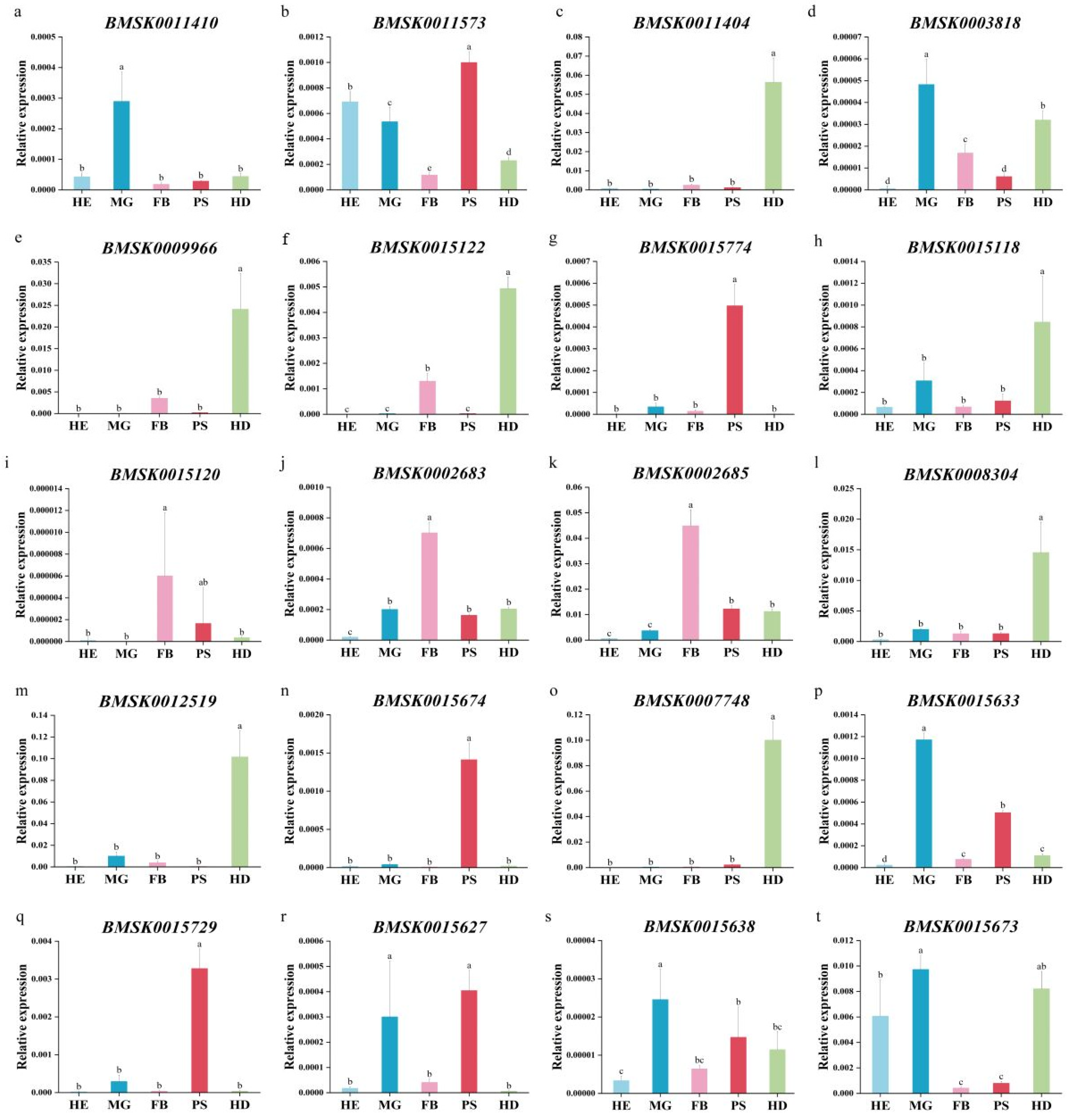
2.5. Expression Profile of Bmtret1s in Different Tissues of Silkworm
2.6. Transcriptional-Level Responses of Bmtret1s to BmNPV Stress
3. Discussion
4. Materials and Methods
4.1. Sericulture Breeding and Virus Preparation
4.2. Identification of the Bmtret1 Gene Family in B. mori
4.3. Chromosomal Localization and Homology Analysis of Bmtret1s
4.4. Sequence Alignment
4.5. Gene Structure and Promoter Analysis of Bmtret1s
4.6. Phylogenetic Analysis of Bmtret1s
4.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Primer Name | Sequence(5′ to 3′) |
|---|---|
| BMSK0015674-F | ACAATAGCATTCGCATTCG |
| BMSK0015674-R | AGTCTCCAGAGTCAGTGAA |
| BMSK0015638-F | ACCTTACGATGCGATGTG |
| BMSK0015638-R | CTCTTCCAACTCCTGTCTG |
| BMSK0008304-F | GCAAGGAACAGCAGATGA |
| BMSK0008304-R | CTATGATGGAAGCCGTGAA |
| BMSK0012519-F | GTTCGCCTTCTGCTCTAA |
| BMSK0012519-R | GGTCTCCGTCCATATCTTC |
| BMSK0015627-F | CGGATTCACTGGACTGTTA |
| BMSK0015627-R | TGGTAAGATTGTTGGCGATA |
| BMSK0009966-F | CTGTGAAGGAATTGCTAACC |
| BMSK0009966-R | AATAGTGACCGAACGAATCT |
| BMSK0015729-F | TTGTTGCCTTCGTTATGTTG |
| BMSK0015729-R | CACTGTTCACTTCGCTACT |
| BMSK0011446-F | GCACGCATTGTCCTTATC |
| BMSK0011446-R | GCACGCATTGTCCTTATC |
| BMSK0011410-F | AGCCAAGACAACATCAGAG |
| BMSK0011410-R | TCAGCATCAACAACACAGT |
| BMSK0015118-F | GCAGTATGTCGGAGTAGC |
| BMSK0015118-R | GAGATGTAGAACGCCTTGA |
| BMSK0015120-F | AATGGCAACGCAGTAGTC |
| BMSK0015120-R | ACAGAAGGCAGTGGCTAT |
| BMSK0007748-F | CGTCCTTATACCACAACTGA |
| BMSK0007748-R | AACACCAACTGCGATTCC |
| BMSK0011573-F | ACAAGAGACCTGCGGATA |
| BMSK0011573-R | GACACTGGAGAAGATGATGA |
| BMSK0015774-F | GTCCACCGTAACCAGAGA |
| BMSK0015774-R | GATGCGTATAACCAGAATGC |
| BMSK0015122-F | GGACTATGCGTAGGATTGAT |
| BMSK0015122-R | TGATATGAATGTGCCGAGAA |
| BMSK0011404-F | GCTTCTGTTCGTCTCCTC |
| BMSK0011404-R | AGTCCTCGCTGATCTCTT |
| BMSK0003818-F | CATCTATCGCACGCTACA |
| BMSK0003818-R | CGCTGAAGACGCTATTATC |
| BMSK0002683-F | TAGGAAGGCGTAGAACTATAC |
| BMSK0002683-R | CACCAACACATAATCCACAA |
| BMSK0002685-F | TGCCTCGTTATCTATGTGTT |
| BMSK0002685-R | TCCAGTTGAAGCCAGTTG |
| BMSK0015633-F | TATGGCGATGCTCCTAATAG |
| BMSK0015633-R | TCTCCTGTAGACTCCTTCC |
| BMSK0015673-F | GCTGGTTCCTGTTTCTAC |
| BMSK0015673-R | TTGGGTCCGTCATTTCTC |
References
