Comprehensive Analysis of Highbush Blueberry Plants Propagated In Vitro and Conventionally
Abstract
:1. Introduction
2. Results
2.1. Plant Analysis
Morphological Measurements
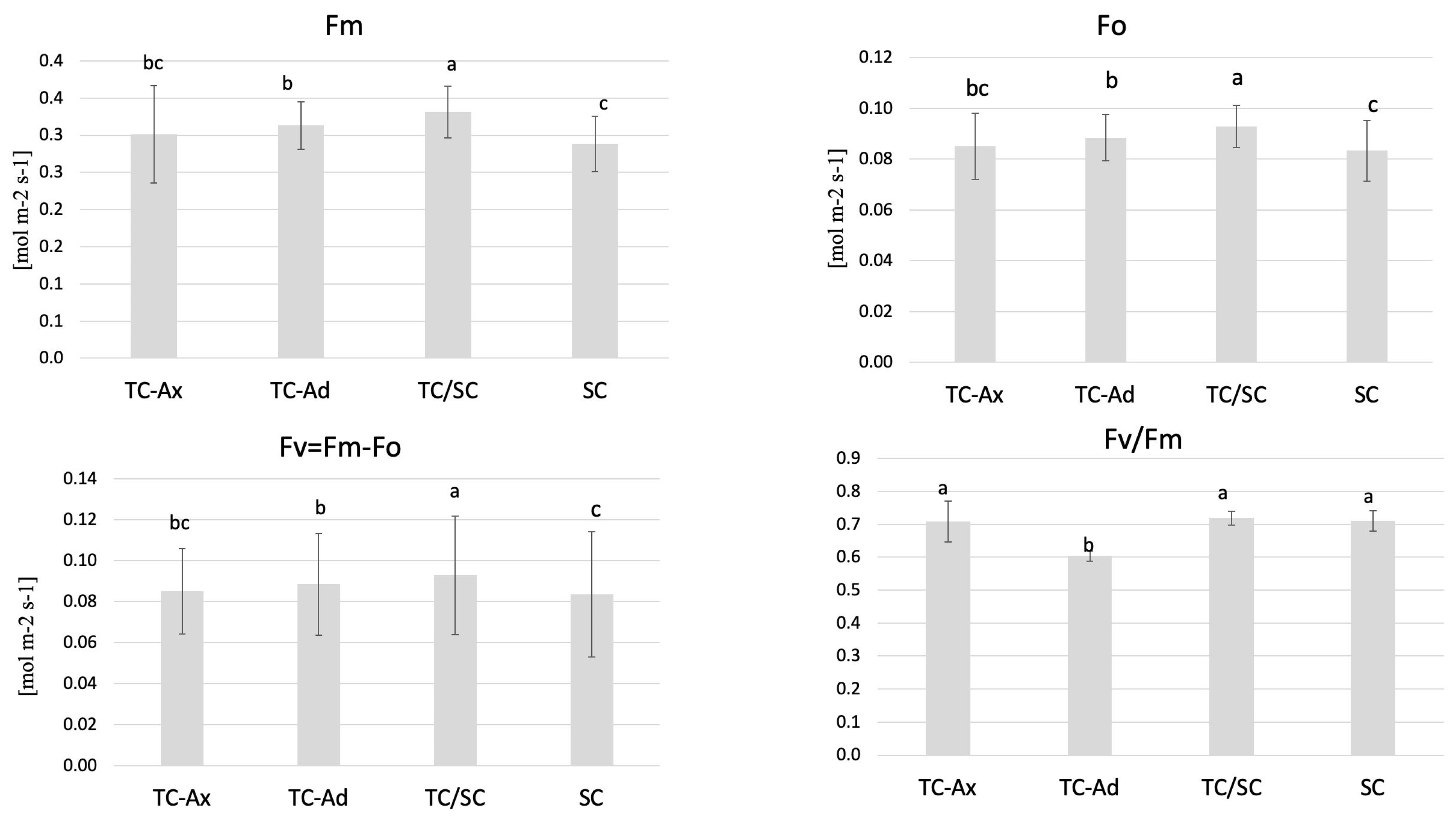
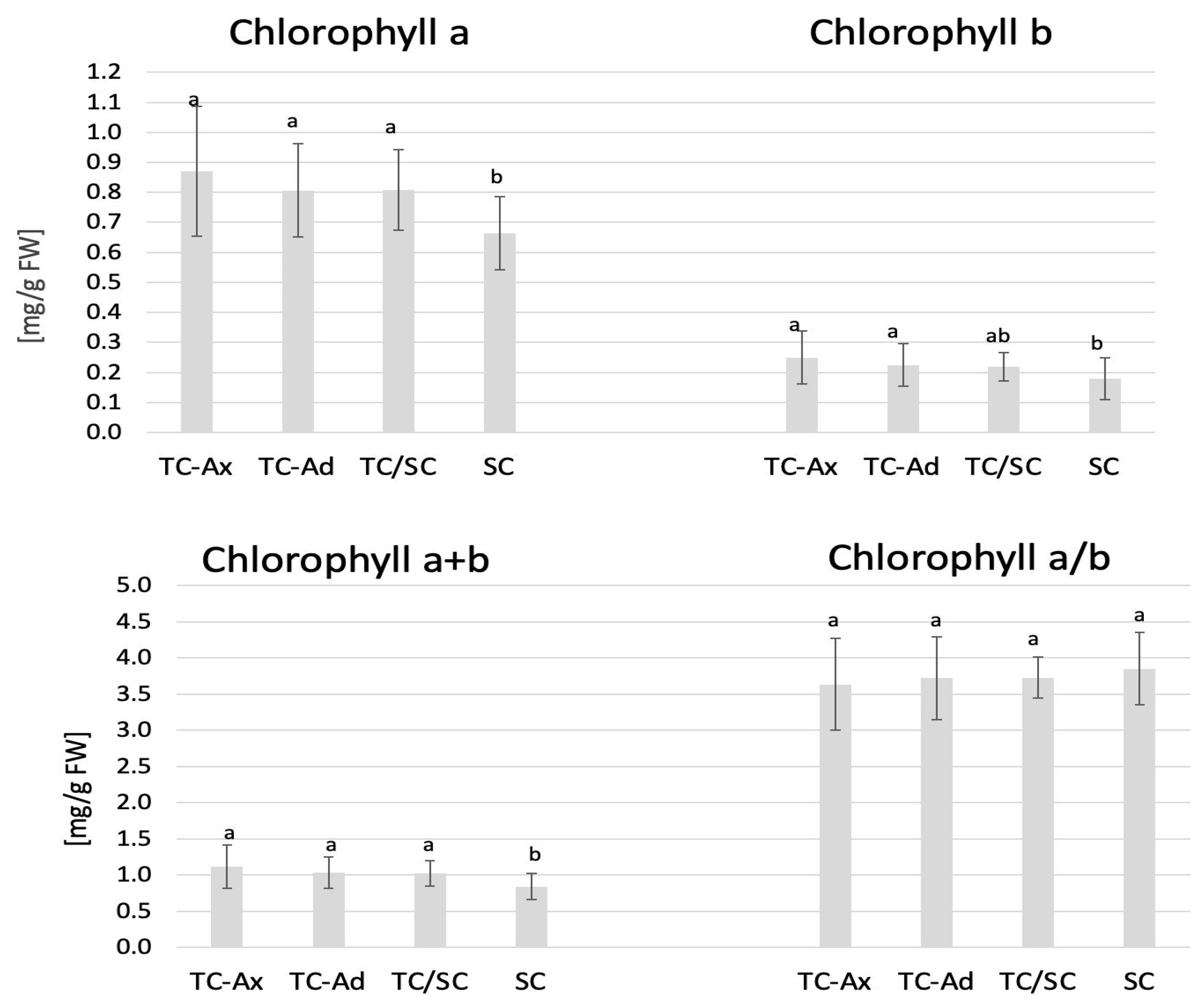
