Over-Production of the Human SLC7A10 in E. coli and Functional Assay in Proteoliposomes
Abstract
:1. Introduction
2. Results
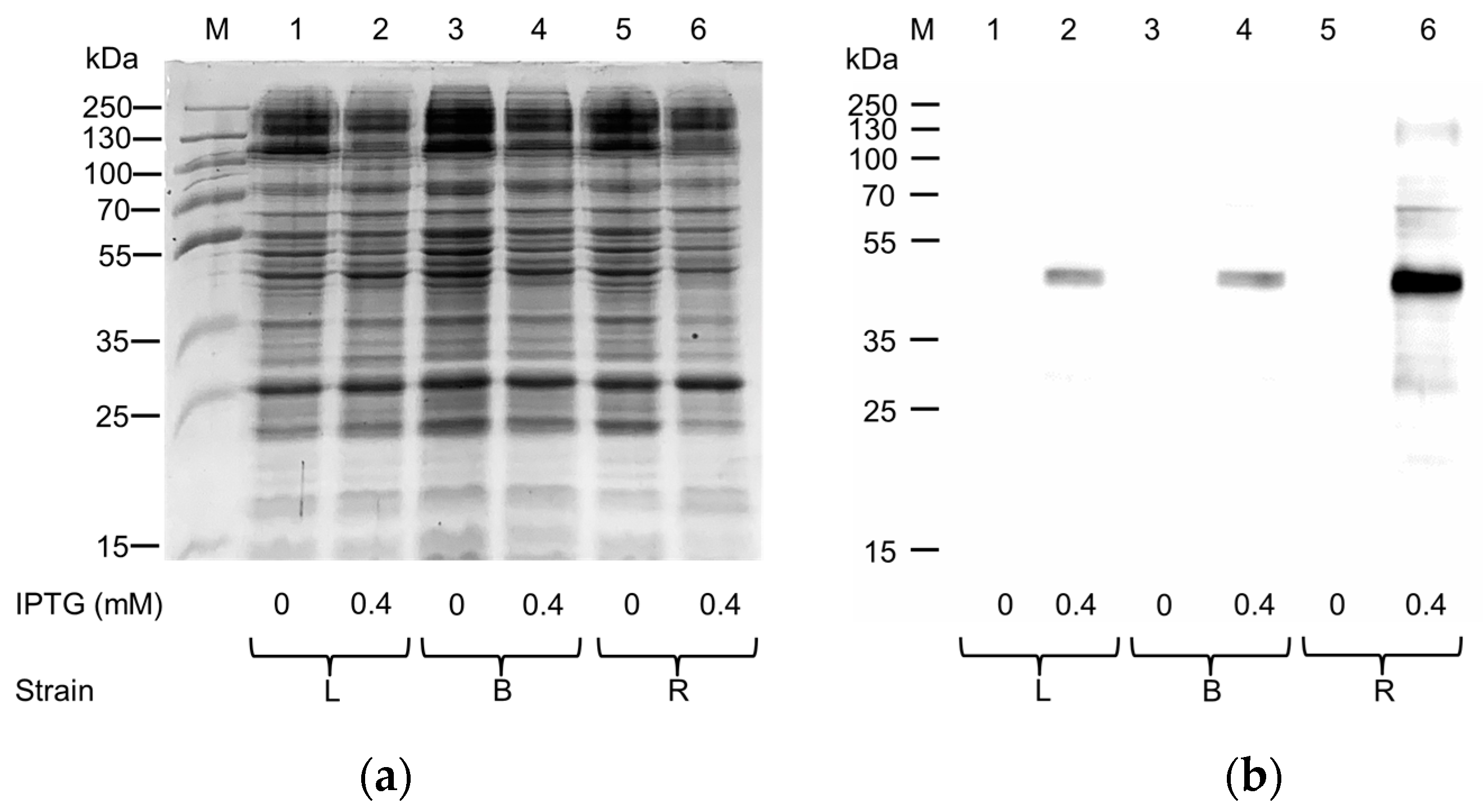
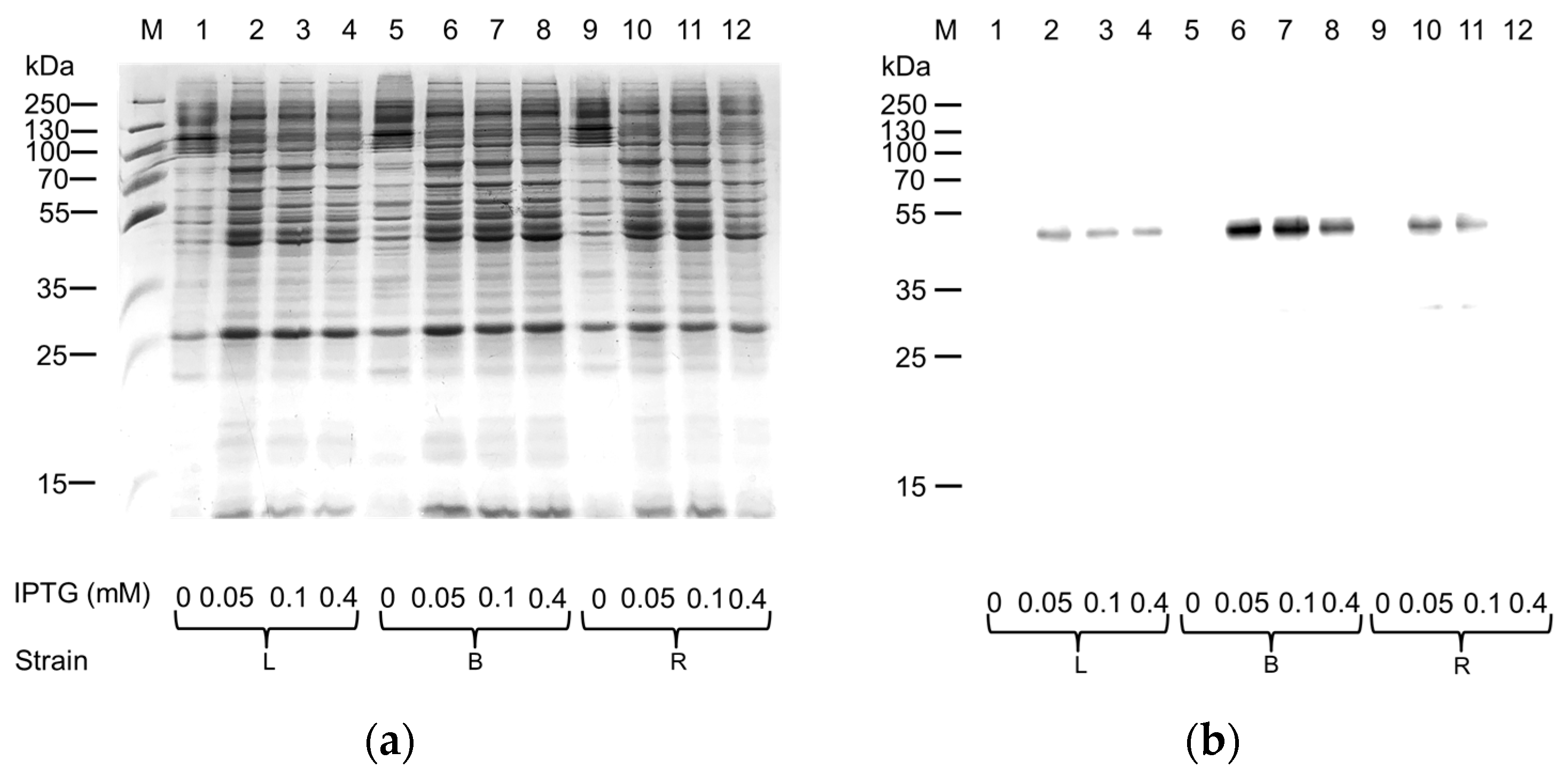
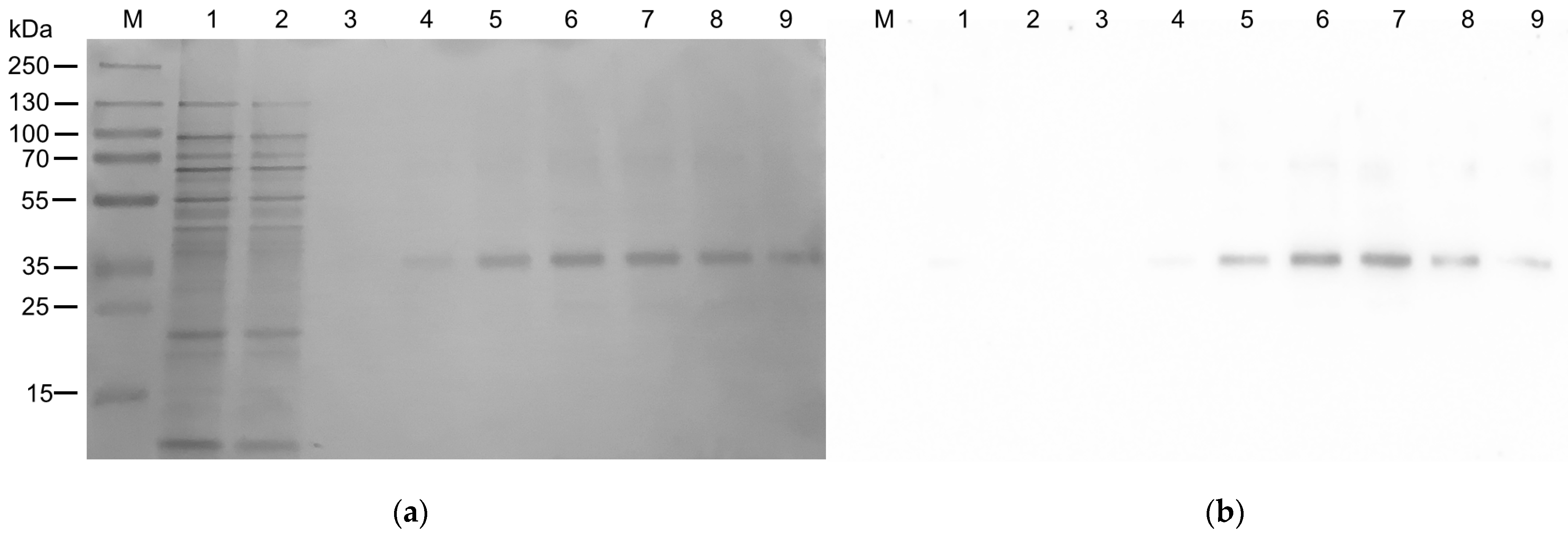
2.1. Expression in E. coli and Purification of hASC-1 Transporter
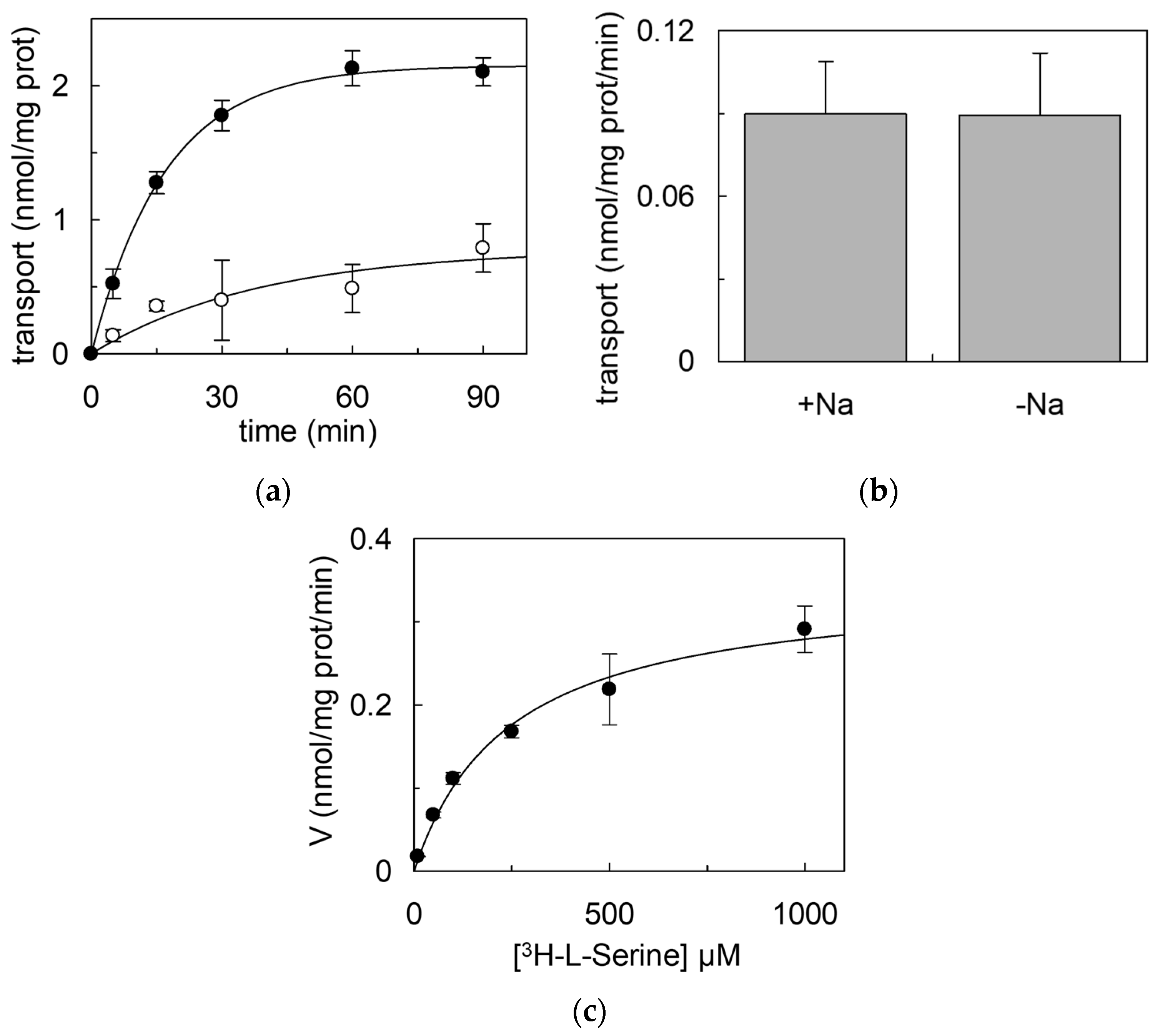
2.2. Reconstitution in Proteoliposomes of hASC-1
3. Discussion
3.1. Expression of ASC-1
3.2. Reconstitution of ASC-1
4. Materials and Methods
4.1. Cloning of Human hASC-1
4.2. Expression of Human ASC-1 Transporter
Effect of O2 on Protein Expression
4.3. Protein Quantification and Analysis
4.4. Purification of the hASC-1 Protein
4.5. Reconstitution of hASC-1 in Proteoliposomes
4.6. Transport Measurements in Proteoliposomes Reconstituted with hASC-1
4.7. Western Blotting
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fotiadis, D.; Kanai, Y.; Palacin, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, J.; Matsuo, H.; Kim, D.K.; Goto, A.; Chairoungdua, A.; Cha, S.H.; Inatomi, J.; Shiokawa, Y.; Yamaguchi, K.; Saito, I.; et al. Cloning and characterization of a human brain Na(+)-independent transporter for small neutral amino acids that transports D-serine with high affinity. Neurosci. Lett. 2000, 287, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tsien, J.Z. Memory and the NMDA receptors. N. Engl. J. Med. 2009, 361, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, I.R.; Conde-Ceide, S.; de Lucas, A.I.; Garci, A.M.A.; Trabanco, A.A.; Lavreysen, H.; Pardo, L.; Tresadern, G. Inhibition of the Alanine-Serine-Cysteine-1 Transporter by BMS-466442. ACS Chem. Neurosci. 2019, 10, 2510–2517. [Google Scholar] [CrossRef] [PubMed]
