Effect of THz Waves of Different Orientations on K+ Permeation Efficiency in the KcsA Channel
Abstract
:1. Introduction
2. Results
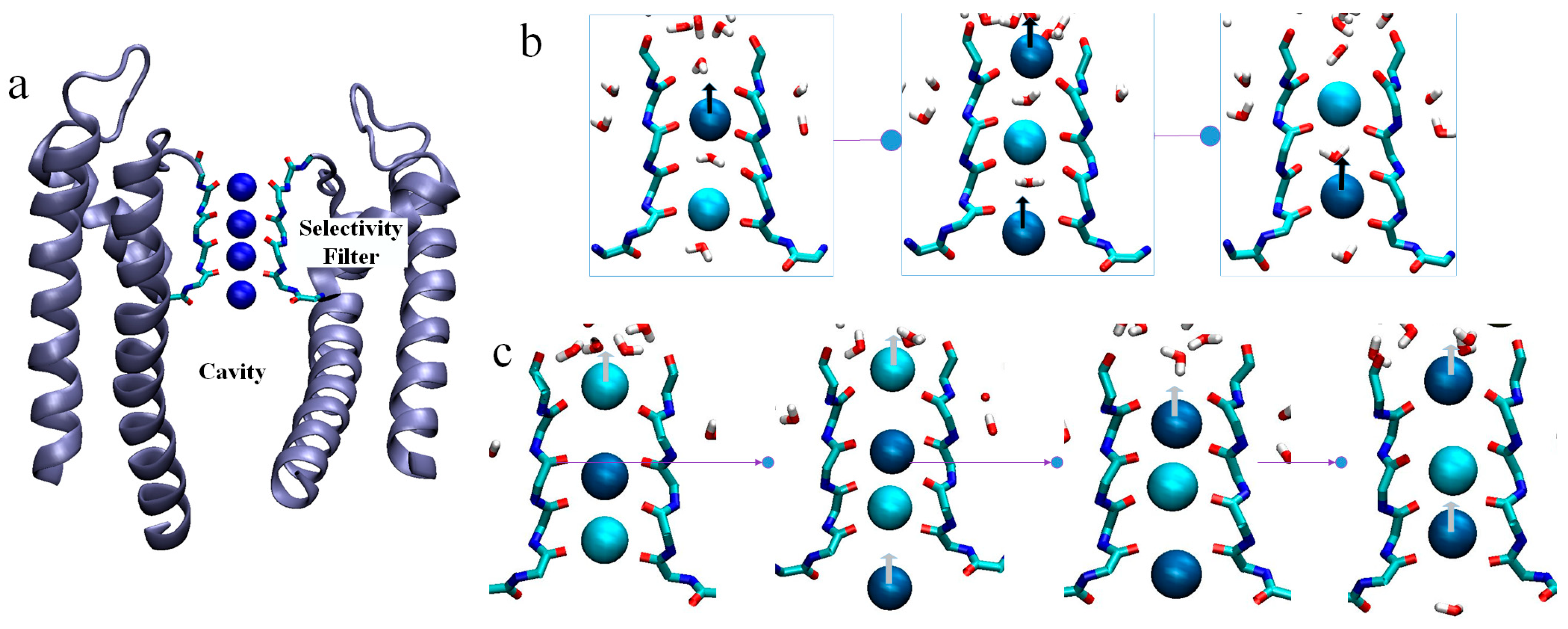
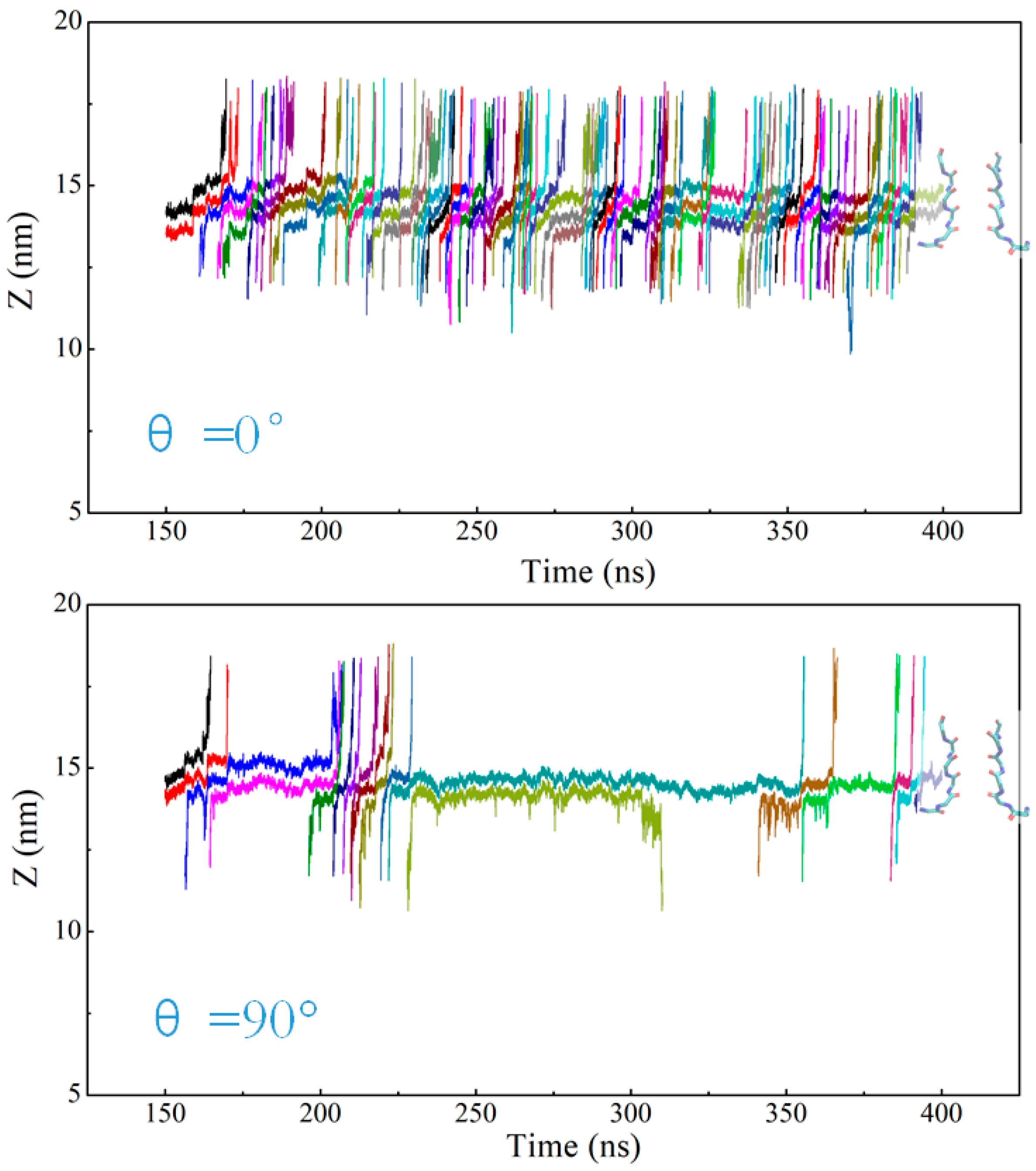
2.1. Ion Flux in the KcsA Channel
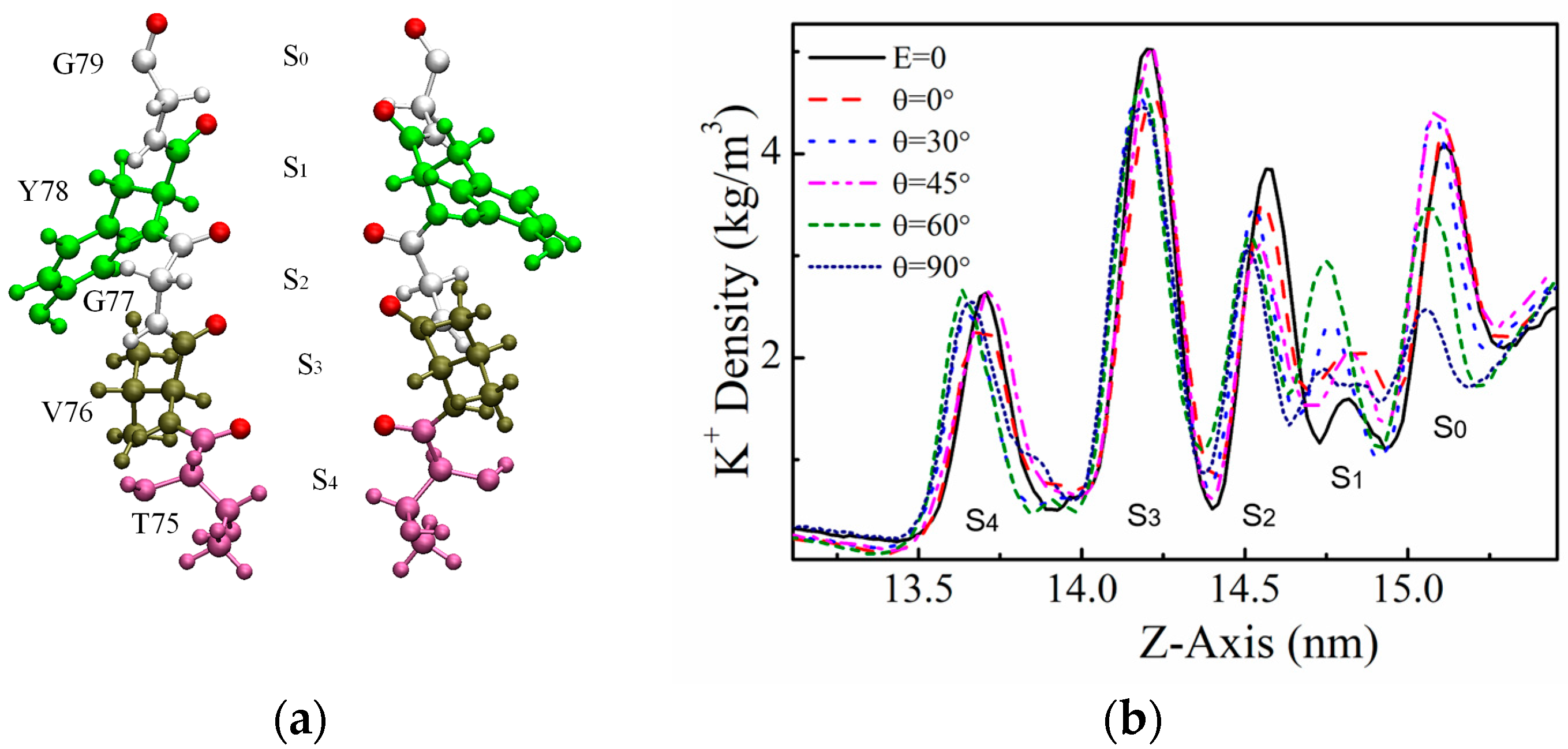
2.2. Density Distribution of K+ at SF Sites
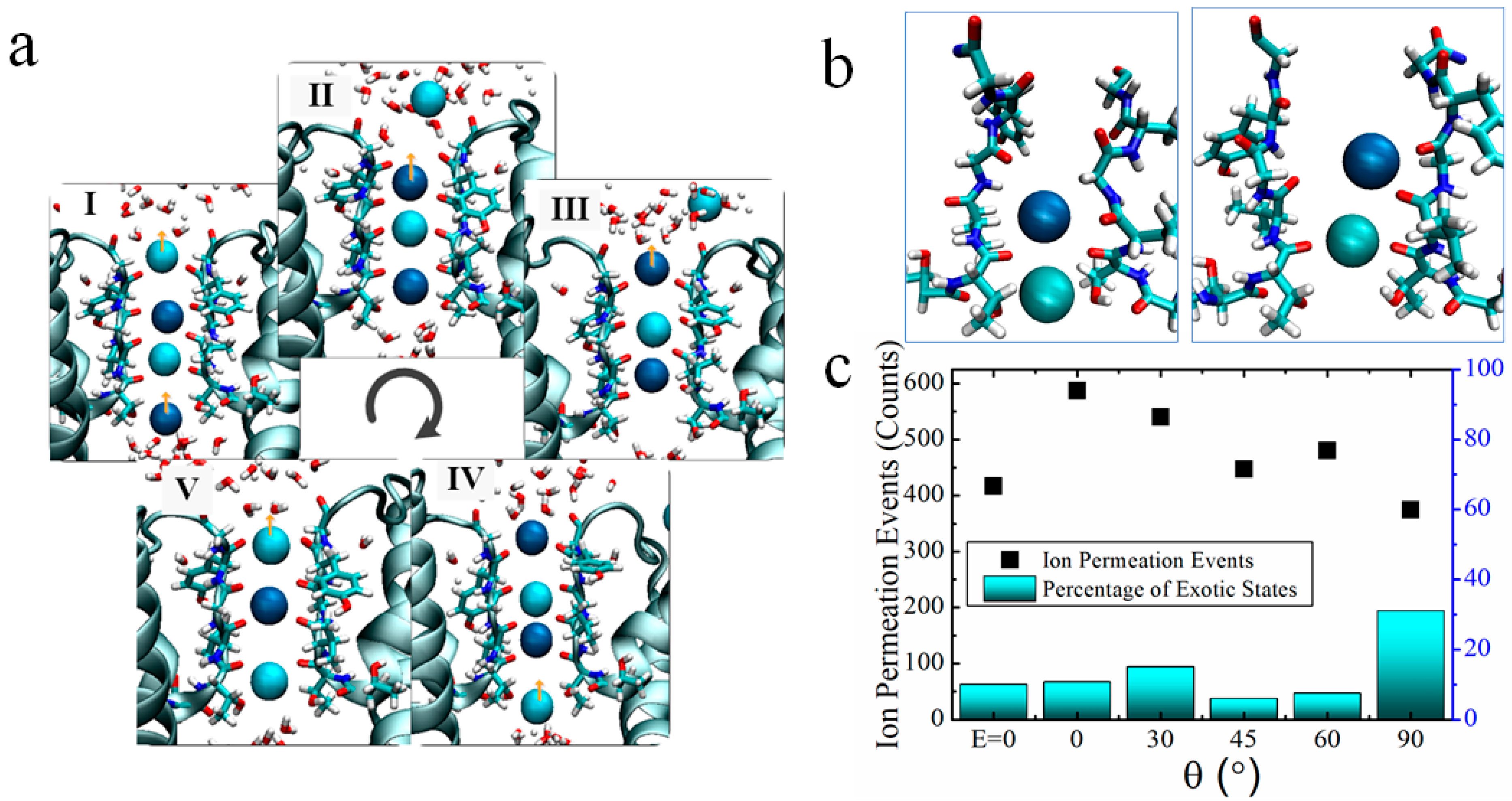
2.3. Two Exotic States Slow down the Rate of Ion Permeation
2.4. The Dihedral Angle of the GLY77 Residue
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Accociates: Sunderland, MA, USA, 2001. [Google Scholar]
- Kuang, Q.; Hebert, H. Structure of potassium channels. Cell Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef] [PubMed]
- Bates, E. Ion channels in development and cancer. Annu. Rev. Cell Dev. Biol. 2015, 31, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [PubMed]
- Litan, A.; Langhans, S.A. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front. Cell. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Lastraioli, E.; Iorio, J.; Arcangeli, A. Ion channel expression as promising cancer biomarker. Biochim. Biophys. Acta 2015, 1848, 2685–2702. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A.; Stühmer, W. The roles of K+ channel in cancer. Nat. Rev. Cancer 2014, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, S.; Bischof, H.; Matt, L.; Lukowski, R. Assessing K+ ions and K+ channel functions in cancer cell metab-olism using fluorescent biosensors. Free Radic. Bio Med. 2022, 181, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Castle, N. Therapeutic potential of KCa3.1 blockers: Recent advances and promising trends. Expert Rev. Clin. Pharmacol. 2010, 3, 385–396. [Google Scholar] [CrossRef]