2.2. Chlorophyll Content and Fluorescence
2.3. Fruit Analysis
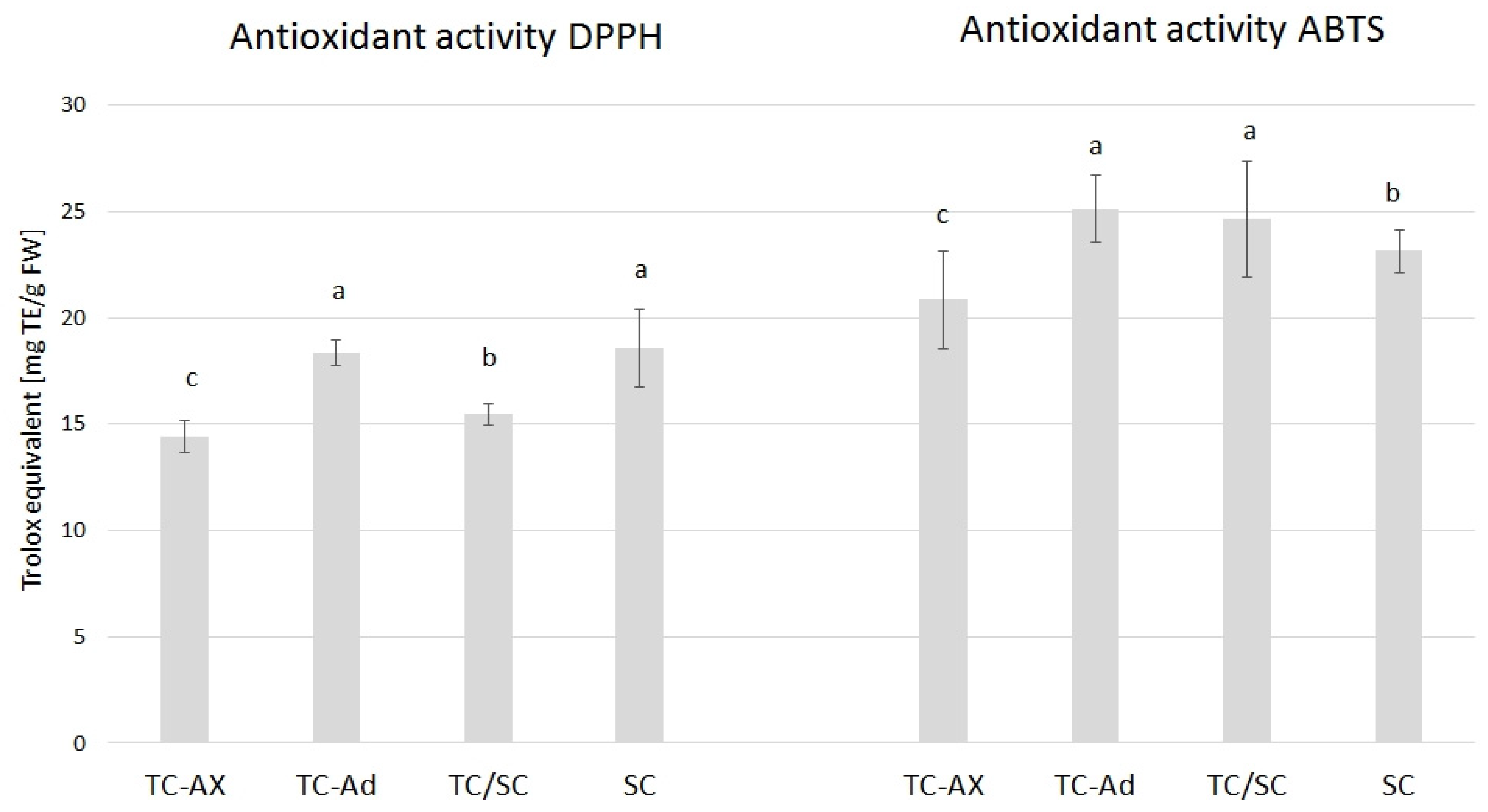
2.3.1. Antioxidant Activity
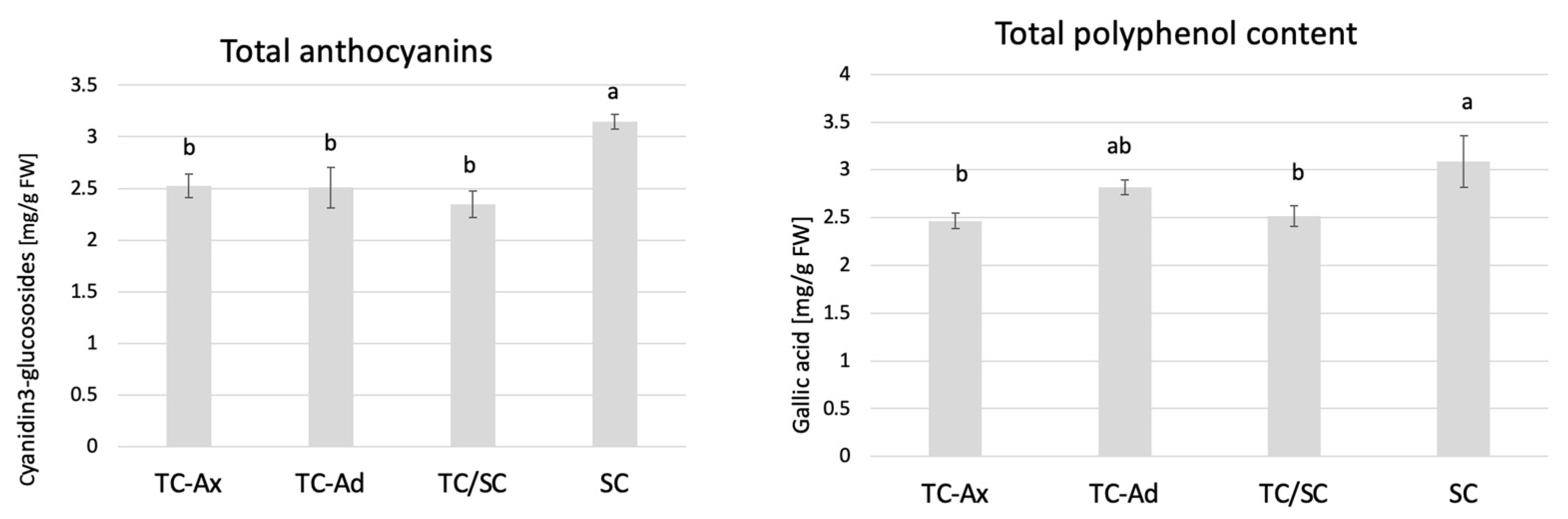
2.3.2. Polyphenols and Anthocyanins
2.3.3. Ascorbic Acid
2.3.4. DNA Methylation
3. Discussion
4. Materials and Methods
4.1. In Vitro Conditions
4.2. In Vivo and Field Conditions
4.3. Plant Analysis
4.3.1. Morphological Measurements
4.3.2. Chlorophyll Content and Fluorescence
- -
- chlorophyll a = [(12.9 · A663) − (3.45 · A649)].