- Ussar, S.; Lee, K.Y.; Dankel, S.N.; Boucher, J.; Haering, M.F.; Kleinridders, A.; Thomou, T.; Xue, R.; Macotela, Y.; Cypess, A.M.; et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 2014, 6, 247ra103. [Google Scholar] [CrossRef]
- Small, K.S.; Hedman, A.K.; Grundberg, E.; Nica, A.C.; Thorleifsson, G.; Kong, A.; Thorsteindottir, U.; Shin, S.Y.; Richards, H.B.; TheGiantConsortium; et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat. Genet. 2011, 43, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Jersin, R.A.; Tallapragada, D.S.P.; Madsen, A.; Skartveit, L.; Fjaere, E.; McCann, A.; Lawrence-Archer, L.; Willems, A.; Bjune, J.I.; Bjune, M.S.; et al. Role of the Neutral Amino Acid Transporter SLC7A10 in Adipocyte Lipid Storage, Obesity, and Insulin Resistance. Diabetes 2021, 70, 680–695. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Drehmann, P.; Milanos, S.; Schaefer, N.; Kasaragod, V.; Herterich, S.; Holzbach-Eberle, U.; Harvey, R.J.; Villmann, C. Dual role of dysfunctional Asc-1 transporter in distinct human pathologies—Human startle disease and developmental delay. eNeuro 2023, 10, 263. [Google Scholar] [CrossRef]
- Zafra, F.; Ibanez, I.; Bartolome-Martin, D.; Piniella, D.; Arribas-Blazquez, M.; Gimenez, C. Glycine Transporters and Its Coupling with NMDA Receptors. Adv. Neurobiol. 2017, 16, 55–83. [Google Scholar] [PubMed]
- Sakimura, K.; Nakao, K.; Yoshikawa, M.; Suzuki, M.; Kimura, H. A novel Na(+) -Independent alanine-serine-cysteine transporter 1 inhibitor inhibits both influx and efflux of D-Serine. J. Neurosci. Res. 2016, 94, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Mikou, A.; Cabaye, A.; Goupil, A.; Bertrand, H.O.; Mothet, J.P.; Acher, F.C. Asc-1 Transporter (SLC7A10): Homology Models and Molecular Dynamics Insights into the First Steps of the Transport Mechanism. Sci. Rep. 2020, 10, 3731. [Google Scholar] [CrossRef] [PubMed]
