A Comprehensive Identification and Expression Analysis of the WUSCHEL Homeobox-Containing Protein Family Reveals Their Special Role in Development and Abiotic Stress Response in Zea mays L.
Abstract
:1. Introduction
2. Results
2.1. Identification and Characterization of ZmWOX Genes
2.2. Phylogenetic Analysis of ZmWOX Proteins
2.3. Structural Analysis of ZmWOX Genes
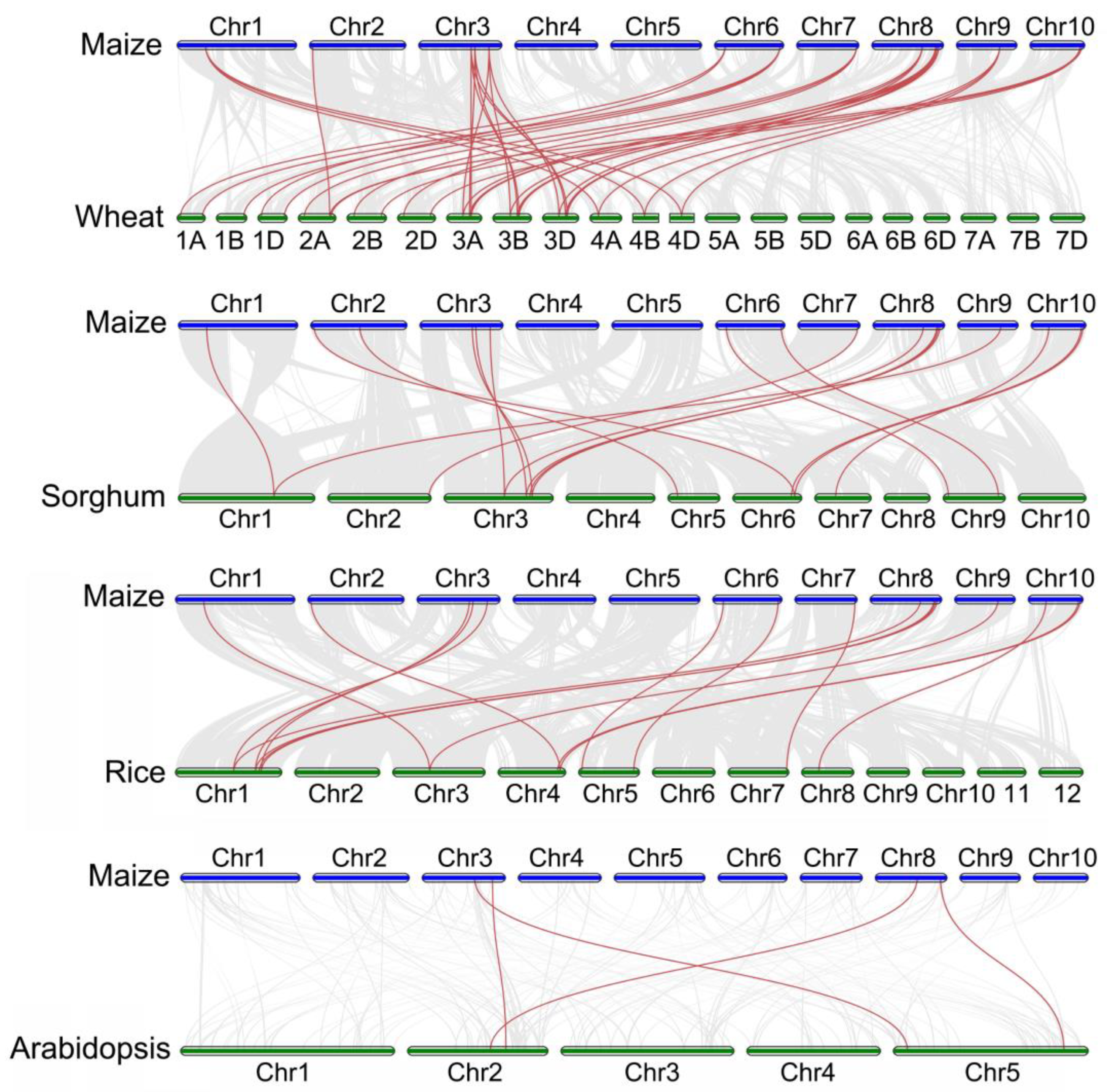
2.4. Gene Duplication and Synteny Analysis of the ZmWOX Genes
2.5. The Cis-Element Analysis of the ZmWOX Promoter
2.6. Expression Patterns of ZmWOX Genes in Different Maize Tissues
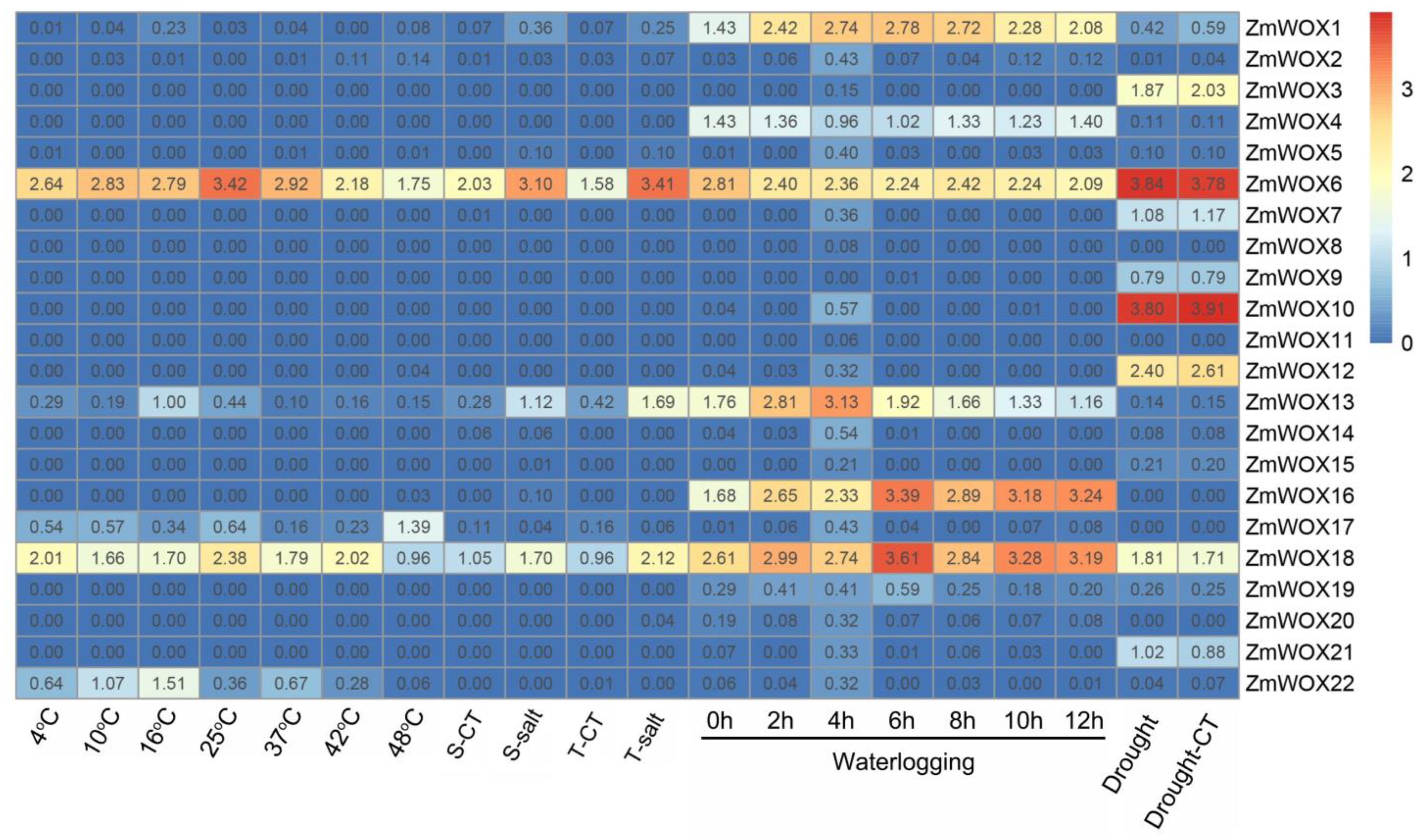
2.7. Expression Patterns of ZmWOXs under Abiotic Stress Conditions
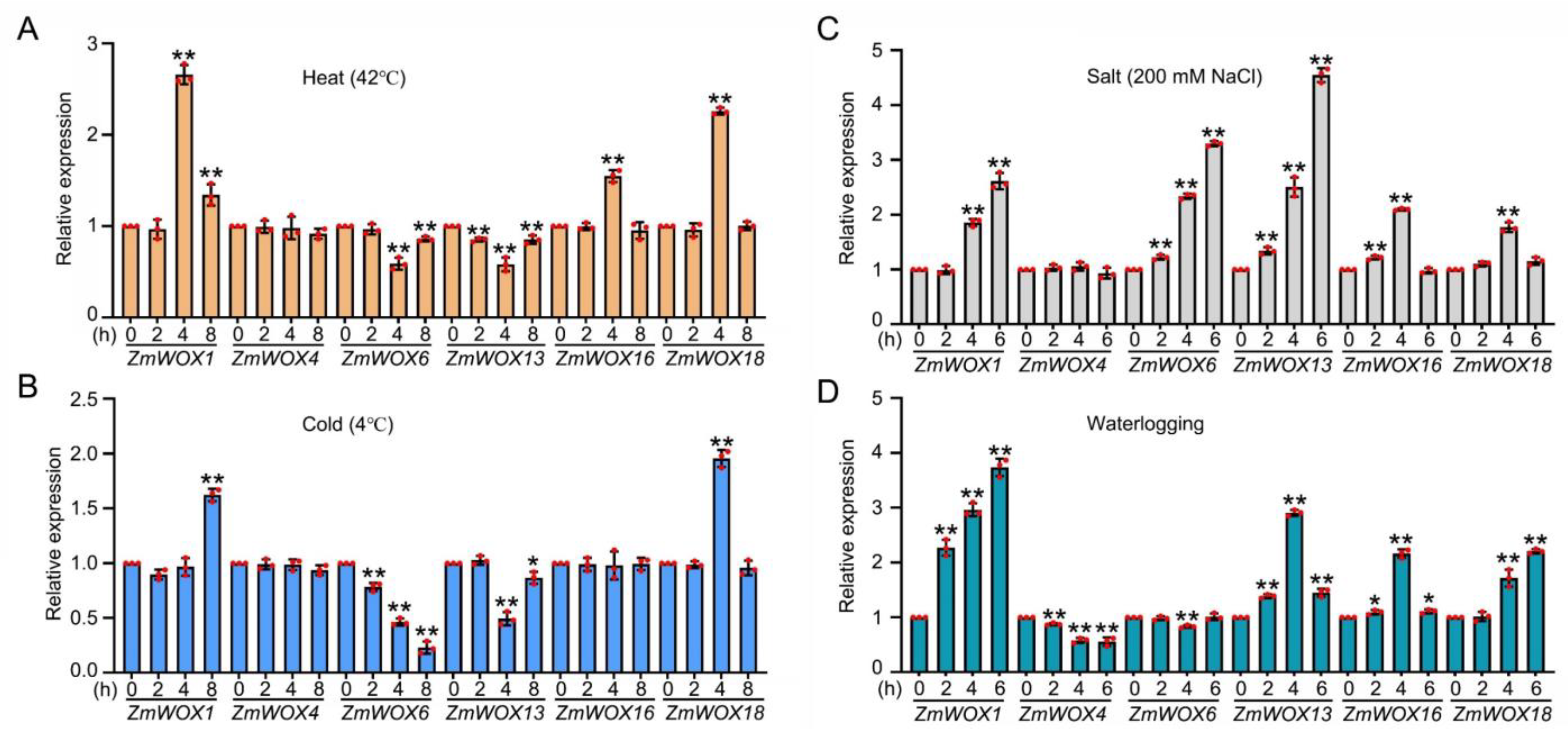
2.8. RT-qPCR Analysis of ZmWOX Genes under Abiotic Stresses
2.9. Subcellular Localization and Transactivation Activity Assays of ZmWOX1 and 18
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Stress Treatments
4.2. Identification of ZmWOX Genes
4.3. Phylogenetic and Structural Analyses of ZmWOX Genes
4.4. Chromosomal Localization and Collinearity Analysis
4.5. Cis-Acting Element Analysis
4.6. Transcriptome Data Analysis
4.7. RT-qPCR Analysis of Gene Expression
4.8. Subcellular Localization
4.9. Transactivation Activity Assays
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robert, J.S. Interpreting the homeobox: Metaphors of gene action and activation in development and evolution. Evol. Dev. 2001, 3, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Wolberger, C. Homeodomain interactions. Curr. Opin. Struct. Biol. 1996, 6, 62–68. [Google Scholar] [CrossRef]
- Dolzblasz, A.; Nardmann, J.; Clerici, E.; Causier, B.; van der Graaff, E.; Chen, J.; Davies, B.; Werr, W.; Laux, T. Stem Cell Regulation by Arabidopsis WOX Genes. Mol. Plant 2016, 9, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Li, F.W.; Kramer, E.M. Large-scale phylogenomic analysis suggests three ancient superclades of the WUSCHEL-RELATED HOMEOBOX transcription factor family in plants. PLoS ONE 2019, 14, e0223521. [Google Scholar] [CrossRef] [PubMed]