- -
- Chlorophyll b = [(21.91 · A649) – (5.32 · A663).]
4.4. DNA Methylation
Methylation Analysis
4.5. Fruit Analysis
4.5.1. Antioxidant Activity
4.5.2. Total Polyphenol and Anthocyanin Contents
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, G.; Cavenago, B.; Bulgari, R.; Spinardi, A. Benzothiadiazole Enhances Ascorbate Recycling and Polyphenols Accumulation in Blueberry in a Cultivar-Dependent Manner. Front. Plant Sci. 2022, 13, 1032133. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Skóra, B.; Grzelak-Błaszczyk, K.; Sójka, M. Extraction of Antioxidant Compounds from Blueberry Fruit Waste and Evaluation of Their In Vitro Biological Activity in Human Keratinocytes (HaCaT). Food Anal. Methods 2021, 14, 2317–2327. [Google Scholar] [CrossRef]
- Piechowiak, T.; Antos, P.; Józefczyk, R.; Kosowski, P.; Skrobacz, K.; Balawejder, M. Impact of Ozonation Process on the Microbiological Contamination and Antioxidant Capacity of Highbush Blueberry (Vaccinum corymbosum L.) Fruit during Cold Storage. Ozone Sci. Eng. 2019, 41, 376–385. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Huang, C.; Cheng, M. Dietary Blueberry and Bifidobacteria Attenuate Nonalcoholic Fatty Liver Disease in Rats by Affecting SIRT1-Mediated Signaling Pathway. Oxid. Med. Cell Longev. 2014, 2014, 469059. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Lau, F.C.; Josep, J.A. Berry Fruit Supplementation and the Aging Brain. J. Agric. Food Chem. 2008, 56, 636–641. [Google Scholar] [CrossRef]
- Papandreou, M.A.; Dimakopoulou, A.; Linardaki, Z.I.; Cordopatis, P.; Klimis-Zacas, D.; Margarity, M.; Lamari, F.N. Effect of a Polyphenol-Rich Wild Blueberry Extract on Cognitive Performance of Mice, Brain Antioxidant Markers and Acetylcholinesterase Activity. Behav. Brain Res. 2009, 198, 352–358. [Google Scholar] [CrossRef]
- Cheatham, C.L.; Vazquez-Vidal, I.; Medlin, A.; Voruganti, V.S. Blueberry Consumption Affects Serum Uric Acid Concentrations in Older Adults in a Sex-Specific Manner. Antioxidants 2016, 5, 43. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry Anthocyanidins Synergistically Suppress Growth and Invasive Potential of Human Non-Small-Cell Lung Cancer Cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, H.; Zheng, X.; Chen, W. Blueberry and Mulberry Juice Prevent Obesity Development in C57BL/6 Mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry Fruit Valorization and Valuable Constituents: A Review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Q.; Hancock, J.F. Vaccinium. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 197–222. [Google Scholar]
- Goyali, J.C.; Igamberdiev, A.U.; Debnath, S.C. Propagation Methods Affect Fruit Morphology and Antioxidant Properties but Maintain Clonal Fidelity in Lowbush Blueberry. HortScience 2015, 50, 888–896. [Google Scholar] [CrossRef]
- Pliszka, K. Borówka Wysoka; Państwowe Wydawnictwo Rolnicze I Leśne: Warszawa, Poland, 2002. [Google Scholar]
- Cohen, D.; Eliot, D. Micropropagation Methods for Blueberries and Tamarillos. Int. Plant Propagators’ Soc. 1979, 29, 144–146. [Google Scholar]
- Zimmerman, R.H.; Broome, O.C. Blueberry Micropropagatio. In Proceedings of the Conference on Nursery Production of Fruit Plants through Tissue Culture-Applications and Feasibility; Agricultural Research Results ARR-NE; Zimmerman, Ed.; USDASEA: Tucson, AZ, USA, 1980; pp. 44–47. [Google Scholar]
- Debnath, S.C. Clonal Fidelity and Morphological and Chemical Variations in Micropropagated Vaccinium Plants. Acta Hortic. 2017, 1180, 111–116. [Google Scholar] [CrossRef]
- Mohamed, G.; Khusnetdinova, L.; Timofeeva, O. Elaboration of Micropropagation Protocol for Vaccinium Corymbosum Cv. “Sunt Blue Giant.”. Asian J. Plant Sci. Res. 2018, 8, 1–11. [Google Scholar]
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant In Vitro Culture; Delve Publishing: Burlington, ON, Canada, 2012. [Google Scholar]
- Kumar, R.; Raturi, P.; Jat, M.J. Plant Propagation: Importance and Scope. In Plant Propagation Andnursery Management for Fruit Crops; NIPA, Genx Electronic Resources & Solutions P. LTD: New Delhi, India, 2023; pp. 1–15. [Google Scholar]