- Cázares-Ordoñez, V.; Pardo, L. Kv10.1 potassium channel: From the brain to the tumors. Biochem. Cell Biol. 2017, 95, 531–536. [Google Scholar] [CrossRef]
- Staudacher, I.; Jehle, J.; Staudacher, K.; Pledl, H.; Lemke, D.; Schweizer, P. HERG K+ channel-dependent apoptosis and cell cycle arrest in human glioblastoma cells. PLoS ONE 2014, 9, e88164. [Google Scholar] [CrossRef]
- Doyle, D.A.; Cabral, J.M.; Pfuetzner, R.A. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Noskov, S.Y.; Bernéche, S.; Roux, B. Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands. Nature 2004, 431, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; MacKinnon, R. The occupancy of ions in the K+ selectivity filter: Charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J. Mol. Biol. 2003, 333, 965–975. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, R. Potassium channels and the atomic basis of selective ion conduction (Nobel Lecture). Angew. Chem. Int. Ed. 2004, 43, 4265–4277. [Google Scholar] [CrossRef] [PubMed]
- Noskov, S.Y.; Roux, B.T. Ion selectivity in potassium channels. Biophys. Chem. 2006, 124, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bian, X. Preferential binding of K+ ions in the selectivity filter at equilibrium explains high selectivity of K+ channels. J Gen. Physiol. 2012, 140, 671–679. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, R.; Cohen, S.L.; Kuo, A.; Lee, A.; Chait, B.T. Structural conservation in prokaryotic and eukaryotic potassium channels. Science 1998, 280, 106–109. [Google Scholar] [CrossRef] [PubMed]