- van der Graaff, E.; Laux, T.; Rensing, S.A. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Segatto, A.L.; Thompson, C.E.; Freitas, L.B. Molecular evolution analysis of WUSCHEL-related homeobox transcription factor family reveals functional divergence among clades in the homeobox region. Dev. Genes Evol. 2016, 226, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Aichinger, E.; Kornet, N.; Friedrich, T.; Laux, T. Plant stem cell niches. Annu. Rev. Plant Biol. 2012, 63, 615–636. [Google Scholar] [CrossRef]
- Haecker, A.; Gross-Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 2004, 131, 657–668. [Google Scholar] [CrossRef]
- Mukherjee, K.; Brocchieri, L.; Burglin, T.R. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol. Biol. Evol. 2009, 26, 2775–2794. [Google Scholar] [CrossRef]
- Tang, L.; He, Y.; Liu, B.; Xu, Y.; Zhao, G. Genome-Wide Identification and Characterization Analysis of WUSCHEL-Related Homeobox Family in Melon (Cucumis melo L.). Int. J. Mol. Sci. 2023, 24, 12326. [Google Scholar] [CrossRef]
- Chen, G.Z.; Huang, J.; Lin, Z.C.; Wang, F.; Yang, S.M.; Jiang, X.; Ahmad, S.; Zhou, Y.Z.; Lan, S.; Liu, Z.J.; et al. Genome-Wide Analysis of WUSCHEL-Related Homeobox Gene Family in Sacred Lotus (Nelumbo nucifera). Int. J. Mol. Sci. 2023, 24, 14216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hamyat, M.; Liu, C.; Ahmad, S.; Gao, X.; Guo, C.; Wang, Y.; Guo, Y. Identification and Characterization of the WOX Family Genes in Five Solanaceae Species Reveal Their Conserved Roles in Peptide Signaling. Genes 2018, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, X.; Ma, D.; Liu, C. Identification and Evolutionary Analysis of Cotton (Gossypium hirsutum) WOX Family Genes and Their Potential Function in Somatic Embryogenesis. Int. J. Mol. Sci. 2023, 24, 11077. [Google Scholar] [CrossRef]
- Riccucci, E.; Vanni, C.; Vangelisti, A.; Fambrini, M.; Giordani, T.; Cavallini, A.; Mascagni, F.; Pugliesi, C. Genome-Wide Analysis of WOX Multigene Family in Sunflower (Helianthus annuus L.). Int. J. Mol. Sci. 2023, 24, 3352. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Xia, Y.; Li, Z.; Jing, D.; Du, J.; Niu, N.; Ma, S.; Wang, J.; Song, Y.; et al. Identification of the WUSCHEL-Related Homeobox (WOX) Gene Family, and Interaction and Functional Analysis of TaWOX9 and TaWUS in Wheat. Int. J. Mol. Sci. 2020, 21, 1581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zong, J.; Liu, J.; Yin, J.; Zhang, D. Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar. J. Integr. Plant Biol. 2010, 52, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Nagasaki, H.; Morikami, A.; Sato, Y.; Matsuoka, M. Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. Plant J. 2003, 35, 429–441. [Google Scholar] [CrossRef]
- Yang, Z.E.; Gong, Q.; Qin, W.Q.; Yang, Z.R.; Cheng, Y.; Lu, L.L.; Ge, X.Y.; Zhang, C.J.; Wu, Z.X.; Li, F.G. Genome-wide analysis of WOX genes in upland cotton and their expression pattern under different stresses. BMC Plant Biol. 2017, 17, 113. [Google Scholar] [CrossRef]
- Deveaux, Y.; Toffano-Nioche, C.; Claisse, G.; Thareau, V.; Morin, H.; Laufs, P.; Moreau, H.; Kreis, M.; Lecharny, A. Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol. Biol. 2008, 8, 291. [Google Scholar] [CrossRef]
- Fiume, E.; Fletcher, J.C. Regulation of Arabidopsis embryo and endosperm development by the polypeptide signaling molecule CLE8. Plant Cell 2012, 24, 1000–1012. [Google Scholar] [CrossRef]
- Nardmann, J.; Reisewitz, P.; Werr, W. Discrete shoot and root stem cell-promoting WUS/WOX5 functions are an evolutionary innovation of angiosperms. Mol. Biol. Evol. 2009, 26, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.F.; Schoof, H.; Haecker, A.; Lenhard, M.; Jurgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Perales, M.; Gruel, J.; Girke, T.; Jonsson, H.; Reddy, G.V. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev. 2011, 25, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.P.; Lu, S.C.; Tian, H.Y.; Ding, Z.J. WOX5 is Shining in the Root Stem Cell Niche. Trends Plant Sci. 2015, 20, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Ji, J.; Kelsey, E.; Ohtsu, K.; Schnable, P.S.; Scanlon, M.J. Tissue specificity and evolution of meristematic WOX3 function. Plant Physiol. 2009, 149, 841–850. [Google Scholar] [CrossRef]
- Etchells, J.P.; Provost, C.M.; Mishra, L.; Turner, S.R. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 2013, 140, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Denis, E.; Kbiri, N.; Mary, V.; Claisse, G.; Conde, E.S.N.; Kreis, M.; Deveaux, Y. WOX14 promotes bioactive gibberellin synthesis and vascular cell differentiation in Arabidopsis. Plant J. 2017, 90, 560–572. [Google Scholar] [CrossRef]