- Sharma, K. Plant Tissue Culture and Micropropagation. In Plant Propagation Andnursery Management for Fruit Crops; Genx Electronic Resources & Solutions P. LTD: New Delhi, India, 2023; pp. 83–95. [Google Scholar]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal Variation—A Novel Source of Variability from Cell Cultures for Plant Improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic Aspects of Somaclonal Variation in Plants. Plant Mol. Biol. 2000, 43, 179–188. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Phillips, R.L.; Olhoft, P. Molecular Basis of Heritable Tissue Culture-Induced Variation in Plants. Somaclonal Var. Induc. Mutat. Crop Improv. 1998, 465–484. [Google Scholar]
- Duta-Cornescu, G.; Constantin, N.; Pojoga, D.M.; Nicuta, D.; Simon-Gruita, A. Somaclonal Variation—Advantage or Disadvantage in Micropropagation of the Medicinal Plants. Int. J. Mol. Sci. 2023, 24, 838. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Tissue Culture-Induced DNA Methylation in Crop Plants: A Review. Mol. Biol. Rep. 2021, 48, 823–841. [Google Scholar] [CrossRef] [PubMed]
- Bairu, M.W.; Aremu, A.O.; van Staden, J. Somaclonal Variation in Plants: Causes and Detection Methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Jain, S.M. Tissue Culture-Derived Variation in Crop Improvement. Euphytica 2001, 118, 153–166. [Google Scholar] [CrossRef]
- Leva, A.R.; Petruccelli, R.; Rinaldi, L.M.R. Somaclonal Variation in Tissue Culture: A Case Study with Olive. In Recent Advances in Plant In Vitro Culture; INTECH Open Access Publisher: Croatia, Balkans, 2012. [Google Scholar]
- Bhatia, R.; Singh, K.P.; Jhang, T.; Sharma, T.R. Assessment of Clonal Fidelity of Micropropagated Gerbera Plants by ISSR Markers. Sci. Hortic. 2009, 119, 208–211. [Google Scholar] [CrossRef]
- Kawiak, A.; Łojkowska, E. Application of RAPD in the Determination of Genetic Fidelity in Micropropagated Drosera Plantlets. Vitr. Cell. Dev. Biol.-Plant 2004, 40, 592–595. [Google Scholar] [CrossRef]
- Skirvin, R.M.; McPheeters, K.D.; Norton, M. Sources and Frequency of Somaclonal Variation. HortScience 2019, 29, 1232–1237. [Google Scholar] [CrossRef]
- Bouman, H.; De Klerk, G.J. Measurement of the Extent of Somaclonal Variation in Begonia Plants Regenerated under Various Conditions. Comparison of Three Assays. Theor. Appl. Genet. 2001, 102, 111–117. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Szüčová, L.; Doležal, K.; Finnie, J.F.; Van Staden, J. Genetic Fidelity in Tissue-Cultured “Williams” Bananas—The Effect of High Concentration of Topolins and Benzyladenine. Sci. Hortic. 2013, 161, 324–327. [Google Scholar] [CrossRef]
- Razani, M.; Kayat, F.; Mohamed Re, R.; Susanto, D. Effect of Somaclonal Variation in Musa Acuminata Cv. Berangan through Micropropagation Using RAPD. Biotechnology 2018, 18, 9–14. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R. Plant Tissue Culture Environment as a Switch-Key of (Epi)Genetic Changes. Plant Cell Tissue Organ. Cult. 2020, 140, 245–257. [Google Scholar] [CrossRef]
- Gimenez, M.D.; Yañez-Santos, A.M.; Paz, R.C.; Quiroga, M.P.; Marfil, C.F.; Conci, V.C.; García-Lampasona, S.C. Assessment of Genetic and Epigenetic Changes in Virus-Free Garlic (Allium Sativum L.) Plants Obtained by Meristem Culture Followed by in Vitro Propagation. Plant Cell Rep. 2016, 35, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, Y.; Gong, L.; Li, F.; Dong, Y.; Liu, B. Efficient Micropropagation of Robinia Ambigua Var. Idahoensis (Idaho Locust) and Detection of Genomic Variation by ISSR Markers. Plant Cell Tissue Organ. Cult. 2006, 84, 343–351. [Google Scholar] [CrossRef]
- Guo, W.L.; Gong, L.; Ding, Z.F.; Li, Y.D.; Li, F.X.; Zhao, S.P.; Liu, B. Genomic Instability in Phenotypically Normal Regenerants of Medicinal Plant Codonopsis Lanceolata Benth. et Hook. f., as Revealed by ISSR and RAPD Markers. Plant Cell Rep. 2006, 25, 896–906. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.-J. How to Measure Somaclonal Variation. Acta Bot. Neerl. 1990, 39, 129–144. [Google Scholar] [CrossRef]