- Li, K.; Dong, Z.; Pan, M. Common strategies in silkworm disease resistance breeding research. Pest Manag. Sci. 2023, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.B.; Li, J.M.; Varela, N.; Wang, Y.L.; Li, F.F.; Bao, Y.Y.; Zhang, C.X.; Liu, S.S.; Wang, X.W. Global analysis of the transcriptional response of whitefly to tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J. Virol. 2011, 85, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Yao, Q.; Bao, F.; Chen, K.P.; Liu, X.Y.; Li, J.; Wang, L. Comparative proteome analysis of silkworm in its susceptibility and resistance responses to Bombyx mori densonucleosis virus. Intervirology 2011, 55, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fei, S.; Xia, J.; Wang, Y.; Wu, H.; Li, X.; Guo, Y.; Swevers, L.; Sun, J.; Feng, M. Sirt5 Inhibits BmNPV replication by promoting a relish-Mediated antiviral pathway in Bombyx mori. Front. Immunol. 2022, 13, 906738. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dong, Z.Q.; Dong, F.F.; Yu, X.B.; Hu, Z.G.; Liao, N.C.; Chen, P.; Lu, C.; Pan, M.H. Gene editing the BmNPV inhibitor of apoptosis protein 2 (iap2) as an antiviral strategy in transgenic silkworm. Int. J. Biol. Macromol. 2021, 166, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Zhang, S.Z.; Zhu, L.B.; Wang, J.; Liu, Y.X.; Wang, Y.L.; Kong, X.; You, L.L.; Toufeeq, S.; Liu, S.H.; et al. The digestive proteinase trypsin, alkaline A contributes to anti-BmNPV activity in silkworm (Bombyx mori). Dev. Comp. Immunol. 2021, 119, 104035. [Google Scholar] [CrossRef] [PubMed]
- Katsuma, S.; Mita, K.; Shimada, T. ERK and JNK-dependent signaling pathways contribute to Bombyx mori nucleopolyhedrovirus infection. J. Virol. 2007, 81, 13700–13709. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gopinathan, K.P. Systemic and in vitro infection process of Bombyx mori nucleopolyhedrovirus. Virus Res. 2004, 101, 109–118. [Google Scholar] [CrossRef]
- Braunagel, S.C.; Summers, M.D. Molecular biology of the baculovirus occlusion-derived virus envelope. Curr. Drug Targets 2007, 8, 1084–1095. [Google Scholar] [CrossRef]
- Blissard, G.W. Baculovirus—Insect cell interactions. Cytotechnology 1996, 20, 73–93. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Liang, C.; Song, J.; Li, N.; Shi, H.; Chen, X. Autographa californica multiple nucleopolyhedrovirus nucleocapsid protein BV/ODV-C42 mediates the nuclear entry of P78/83. J. Virol. 2008, 82, 4554–4561. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Tang, X.D.; Lv, Z.Y.; Wang, X.Y.; Tian, C.H.; Xu, Y.P.; Zhang, C.X. Gene expression profiling of resistant and susceptible Bombyx mori strains reveals nucleopolyhedrovirus-associated variations in host gene transcript levels. Genomics 2009, 82, 138–145. [Google Scholar] [CrossRef]