- Romera-Branchat, M.; Ripoll, J.J.; Yanofsky, M.F.; Pelaz, S. The WOX13 homeobox gene promotes replum formation in the Arabidopsis thaliana fruit. Plant J. 2013, 73, 37–49. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, Y.; Zhu, N.; Zhao, Y. The rice WUSCHEL-related homeobox genes are involved in reproductive organ development, hormone signaling and abiotic stress response. Gene 2014, 549, 266–274. [Google Scholar] [CrossRef]
- Yasui, Y.; Ohmori, Y.; Takebayashi, Y.; Sakakibara, H.; Hirano, H.Y. WUSCHEL-RELATED HOMEOBOX4 acts as a key regulator in early leaf development in rice. PLoS Genet. 2018, 14, e1007365. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Dai, M.; Huang, L.; Zhou, D.X. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell. 2009, 21, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Zhang, L.; Yang, Y.; Shan, Z.; Zhou, X.A. Genome-Wide Analysis of the WOX Gene Family and Function Exploration of GmWOX18 in Soybean. Plants 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Tang, R.; Chen, X.; Xu, Z.; Ren, Z.; Wang, L. Genome-wide identification and characterization of WOX genes in Cucumis sativus. Genome 2021, 64, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Minh-Thu, P.T.; Kim, J.S.; Chae, S.; Jun, K.M.; Lee, G.S.; Kim, D.E.; Cheong, J.J.; Song, S.I.; Nahm, B.H.; Kim, Y.K. A WUSCHEL Homeobox Transcription Factor, OsWOX13, Enhances Drought Tolerance and Triggers Early Flowering in Rice. Mol. Cells 2018, 41, 781–798. [Google Scholar] [PubMed]
- Lv, J.; Feng, Y.; Jiang, L.; Zhang, G.; Wu, T.; Zhang, X.; Xu, X.; Wang, Y.; Han, Z. Genome-wide identification of WOX family members in nine Rosaceae species and a functional analysis of MdWOX13-1 in drought resistance. Plant Sci. 2023, 328, 111564. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, M.J.; Schneeberger, R.G.; Freeling, M. The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 1996, 122, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Nardmann, J.; Werr, W. The shoot stem cell niche in angiosperms: Expression patterns of WUS orthologues in rice and maize imply major modifications in the course of mono- and dicot evolution. Mol. Biol. Evol. 2006, 23, 2492–2504. [Google Scholar] [CrossRef]