- Errasti-Murugarren, E.; Fort, J.; Bartoccioni, P.; Diaz, L.; Pardon, E.; Carpena, X.; Espino-Guarch, M.; Zorzano, A.; Ziegler, C.; Steyaert, J.; et al. L amino acid transporter structure and molecular bases for the asymmetry of substrate interaction. Nat. Commun. 2019, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Galluccio, M.; Console, L.; Pochini, L.; Scalise, M.; Giangregorio, N.; Indiveri, C. Strategies for Successful Over-Expression of Human Membrane Transport Systems Using Bacterial Hosts: Future Perspectives. Int. J. Mol. Sci. 2022, 23, 3823. [Google Scholar] [CrossRef] [PubMed]
- Galluccio, M.; Scalise, M.; Pappacoda, G.; Scarpelli, M.; Bonanomi, M.; Gaglio, D.; Indiveri, C. Production of recombinant human xCT (SLC7A11) and reconstitution in proteoliposomes for functional studies. Front. Physiol. 2022, 13, 993626. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Meury, M.; Alvarez-Marimon, E.; Costa, M.; Perez-Cano, L.; Zorzano, A.; Fernandez-Recio, J.; Palacin, M.; Fotiadis, D. Structural bases for the interaction and stabilization of the human amino acid transporter LAT2 with its ancillary protein 4F2hc. Proc. Natl. Acad. Sci. USA 2014, 111, 2966–2971. [Google Scholar] [CrossRef]
- Cosco, J.; Scalise, M.; Colas, C.; Galluccio, M.; Martini, R.; Rovella, F.; Mazza, T.; Ecker, G.F.; Indiveri, C. ATP modulates SLC7A5 (LAT1) synergistically with cholesterol. Sci. Rep. 2020, 10, 16738. [Google Scholar] [CrossRef]
- Galluccio, M.; Pantanella, M.; Giudice, D.; Brescia, S.; Indiveri, C. Low temperature bacterial expression of the neutral amino acid transporters SLC1A5 (ASCT2), and SLC6A19 (B0AT1). Mol. Biol. Rep. 2020, 47, 7283–7289. [Google Scholar] [CrossRef]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular basis for redox control by the human cystine/glutamate antiporter system xc(). Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef]