- Mei, X.; Li, C.; Peng, P.; Wang, J.; He, E.; Qiu, Z.; Xia, D.; Zhao, Q.; Shen, D. Bombyx mori C-Type lectin (BmIML-2) inhibits the proliferation of B.mori nucleopolyhedrovirus (BmNPV) through involvement in apoptosis. Int. J. Mol. Sci. 2022, 23, 8369. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17–27. [Google Scholar] [CrossRef]
- Chen, A.; Tapia, H.; Goddard, J.M.; Gibney, P.A. Trehalose and its applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5004–5037. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Wyatt, G.R.; Kale, G.F. The chemistry of insect hemolymph.II.Trehalose and other carbohydrates. J. Gen. Physiol. 1957, 40, 833–847. [Google Scholar] [CrossRef]
- Candy, D.J.; Kilby, B.A. Site and mode of trehalose biosynthesis in the locust. Nature 1959, 183, 1594–1595. [Google Scholar] [CrossRef]
- Murphy, T.A.; Wyatt, G.R. The enzymes of glycogen and trehalose synthesis in silk moth fat body. J. Biol. Chem. 1965, 240, 1500–1508. [Google Scholar] [CrossRef]
- Kikuta, S.; Nakamura, Y.; Hattori, M.; Sato, R.; Kikawada, T.; Noda, H. Herbivory-induced glucose transporter gene expression in the brown planthopper, Nilaparvata lugens. Insect Biochem. Mol. Biol. 2015, 64, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ashford, D.A.; Smith, W.A.; Douglas, A.E. Living on a high sugar diet: The fate of sucrose ingested by a phloem-feeding insect, the pea aphid acyrthosiphon pisum. J. Insect Physiol. 2000, 46, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Fraga, A.; Ribeiro, L.; Lobato, M.; Santos, V.; Silva, J.R.; Gomes, H.; da Cunha Moraes, J.L.; de Souza Menezes, J.; de Oliveira, C.J.; Campos, E.; et al. Glycogen and glucose metabolism are essential for early embryonic development of the red flour beetle Tribolium castaneum. PLoS ONE 2013, 8, e65125. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, S.; Wang, S.G.; Wang, H.J.; Zhang, J.Y.; Cui, S.Y. Invertebrate trehalose-6-phosphate synthase gene: Genetic architecture, biochemistry, physiological function, and potential applications. Front. Physiol. 2018, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- García de Castro, A.; Tunnacliffe, A. Intracellular trehalose improves osmotolerance but not desiccation tolerance in mammalian cells. FEBS Lett. 2000, 487, 199–202. [Google Scholar] [CrossRef]
- Stambuk, B.U.; Panek, A.D.; Crowe, J.H.; Crowe, L.M.; de Araujo, P.S. Expression of high-affinity trehalose-H+ symport in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1998, 1379, 118–128. [Google Scholar] [CrossRef]