- Hoerster, G.; Wang, N.; Ryan, L.; Wu, E.; Anand, A.; McBride, K.; Lowe, K.; Jones, T.; Gordon-Kamm, B. Use of non-integrating vectors to enhance maize transformation Non-integrating WUS2 enhances transformation. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 265–279. [Google Scholar] [CrossRef]
- Lin, H.; Niu, L.; McHale, N.A.; Ohme-Takagi, M.; Mysore, K.S.; Tadege, M. Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc. Natl. Acad. Sci. USA 2013, 110, 366–371. [Google Scholar] [CrossRef]
- Zhu, W.; Miao, X.; Qian, J.; Chen, S.; Jin, Q.; Li, M.; Han, L.; Zhong, W.; Xie, D.; Shang, X.; et al. A translatome-transcriptome multi-omics gene regulatory network reveals the complicated functional landscape of maize. Genome Biol. 2023, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Yang, J.F.; Li, M.; Xu, Z.S.; Fu, J.D. The Maize WRKY Transcription Factor ZmWRKY40 Confers Drought Resistance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2580. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Zhang, Y.; Li, C.; Gong, S.; Yan, S.; Li, G.; Hu, G.; Ren, H.; Yang, J.; et al. Comparative transcriptome analysis of salt-sensitive and salt-tolerant maize reveals potential mechanisms to enhance salt resistance. Genes Genom. 2019, 41, 781–801. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Li, Y.; Zhang, Y.; Gou, Z.; Qi, X.; Zhang, J. Transcriptomic Analysis Revealed the Common and Divergent Responses of Maize Seedling Leaves to Cold and Heat Stresses. Genes 2020, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Tan, Z.; Fang, T.; Tang, K.; Liang, K.; Qiu, F. A Comprehensive Transcriptomics Analysis Reveals Long Non-Coding RNA to be Involved in the Key Metabolic Pathway in Response to Waterlogging Stress in Maize. Genes 2020, 11, 267. [Google Scholar] [CrossRef]
- Lian, G.; Ding, Z.; Wang, Q.; Zhang, D.; Xu, J. Origins and evolution of WUSCHEL-related homeobox protein family in plant kingdom. Sci. World J. 2014, 2014, 534140. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef]
- Lenhard, M.; Bohnert, A.; Jurgens, G.; Laux, T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 2001, 105, 805–814. [Google Scholar] [CrossRef]