- LeMasurier, M.; Heginbotham, L.; Miller, C. KcsA: It’s a potassium channel. J. Gen. Physiol. 2001, 118, 303–313. [Google Scholar] [CrossRef]
- Cuello, L.G.; Cortes, D.M.; Perozo, E. The gating cycle of a K+ channel at atomic resolution. eLife 2017, 6, e28032. [Google Scholar] [CrossRef]
- Åqvist, J.; Luzhkov, V. Ion permeation mechanism of the potassium channel. Nature 2000, 404, 881–884. [Google Scholar] [CrossRef]
- Medovoy, D.; Perozo, E.; Roux, B.T. Multi-ion free energy landscapes underscore the microscopic mechanism of ion se-lectivity in the KcsA channel. Biochem. Biophys. Acta 2016, 1858, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Hummer, G. Potassium ions line up. Science 2014, 346, 303. [Google Scholar] [CrossRef] [PubMed]
- Köpfer, D.A. Ion permeation in K+ channels occurs by direct coulomb knock-on. Science 2014, 346, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Kopec, W.; Köpfer, D.A.; Vickery, O.N.; Bondarenko, A.S.; Jansen, T.L.C.; de Groot, B.L.; Zachariae, U. Direct knock-on of desolvated ions governs striction selectivity in K+ channels. Nat. Chem. 2018, 10, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Sumikama, T.; Oiki, S. Digitalized K+ occupancy in the nanocavity holds and releases queues of K+ in a channel. J. Am. Chem. Soc. 2016, 138, 10284–10292. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H. Regulation of ion permeation of the KcsA channel by applied midinfrared field. Int. J. Mol. Sci. 2023, 24, 556. [Google Scholar] [CrossRef]