- Galluccio, M.; Mazza, T.; Scalise, M.; Sarubbi, M.C.; Indiveri, C. Bacterial over-expression of functionally active human CT2 (SLC22A16) carnitine transporter. Mol. Biol. Rep. 2022, 49, 8185–8193. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, J.L.; Levy, D. Reconstitution of membrane proteins into liposomes. Methods Enzymol. 2003, 372, 65–86. [Google Scholar] [PubMed]
- Hering, J.; Missel, J.W.; Zhang, L.; Gunnarsson, A.; Castaldo, M.; Pedersen, P.A.; Ek, M.; Gourdon, P.; Snijder, H.J. The rapid “teabag” method for high-end purification of membrane proteins. Sci. Rep. 2020, 10, 16167. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, M.V.; Findlay, H.E.; Booth, P.J. Detergent-free purification and reconstitution of functional human serotonin transporter (SERT) using diisobutylene maleic acid (DIBMA) copolymer. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183602. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.A.; Jarrott, R.J.; Whitten, A.E.; Choudhury, H.G.; Drew, D.; Martin, J.L. Heterologous Expression and Biochemical Characterization of the Human Zinc Transporter 1 (ZnT1) and Its Soluble C-Terminal Domain. Front. Chem. 2021, 9, 667803. [Google Scholar] [CrossRef] [PubMed]
- Hresko, R.C.; Kraft, T.E.; Quigley, A.; Carpenter, E.P.; Hruz, P.W. Mammalian Glucose Transporter Activity Is Dependent upon Anionic and Conical Phospholipids. J. Biol. Chem. 2016, 291, 17271–17282. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Cameron, A.; Newstead, S.; Omote, H.; Moriyama, Y.; Kasahara, M.; Iwata, S.; Drew, D. Tricks of the trade used to accelerate high-resolution structure determination of membrane proteins. FEBS Lett. 2010, 584, 2539–2547. [Google Scholar] [CrossRef]