- Kanamori, Y.; Saito, A.; Hagiwara-Komoda, Y.; Tanaka, D.; Mitsumasu, K.; Kikuta, S.; Watanabe, M.; Cornette, R.; Kikawada, T.; Okuda, T. The trehalose transporter 1 gene sequence is conserved in insects and encodes proteins with different kinetic properties involved in trehalose import into peripheral tissues. Insect Biochem. Mol. Biol. 2010, 40, 30–37. [Google Scholar] [CrossRef]
- Kikawada, T.; Saito, A.; Kanamori, Y.; Nakahara, Y.; Iwata, K.-I.; Tanaka, D.; Watanabe, M.; Okuda, T. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11585–11590. [Google Scholar] [CrossRef]
- Takiguchi, M.; Niimi, T.; Su, Z.H.; Yaginuma, T. Trehalase from male accessory gland of an insect, Tenebrio molitor. cDNA sequencing and developmental profile of the gene expression. Biochem. J. 1992, 266, 19–22. [Google Scholar] [CrossRef]
- Tang, B.; Yang, M.; Shen, Q.; Xu, Y.; Wang, H.; Wang, S. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef]
- Harrison, R.L.; Herniou, E.A.; Jehle, J.A.; Theilmann, D.A.; Burand, J.P.; Becnel, J.J.; Krell, P.J.; van Oers, M.M.; Mowery, J.D.; Bauchan, G.R.; et al. ICTV virus taxonomy profile: Baculoviridae. J. Gen. Virol. 2018, 99, 1185–1186. [Google Scholar] [CrossRef]
- Lange, M.; Wang, H.; Zhihong, H.; Jehle, J.A. Towards a molecular identification and classification system of lepidopteran-specific baculoviruses. Virology 2004, 325, 36–47. [Google Scholar] [CrossRef]
- Zafar, B.; Shabir, A.W.; Malik, M.A.; Ganai, M.A. A review: Disease resistance in mulberry silkworm Bombyx mori L. Asian J. Sci. Technol. 2013, 4, 157–166. [Google Scholar]
- Zhou, Y.; Gao, L.; Shi, H.; Xia, H.; Gao, L.; Lian, C.; Chen, L.; Yao, Q.; Chen, K.; Liu, X. Microarray analysis of gene expression profile in resistant and susceptible Bombyx mori strains reveals resistance-related genes to nucleopolyhedrovirus. Genomics 2013, 101, 256–262. [Google Scholar] [CrossRef]
- Jia-Ping, X.; Ke-Ping, C.; Ming-Hui, L.; Qin, Y.; Gui-Tian, G.; Yuan, Z. Identification and characterization of Bms3a in Bombyx mori L. Afr. J. Biotechnol. 2008, 34, 24–30. [Google Scholar]
- Xu, J.P.; Chen, K.P.; Yao, Q.; Liu, M.H.; Gao, G.T.; Zhao, Y. Identification and characterization of an NPV infection-related gene Bmsop2 in Bombyx mori L. J. Appl. Entomol. 2005, 129, 425–431. [Google Scholar] [CrossRef]
- Yu, W.D.; Pan, B.Y.; Qiu, L.Y.; Huang, Z.; Zhou, T.; Ye, L.; Tang, B.; Wang, S.G. The structure characteristics and biological functions on regulating trehalose metabolism of two NlTret1s in Nilaparvata lugens. Sci. Agric. Sin. 2020, 53, 4802–4812. [Google Scholar]
- Yang, J. Transcriptome Analysis of Midgut Tissue Infected by BmNPV and Functional Identification of BmTret1-like Gene. Master’s Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2017. [Google Scholar]