- Williams, B.S.; Callebaut, H.; Kumar, S.; Hu, Q. 3.4-THz quantum cascade laser based on longitudinal-optical-phonon scattering for depopulation. Appl. Phys. Lett. 2003, 82, 1015–1017. [Google Scholar] [CrossRef]
- Xiang, Z.; Tang, C.; Chang, C.; Liu, G. A new viewpoint and model of neural signal generation and transmission: Signal trans-mission on unmyelinated neurons. Nano Res. 2021, 14, 590–600. [Google Scholar] [CrossRef]
- Liu, G.; Chang, C.; Qiao, Z.; Wu, K.; Zhu, Z.; Cui, G.; Peng, W.; Tang, Y.; Li, J.; Fan, C. Myelin sheath as a dielectric waveguide for signal propagation in the mid-infrared to terahertz spectral range. Adv. Funct. 2019, 29, 1807862. [Google Scholar] [CrossRef]
- Olshevskaya, J.S.; Ratushnyak, A.S.; Petrov, A.K.; Kozlov, A.S.; Zapara, T.A. Effect of terahertz electromagnetic waves on neurons systems. In Proceedings of the 2008 IEEE Region 8 International Conference on Computational Technologies in Electrical and Electronics Engineering, Novosibirsk, Russia, 21–25 July 2008; pp. 210–211. [Google Scholar]
- Wu, K.; Qi, C.; Zhu, Z.; Wang, C.; Song, B.; Chang, C. Terahertz wave accelerates DNA unwinding: A molecular dynamics simulation study. J. Phys. Chem. Lett. 2020, 11, 7002–7008. [Google Scholar] [CrossRef]