- James, Z.M.; Borst, A.J.; Haitin, Y.; Frenz, B.; DiMaio, F.; Zagotta, W.N.; Veesler, D. CryoEM structure of a prokaryotic cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 2017, 114, 4430–4435. [Google Scholar] [CrossRef]
- Tanaka, Y.; Iwaki, S.; Tsukazaki, T. Crystal Structure of a Plant Multidrug and Toxic Compound Extrusion Family Protein. Structure 2017, 25, 1455–1460 e1452. [Google Scholar] [CrossRef]
- Rehan, S.; Paavilainen, V.O.; Jaakola, V.P. Functional reconstitution of human equilibrative nucleoside transporter-1 into styrene maleic acid co-polymer lipid particles. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1059–1065. [Google Scholar] [CrossRef]
- Choy, B.C.; Cater, R.J.; Mancia, F.; Pryor, E.E., Jr. A 10-year meta-analysis of membrane protein structural biology: Detergents, membrane mimetics, and structure determination techniques. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183533. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, C.; Strauss, J.; Stohrer, C.; Naylor, C.; Pryor, E.; Hobbs, J.; Tanley, S.; Goldman, A.; Byrne, B. A novel high-throughput screen for identifying lipids that stabilise membrane proteins in detergent based solution. PLoS ONE 2021, 16, e0254118. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.A.; Liu, J.J.; Chun, E.; Wacker, D.; Wu, H.; Cherezov, V.; Stevens, R.C. GPCR stabilization using the bicelle-like architecture of mixed sterol-detergent micelles. Methods 2011, 55, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Cosco, J.; Regina, T.M.R.; Scalise, M.; Galluccio, M.; Indiveri, C. Regulatory Aspects of the Vacuolar CAT2 Arginine Transporter of S. lycopersicum: Role of Osmotic Pressure and Cations. Int. J. Mol. Sci. 2019, 20, 906. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Epand, R.M.; Barrantes, F.J. Cholesterol-Recognition Motifs in Membrane Proteins. Adv. Exp. Med. Biol. 2019, 1135, 3–25. [Google Scholar] [PubMed]