- Scanlon, M.J.; Chen, K.D.; McKnight, C.I. The narrow sheath duplicate genes: Sectors of dual aneuploidy reveal ancestrally conserved gene functions during maize leaf development. Genetics 2000, 155, 1379–1389. [Google Scholar] [CrossRef]
- Aguirre, L.; Hendelman, A.; Hutton, S.F.; McCandlish, D.M.; Lippman, Z.B. Idiosyncratic and dose-dependent epistasis drives variation in tomato fruit size. Science 2023, 382, 315–320. [Google Scholar] [CrossRef]
- Wang, K.; Shi, L.; Liang, X.; Zhao, P.; Wang, W.; Liu, J.; Chang, Y.; Hiei, Y.; Yanagihara, C.; Du, L.; et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat. Plants 2022, 8, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Cao, Y.; Chen, X.; Wang, H.; Zhu, B.; Du, X.; Sun, Y. The Genome-Wide Identification, Characterization, and Expression Analysis of the Strictosidine Synthase-like Family in Maize (Zea mays L.). Int. J. Mol. Sci. 2023, 24, 14733. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Jeffryes, M.; Bateman, A.; Finn, R.D. The HMMER Web Server for Protein Sequence Similarity Search. Curr. Protoc. Bioinform. 2017, 60, 3-15-1–3-15-23. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Gu, L.; Chen, X.; Hou, Y.; Wang, H.; Wang, H.; Zhu, B.; Du, X. ZmWRKY70 activates the expression of hypoxic responsive genes in maize and enhances tolerance to submergence in Arabidopsis. Plant Physiol. Biochem. 2023, 201, 107861. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zuo, D.; Hu, M.; Zhou, W.; Lei, F.; Zhao, J.; Gu, L. EcAGL enhances cadmium tolerance in transgenic Arabidopsis thaliana through inhibits cadmium transport and ethylene synthesis pathway. Plant Physiol. Biochem. 2023, 201, 107900. [Google Scholar] [CrossRef] [PubMed]






| MazieGDB ID | Gene Name | CDS (bp) | Protein Size (aa) | MW (kDa) | pI | Subcellular Location |
|---|---|---|---|---|---|---|
| Zm00001eb015500 | ZmWOX1 | 945 | 314 | 32.01 | 6.31 | Nucleus |
| Zm00001eb067310 | ZmWOX2 | 963 | 320 | 33.09 | 8.21 | Chloroplast |
| Zm00001eb092480 | ZmWOX3 | 789 | 262 | 27.84 | 8.37 | Nucleus |
| Zm00001eb147630 | ZmWOX4 | 666 | 221 | 24.76 | 8.36 | Nucleus |
| Zm00001eb148390 | ZmWOX5 | 975 | 324 | 34.81 | 9.16 | Nucleus |