- Li, Y.; Chang, C.; Zhu, Z.; Sun, L.; Fan, C. Terahertz wave enhances permeability of the voltage-gated Calcium Channel. J. Am. Chem. Soc. 2021, 143, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiao, Z.; Chai, Y.; Zhu, Z.; Wu, K.; Ji, W.; Li, D.; Xiao, Y.; Mao, L.; Chang, C.; et al. Nonthermal and reversible control of neuronal signaling and behavior by midinfrared stimulation. Proc. Natl. Acad. Sci. USA 2021, 118, e2015685118. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ivetac, A.; Sansom, M.S.P. Molecular dynamics simulations and membrane protein structure quality. Eur. Biophys. J. 2008, 37, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Omori, S.; Fuchigami, A.; Kidera, A. Latent dynamics of a protein molecule observed in dihedral angle space. J. Math. Phys. 2010, 132, 115103. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Piazena, H. Strong correlation between specific heat capacity and water content in human tissues suggests preferred heat deposition in malignant tumors upon electromagnetic irradiation. Int. J. Hyperth. 2022, 39, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.F.A.; Gordon, R.E. Water in malignant tissue, measured by cell refractometry and nuclear magnetic resonance. J. Microsc. 1982, 128, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Melcr, J.; Bonhenry, D.; Timr, Š.; Jungwirth, P. Transmembrane Potential Modeling: Comparison between methods of constant electric field and ion imbalance. J. Chem. Theory Comput. 2016, 12, 2418–2425. [Google Scholar] [CrossRef]
- Roux, B. The membrane potential and its representation by a constant electric field in computer simulations. Biophys. J. 2008, 95, 4205–4216. [Google Scholar] [CrossRef]