- Debnath, S.C. Strategies to Propagate Vaccinium Nuclear Stocks for the Canadian Berry Industry. Can. J. Plant Sci. 2007, 87, 911–922. [Google Scholar] [CrossRef]
- Litwińczuk, W. Rozmnażanie Borówki Wysokiej (Vaccinium corymbosum L.) w Kulturach In Vitro. Wpływ Mikrorozmnażania Na Wzrost i Owocowanie Krzewów; Wydawnictwo Uniwersytetu Rzeszowskiego: Rzeszów, Poland, 2007. [Google Scholar]
- Jamieson, A.R.; Nickerson, N.L. Field Performance of the Lowbush Blueberry Propagated by Seed, Stem Cuttings and Micropropagation. Proc. Acta Hortic. 2003, 626, 423–428. [Google Scholar] [CrossRef]
- Goyali, J.C.; Igamberdiev, A.U.; Debnath, S.C. Morphology, Phenolic Content and Antioxidant Capacity of Lowbush Blueberry (Vaccinium angustifolium Ait.) Plants as Affected by in Vitro and Ex Vitro Propagation Methods. Can. J. Plant Sci. 2013, 93, 1001–1008. [Google Scholar] [CrossRef]
- Morrison, S.; Smagula, J.M.; Litten, W. Morphology, Growth, and Rhizome Development of Vaccinium Angustifolium Ait. Seedlings, Rooted Softwood Cuttings, and Micropropagated Plantlets. HortScience 2000, 35, 738–741. [Google Scholar] [CrossRef]
- Foley, S.L.; Debnath, S.C. Influence of in Vitro and Ex. Vitro Propagation on Anthocyanin Content and Anti-Oxidant Activity of Lingonberries. J. Hortic. Sci. Biotechnol. 2007, 82, 114–118. [Google Scholar] [CrossRef]
- Peraza-Echeverria, S.; Herrera-Valencia, V.A.; Kay, A.J. Detection of DNA Methylation Changes in Micropropagated Banana Plants Using Methylation-Sensitive Amplification Polymorphism (MSAP). Plant Sci. 2001, 161, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kimatu, J.N.; Xu, K.; Liu, B. DNA Cytosine Methylation in Plant Development. J. Genet. Genom. 2010, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goyali, J.C.; Igamberdiev, A.U.; Debnath, S.C. DNA Methylation in Lowbush Blueberry (Vaccinium Angustifolium Ait.) Propagated by Softwood Cutting and Tissue Culture. Can. J. Plant Sci. 2018, 98, 1035–1044. [Google Scholar] [CrossRef]
- Xu, M.; Li, X.; Korban, S.S. AFLP-Based Detection of DNA Methylation. Plant Mol. Biol. Report. 2000, 18, 361–368. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Korban, S.S. DNA Methylation Profiles Differ between Fiel and in Vitro Grown Leaves of Apple. J. Plant Physiol. 2002, 159, 1229–1234. [Google Scholar] [CrossRef]
- Xiong, L.Z.; Xu, C.G.; Saghai Maroof, M.A.; Zhang, Q. Patterns of Cytosine Methylation in an Elite Rice Hybrid and Its Parental Lines, Detected by a Methylation-Sensitive Amplification Polymorphism Technique. Mol. General. Genet. 1999, 261, 439–446. [Google Scholar] [CrossRef]
- Litwińczuk, W.; Szczerba, G.; Wrona, D. Field Performance of Highbush Blueberries (Vaccinium×corymbosum L.) Cv. ‘Herbert’ Propagated by Cuttings and Tissue Culture. Sci. Hortic. 2005, 106, 162–169. [Google Scholar] [CrossRef]
- Mazurek, M.; Siekierzyńska, A.; Jacek, B.; Litwińczuk, W. Differences in Response to Drought Stress among Highbush Blueberry Plants Propagated Conventionally and by Tissue Culture. Plant Biosyst. 2021, 155, 172–178. [Google Scholar] [CrossRef]
- Debnath, S.C.; Ghosh, A. Phenotypic Variation and Epigenetic Insight into Tissue Culture Berry Crops. Front. Plant Sci. 2022, 13, 1042726. [Google Scholar] [CrossRef]
- Walder, R.Y.; Langtimm, C.J.; Chaterjee, R.; Walder, J.A. Cloning of the MspI modification enzyme. The site of modification andits effect on cleavage by MspI and HpaII. J. Biol. Chem. 1982, 258, 1235–1241. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal Variations and Their Applications in Horticultural Crops Improvement. Biotech 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Grout, J.M.; Read, P.E.; Wildung, D.K. Influence of Tissue Culture and Leaf-Bud Propagation on the Growth Habit of ‘Northblue’ Blueberry. J. Am. Soc. Hortic. Sci. 2022, 111, 372–375. [Google Scholar] [CrossRef]