- Stieger, B.; Steiger, J.; Locher, K.P. Membrane lipids and transporter function. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166079. [Google Scholar] [CrossRef]
- Dulley, J.R.; Grieve, P.A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal. Biochem. 1975, 64, 136–141. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluccio, M.; Mazza, T.; Scalise, M.; Tripicchio, M.; Scarpelli, M.; Tolomeo, M.; Pochini, L.; Indiveri, C. Over-Production of the Human SLC7A10 in E. coli and Functional Assay in Proteoliposomes. Int. J. Mol. Sci. 2024, 25, 536. https://doi.org/10.3390/ijms25010536
Galluccio M, Mazza T, Scalise M, Tripicchio M, Scarpelli M, Tolomeo M, Pochini L, Indiveri C. Over-Production of the Human SLC7A10 in E. coli and Functional Assay in Proteoliposomes. International Journal of Molecular Sciences. 2024; 25(1):536. https://doi.org/10.3390/ijms25010536
Chicago/Turabian StyleGalluccio, Michele, Tiziano Mazza, Mariafrancesca Scalise, Martina Tripicchio, Martina Scarpelli, Maria Tolomeo, Lorena Pochini, and Cesare Indiveri. 2024. "Over-Production of the Human SLC7A10 in E. coli and Functional Assay in Proteoliposomes" International Journal of Molecular Sciences 25, no. 1: 536. https://doi.org/10.3390/ijms25010536
APA StyleGalluccio, M., Mazza, T., Scalise, M., Tripicchio, M., Scarpelli, M., Tolomeo, M., Pochini, L., & Indiveri, C. (2024). Over-Production of the Human SLC7A10 in E. coli and Functional Assay in Proteoliposomes. International Journal of Molecular Sciences, 25(1), 536. https://doi.org/10.3390/ijms25010536