- Gumbart, J.; Khalili-Araghi, F.; Sotomayor, M.; Roux, B. Constant electric field simulations of the membrane potential Illustrated with simple systems. Biochim. Biophys. Acta Biomembr. 2012, 1818, 294–302. [Google Scholar] [CrossRef]
- Kutzner, C.; Grubmüller, H.; de Groot, B.L.; Zachariae, U. Computational Electrophysiology: The molecular dynamics of ion channel permeation and selectivity in atomistic detail. Biophys. J. 2011, 101, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.N.; Crozier, P.S.; Woolf, T.B. Atomistic simulations of biologically realistic transmembrane potential gradients. J. Chem. Phys. 2004, 121, 10847–10851. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Delemotte, L.; Dehez, F.; Treptow, W.; Tarek, M.J. Modeling Membranes under a Transmembrane Potential. Phys. Chem. B 2008, 112, 5547–5550. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, H.; Ding, W.; Zhao, X.; Li, Y.; Liu, C. Effect of THz Waves of Different Orientations on K+ Permeation Efficiency in the KcsA Channel. Int. J. Mol. Sci. 2024, 25, 429. https://doi.org/10.3390/ijms25010429
Wang Y, Wang H, Ding W, Zhao X, Li Y, Liu C. Effect of THz Waves of Different Orientations on K+ Permeation Efficiency in the KcsA Channel. International Journal of Molecular Sciences. 2024; 25(1):429. https://doi.org/10.3390/ijms25010429
Chicago/Turabian StyleWang, Yize, Hongguang Wang, Wen Ding, Xiaofei Zhao, Yongdong Li, and Chunliang Liu. 2024. "Effect of THz Waves of Different Orientations on K+ Permeation Efficiency in the KcsA Channel" International Journal of Molecular Sciences 25, no. 1: 429. https://doi.org/10.3390/ijms25010429