- El-Shiekh, A.; Wildung, D.K.; Luby, J.J.; Sargent, K.L.; Read, P.E. Long-Term Effects of Propagation by Tissue Culture or Softwood Single-Node Cuttings on Growth Habit, Yield, and Berry Weight of “northblue” Blueberry. J. Am. Soc. Hortic. Sci. 1996, 121, 339–342. [Google Scholar] [CrossRef]
- Debnath, S.C. Effects of Carbon Source and Concentration on Development of Lingonberry (Vaccinium Vitis-Idaea L.) Shoots Cultivated in Vitro from Nodal Explants. In Vitro Cell. Dev. Biol.-Plant 2005, 41, 145–150. [Google Scholar] [CrossRef]
- Gustavsson, B.A.; Stanys, V. Field Performance of “Sanna” Lingonberry Derived by Micropropagation vs. Stem Cuttings. HortScience 2000, 35, 742–744. [Google Scholar] [CrossRef]
- Debnath, S.C. Zeatin-Induced One-Step in Vitro Cloning Affects the Vegetative Growth of Cranberry (Vaccinium Macrocarpon Ait.) Micropropagules over Stem Cuttings. Plant Cell Tissue Organ. Cult. 2008, 93, 231–240. [Google Scholar] [CrossRef]
- Serres, R.; McCown, B. Rapid Flowering of Microcultured Cranberry Plants. HortScience 1994, 29, 159–161. [Google Scholar] [CrossRef]
- Dobránszki, J.; Mendler-Drienyovszki, N. Cytokinin-Induced Changes in the Chlorophyll Content and Fluorescence of in Vitro Apple Leaves. J. Plant Physiol. 2014, 171, 1472–1478. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Rodrigues, L.C.A.; Santos, E.R.; Batista, B.G.; Gontijo, A.B.P.L.; Falqueto, A.R. Anatomy and Photosystem II Activity of in Vitro Grown Aechmea Blanchetiana as Affected by 1-Naphthaleneacetic Acid. Biol. Plant 2018, 62, 211–221. [Google Scholar] [CrossRef]
- Stefanova, M.; Koleva, D.P.; Ganeva, T.G.; Dimitrova, M. Effect of Plant Growth Regulators on the Regeneration of in Vitro Propagated Lamium album L. Plants. Bulg. J. Agric. Sci. 2013, 19, 1208–1212. [Google Scholar]
- Majada, J.P.; Fal, M.A.; Tadeo, F.; Sánchez-Tamés, R. Effects of Natural Ventilation on Leaf Ultrastructure of Dianthus caryophyllus L. Cultured in Vitro. Vitr. Cell. Dev. Biol.-Plant 2002, 38, 272–278. [Google Scholar] [CrossRef]
- Hazarika, B.N. Morpho-Physiological Disorders in in Vitro Culture of Plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Debnath, S.C.; Goyali, J.C. In Vitro Propagation and Variation of Antioxidant Properties in Micropropagated Vaccinium Berry Plants—A Review. Molecules 2020, 25, 788. [Google Scholar] [CrossRef] [PubMed]
- Palacio, L.; Baeza, M.C.; Cantero, J.J.; Cusidó, R.; Goleniowski, M.E. In Vitro Propagation of “Jarilla” (Larrea divaricata Cav.) and Secondary Metabolite Production. Biol. Pharm. Bull. 2008, 31, 2321–2325. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Ncube, B.; van Staden, J. In Vitro Propagation and Secondary Product Production by Merwilla plumbea (Lindl.) Speta. Plant Growth Regul. 2012, 67, 235–245. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Naik, P.M. Influence of 2iP and 2,4-D Concentrations on Accumulation of Biomass, Phenolics, Flavonoids and Radical Scavenging Activity in Date Palm (Phoenix dactylifera L.) Cell Suspension Culture. Horticulturae 2022, 8, 683. [Google Scholar] [CrossRef]
- Bienaimé, C.; Melin, A.; Bensaddek, L.; Attoumbré, J.; Nava-Saucedo, E.; Baltora-Rosset, S. Effects of plant growth regulators on cell growth and alkaloids production by cell cultures of Lycopodiella inundata. Plant Cell Tissue Organ. Cult. 2015, 123, 523–533. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Vitamvas, J.; Viehmannova, I.; Cepkova, P.H.; Mrhalova, H.; Eliasova, K. Assessment of Somaclonal Variation in Indirect Morphogenesis-Derived Plants of Arracacia Xanthorrhiza. Pesqui. Agropecu. Bras. 2019, 54. [Google Scholar] [CrossRef]
- Debnath, S.C. Adventitious Shoot Regeneration in a Bioreactor System and EST-PCR Based Clonal Fidelity in Lowbush Blueberry (Vaccinium angustifolium Ait.). Sci. Hortic. 2011, 128, 124–130. [Google Scholar] [CrossRef]
- Soneji, J.R.; Rao, P.S.; Mhatre, M. Somaclonal Variation in Micropropagated Dormant Axillary Buds of Pineapple (Ananas comosus L., Merr.). J. Hortic. Sci. Biotechnol. 2002, 77, 28–32. [Google Scholar] [CrossRef]