| Zm00001eb149680 | ZmWOX6 | 822 | 273 | 30.39 | 6.06 | Nucleus |
| Zm00001eb157360 | ZmWOX7 | 1551 | 516 | 52.90 | 7.15 | Nucleus |
| Zm00001eb180280 | ZmWOX8 | 252 | 83 | 10.01 | 10.52 | Cytosol/Nucleus |
| Zm00001eb197430 | ZmWOX9 | 399 | 132 | 14.92 | 9.85 | Mitochondrion |
| Zm00001eb265710 | ZmWOX10 | 777 | 258 | 27.47 | 7.13 | Nucleus |
| Zm00001eb280440 | ZmWOX11 | 381 | 126 | 14.26 | 9.55 | Nucleus |
| Zm00001eb295920 | ZmWOX12 | 1518 | 505 | 53.26 | 7.21 | Nucleus |
| Zm00001eb330990 | ZmWOX13 | 780 | 259 | 27.52 | 7.02 | Nucleus |
| Zm00001eb355310 | ZmWOX14 | 525 | 174 | 19.38 | 9.60 | Nucleus |
| Zm00001eb359810 | ZmWOX15 | 1545 | 514 | 53.24 | 7.27 | Mitochondrion |
| Zm00001eb367200 | ZmWOX16 | 708 | 235 | 26.47 | 9.72 | Nucleus |
| Zm00001eb367990 | ZmWOX17 | 1026 | 341 | 36.77 | 9.18 | Nucleus |
| Zm00001eb368970 | ZmWOX18 | 849 | 282 | 30.98 | 6.46 | Nucleus |
| Zm00001eb395430 | ZmWOX19 | 948 | 315 | 32.41 | 6.84 | Nucleus |
| Zm00001eb414580 | ZmWOX20 | 885 | 294 | 31.37 | 7.91 | Nucleus |
| Zm00001eb432140 | ZmWOX21 | 753 | 250 | 27.72 | 8.63 | Chloroplast |
| Zm00001eb433010 | ZmWOX22 | 978 | 325 | 33.17 | 5.56 | Chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hou, Y.; Cao, Y.; Wei, B.; Gu, L. A Comprehensive Identification and Expression Analysis of the WUSCHEL Homeobox-Containing Protein Family Reveals Their Special Role in Development and Abiotic Stress Response in Zea mays L. Int. J. Mol. Sci. 2024, 25, 441. https://doi.org/10.3390/ijms25010441
Chen X, Hou Y, Cao Y, Wei B, Gu L. A Comprehensive Identification and Expression Analysis of the WUSCHEL Homeobox-Containing Protein Family Reveals Their Special Role in Development and Abiotic Stress Response in Zea mays L. International Journal of Molecular Sciences. 2024; 25(1):441. https://doi.org/10.3390/ijms25010441
Chicago/Turabian StyleChen, Xuanxuan, Yunyan Hou, Yongyan Cao, Bo Wei, and Lei Gu. 2024. "A Comprehensive Identification and Expression Analysis of the WUSCHEL Homeobox-Containing Protein Family Reveals Their Special Role in Development and Abiotic Stress Response in Zea mays L." International Journal of Molecular Sciences 25, no. 1: 441. https://doi.org/10.3390/ijms25010441