- Song, Q. Identification of Resistance of Silkworm BmTret1-X1 Gene to Bombyx mori nucleopolyhedrovirus (BmNPV). Master’s Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2022. [Google Scholar]


| Gene ID | CDS Size (bp) | Protein Physicochemical Characteristics | TMHs | Subcellular Localization * | |||
|---|---|---|---|---|---|---|---|
| Length (aa) | MW (kDa) | pI | Aliphatic Index | ||||
| BMSK0011410 | 1443 | 480 | 51.74 | 9.31 | 116.67 | 12 | PM |
| BMSK0011573 | 1155 | 384 | 42.90 | 6.02 | 107.16 | 7 | PM |
| BMSK0011404 | 1401 | 466 | 50.77 | 8.31 | 111.33 | 12 | PM |
| BMSK0011446 | 1632 | 543 | 58.85 | 7.84 | 100.72 | 9 | PM |
| BMSK0003818 | 1524 | 507 | 56.44 | 7.55 | 112.76 | 11 | PM |
| BMSK0009966 | 615 | 204 | 23.18 | 8.28 | 101.18 | 4 | EX |
| BMSK0015122 | 1635 | 544 | 58.66 | 9.48 | 113.86 | 11 | PM |
| BMSK0015774 | 1233 | 410 | 44.81 | 8.17 | 108.24 | 10 | PM |
| BMSK0015118 | 1275 | 424 | 46.07 | 4.83 | 115.26 | 11 | PM |
| BMSK0015120 | 1374 | 457 | 49.34 | 6.59 | 117.13 | 12 | PM |
| BMSK0002683 | 1353 | 450 | 49.19 | 8.61 | 116.42 | 11 | PM |
| BMSK0002685 | 1776 | 591 | 65.47 | 9.15 | 98.65 | 10 | PM |
| BMSK0008304 | 1359 | 452 | 49.90 | 8.74 | 102.23 | 10 | PM |
| BMSK0012519 | 1368 | 455 | 49.99 | 9.42 | 100.26 | 10 | PM |
| BMSK0015674 | 1494 | 497 | 54.72 | 9.05 | 107.95 | 10 | MT |
| BMSK0007748 | 1398 | 465 | 51.74 | 9.05 | 108.04 | 12 | PM |
| BMSK0015633 | 1608 | 535 | 58.76 | 8.92 | 103.20 | 12 | PM |
| BMSK0015729 | 684 | 227 | 26.00 | 5.21 | 92.69 | 2 | CY |
| BMSK0015627 | 1368 | 455 | 50.32 | 9.08 | 125.34 | 11 | PM |
| BMSK0015638 | 1512 | 503 | 56.54 | 9.28 | 104.10 | 12 | PM |
| BMSK0015673 | 1515 | 504 | 55.87 | 9.08 | 113.57 | 10 | PM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Qian, Y.; Chen, E.; Wang, M.; Ouyang, G.; Xu, Y.; Zhao, G.; Qian, H. The Bmtret1 Gene Family and Its Potential Role in Response to BmNPV Stress in Bombyx mori. Int. J. Mol. Sci. 2024, 25, 402. https://doi.org/10.3390/ijms25010402
Lin M, Qian Y, Chen E, Wang M, Ouyang G, Xu Y, Zhao G, Qian H. The Bmtret1 Gene Family and Its Potential Role in Response to BmNPV Stress in Bombyx mori. International Journal of Molecular Sciences. 2024; 25(1):402. https://doi.org/10.3390/ijms25010402
Chicago/Turabian StyleLin, Mingjun, Yixuan Qian, Enxi Chen, Mengjiao Wang, Gui Ouyang, Yao Xu, Guodong Zhao, and Heying Qian. 2024. "The Bmtret1 Gene Family and Its Potential Role in Response to BmNPV Stress in Bombyx mori" International Journal of Molecular Sciences 25, no. 1: 402. https://doi.org/10.3390/ijms25010402