- Gajdošová, A.; Ostrolucká, M.G.; Libiaková, G.; Ondrušková, E.; Šimala, D. Microclonal Propagation of Vaccinium sp. and Rubus sp. and Detection of Genetic Variability in Culture in vitro. J. Fruit. Ornam. Plant Res. 2006, 14, 1. [Google Scholar]
- Orłowska, R.; Machczyńska, J.; Oleszczuk, S.; Zimny, J.; Bednarek, P.T. DNA Methylation Changes and TE Activity Induced in Tissue Cultures of Barley (Hordeum vulgare L.). J. Biol. Res. 2016, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, M.; Lewinska, A.; Gurgul, A.; Zabek, T.; Potocki, L.; Oklejewicz, B.; Bugno-Poniewierska, M.; Wegrzyn, M.; Slota, E. Changes in DNA Methylation Patterns and Repetitive Sequences in Blood Lymphocytes of Aged Horses. Age 2014, 36, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Stephens, K.E.; Miaskowski, C.A.; Levine, J.D.; Pullinger, C.R.; Aouizerat, B.E. Epigenetic Regulation and Measurement of Epigenetic Changes. Biol. Res. Nurs. 2013, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, H.; Umeda, M. Epigenetic Control of Cell Division and Cell Differentiation in the Root Apex. Front. Plant Sci. 2015, 6, 1178. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Bassam, B.J.; Gresshoff, P.M. Silver Staining Dna in Polyacrylamide Gels. Nat. Protoc. 2007, 2, 2649–2654. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]


| Parameters | TC-Ax | TC-Ad | TC/SC | SC |
|---|---|---|---|---|
| Average length of main shoots (cm) | 39.4 a | 33.6 c | 37.8 ab | 34.5 bc |
| Maximum length of main shoots (cm) | 61.5 b | 57.2 bc | 67.3 a | 53.3 c |
| Number of main shoots | 5.5 a | 6.1 a | 6.1 a | 3.8 b |
| Number of lateral shoots | 11.9 b | 11.5 b | 16.0 a | 11.0 b |
| Total number of shoots | 17.5 b | 17.6 b | 22.1 a | 14.8 c |
| Type of Propagation | TC-Ax | TC-Ad | TC/SC | SC |
|---|---|---|---|---|
| Types of Methylation | Methylation Frequency (%) | |||
| M | 13.2 | 14.3 | 14.0 | 13.0 |
| H | 10.6 | 10.5 | 10.5 | 10.6 |
| M + H | 23.8 | 24.8 | 24.5 | 23.6 |
| MSAP Stage | Primer/Adapter | Sequence |
|---|---|---|
| Ligation | EcoRI-Adapter MspI-HpaII-Adapter | 5′CTCGTAGACTGCGTACC 3′ |
| 3′CATCTGACGCATGGTTAA 5′ 5′CGACTCAGGACTCAT3′ 3′TGAGTCCTGAGTAGCAG5′ | ||
| Preamplification | Pre-EcoRI | 5′GACTGCGTACCAATTC 3′ |
| Pre-MspI-HpaII | 5′GATGAGTCCTGAGTCGG 3′ | |
| Selective amplification | EcoRI-ACT | 5′GACTGCGTACCAATTCACT 3′ |
| EcoRI-AG | 5′GACTGCGTACCAATTCAG3′ | |
| EcoRI-AC | 5′GACTGCGTACCAATTCAC 3′ | |
| EcoRI-AT | 5′GACTGCGTACCAATTCAT 3′ | |
| MspI/HpaII-ATG | 5′GATGAGTCCTGAGTCGGATG3′ | |
| MspI/HpaII-CTA | 5′GATGAGTCCTGAGTCGGCTA3′ | |
| MspI/HpaII-CTC | 5′GATGAGTCCTGAGTCGGCTC3′ | |
| MspI/HpaII-CAT | 5′GATGAGTCCTGAGTCGGCAT3′ | |
| MspI/HpaII-CT | 5′GATGAGTCCTGAGTCGGCT3′ | |
| MspI/HpaII-GT | 5′GATGAGTCCTGAGTCGGGT3′ | |
| MspI/HpaII-CA | 5′GATGAGTCCTGAGTCGGCA3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, M.; Siekierzyńska, A.; Piechowiak, T.; Spinardi, A.; Litwińczuk, W. Comprehensive Analysis of Highbush Blueberry Plants Propagated In Vitro and Conventionally. Int. J. Mol. Sci. 2024, 25, 544. https://doi.org/10.3390/ijms25010544
Mazurek M, Siekierzyńska A, Piechowiak T, Spinardi A, Litwińczuk W. Comprehensive Analysis of Highbush Blueberry Plants Propagated In Vitro and Conventionally. International Journal of Molecular Sciences. 2024; 25(1):544. https://doi.org/10.3390/ijms25010544
Chicago/Turabian StyleMazurek, Marzena, Aleksandra Siekierzyńska, Tomasz Piechowiak, Anna Spinardi, and Wojciech Litwińczuk. 2024. "Comprehensive Analysis of Highbush Blueberry Plants Propagated In Vitro and Conventionally" International Journal of Molecular Sciences 25, no. 1: 544. https://doi.org/10.3390/ijms25010544
APA StyleMazurek, M., Siekierzyńska, A., Piechowiak, T., Spinardi, A., & Litwińczuk, W. (2024). Comprehensive Analysis of Highbush Blueberry Plants Propagated In Vitro and Conventionally. International Journal of Molecular Sciences, 25(1), 544. https://doi.org/10.3390/ijms25010544









