Breg-Mediated Immunoregulation in the Skin
Abstract
1. Introduction
2. Breg Homing and Induction in the Skin
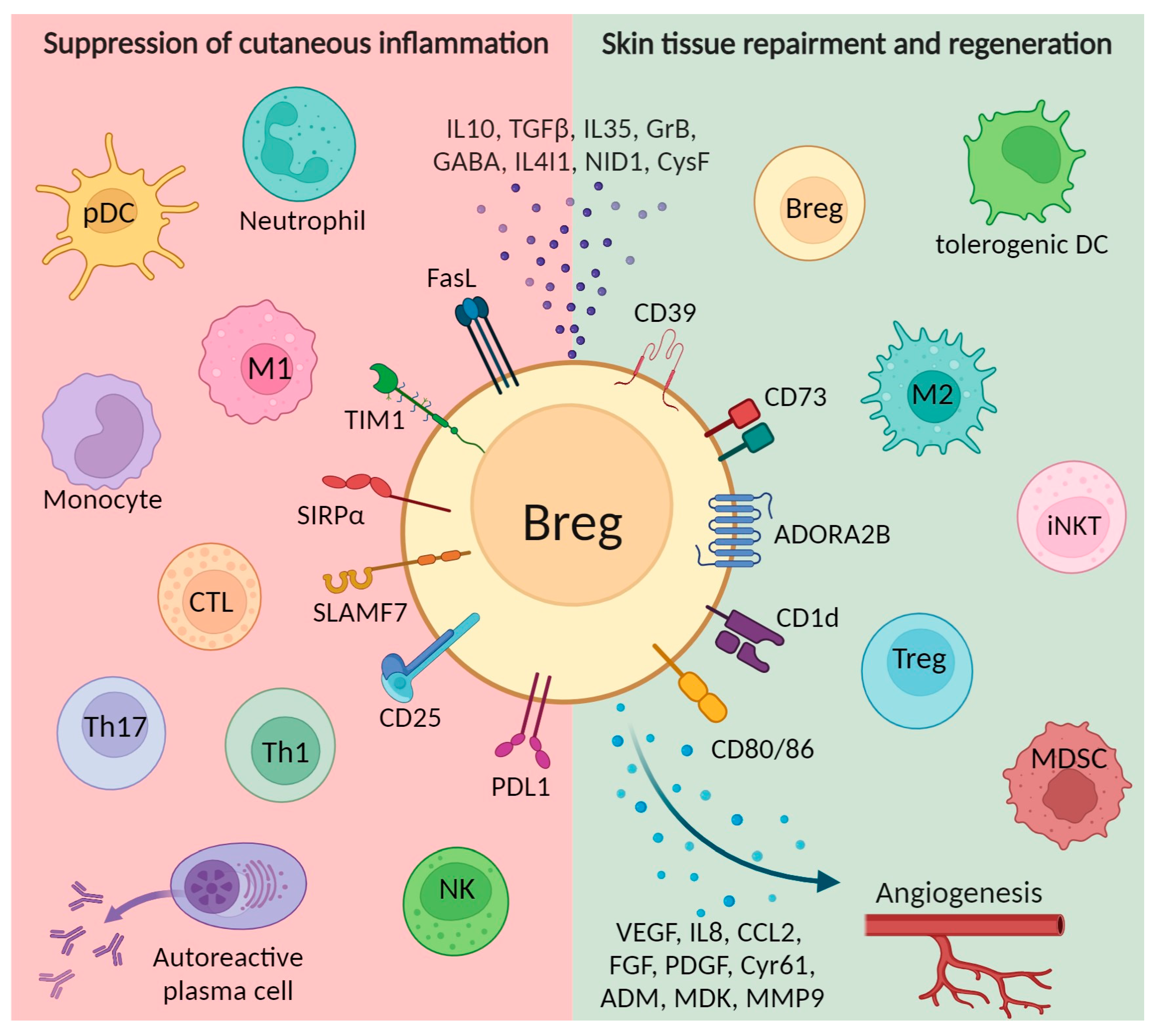
3. Bregs Promote Cutaneous Wound Healing via Several Mechanisms in a Variety of Pathological and Healthy States
3.1. Maintaining Tissue Homeostasis
3.2. Promotion of Angiogenesis
3.3. Interaction with Other Types of Suppressor Cells
3.3.1. Regulatory T Cells
3.3.2. Alternatively Activated (M2) Macrophages
3.3.3. Other Immune Suppressor Cells
3.4. Suppression of Effector Cells
3.4.1. Cytotoxic T Cells
3.4.2. Helper T Cells
3.4.3. Natural Killer Cells
3.4.4. Dendritic Cells
3.4.5. Neutrophils
3.4.6. Effector B Cells
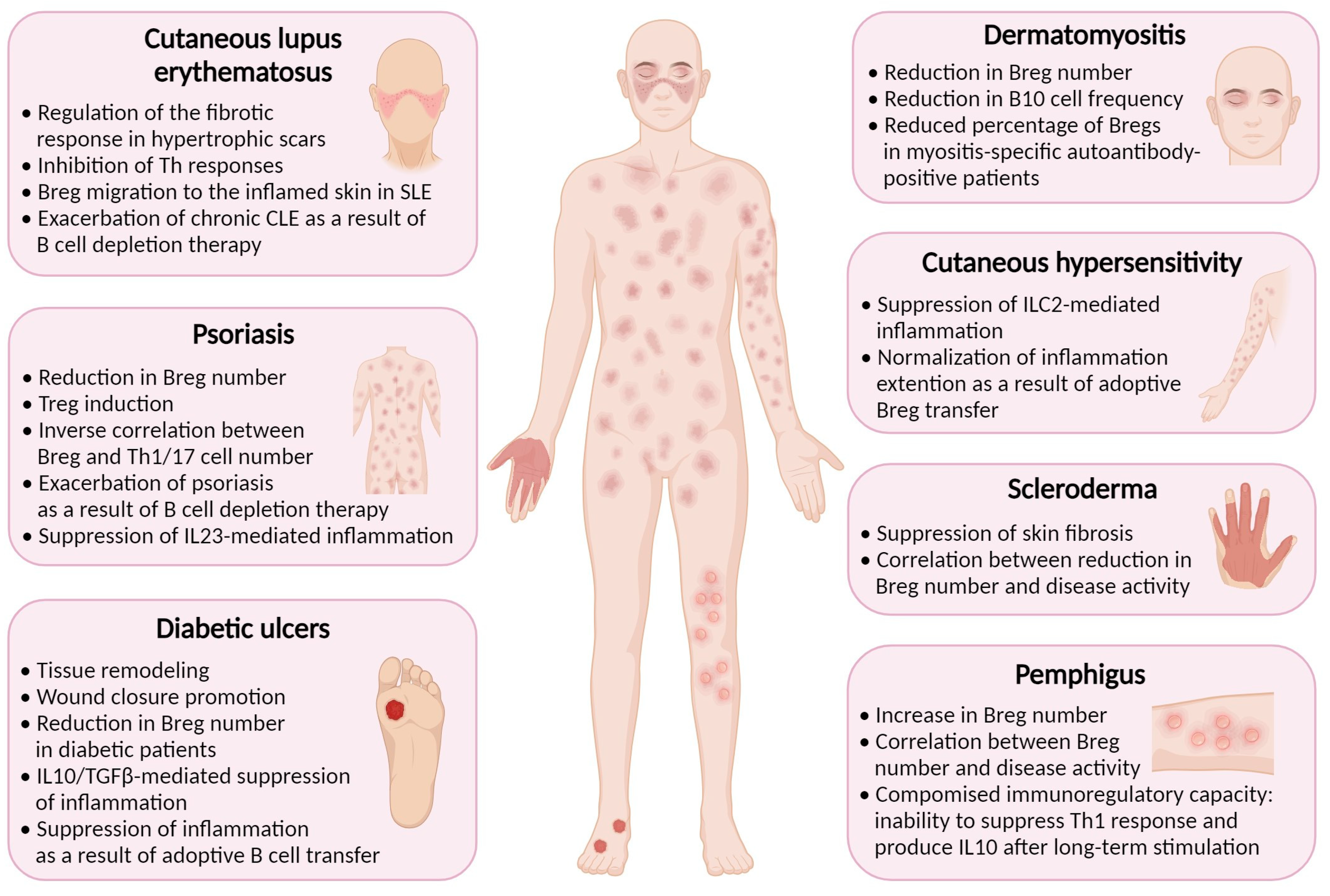
4. Breg-Mediated Wound Healing in Inflammatory Skin Diseases
4.1. Diabetes Mellitus
4.2. Psoriasis
4.3. Contact Hypersensitivity
4.4. Systemic Sclerosis (Scleroderma)
4.5. Cutaneous Lupus Erythematosus
4.6. Pemphigus
4.7. Dermatomyositis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiricozzi, A.; Zhang, S.; Dattola, A.; Cannizzaro, M.V.; Gabellini, M.; Chimenti, S.; Nistico, S.P. New Insights into the Pathogenesis of Cutaneous Autoimmune Disorders. J. Biol. Regul. Homeost. Agents 2012, 26, 165–170. [Google Scholar]
- Vesely, M.D. Getting Under the Skin: Targeting Cutaneous Autoimmune Disease. Yale J. Biol. Med. 2020, 93, 197–206. [Google Scholar]
- Lux, C.N. Wound Healing in Animals: A Review of Physiology and Clinical Evaluation. Vet. Dermatol. 2022, 33, 91-e27. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound Bed Preparation: A Systematic Approach to Wound Management. Wound Repair Regen. 2003, 11 (Suppl. S1), S1–S28. [Google Scholar] [CrossRef]
- Visha, M.G.; Karunagaran, M. A Review on Wound Healing. Int. J. Clin. Correl. 2019, 3, 50–59. [Google Scholar]
- Nosbaum, A.; Prevel, N.; Truong, H.-A.; Mehta, P.; Ettinger, M.; Scharschmidt, T.C.; Ali, N.H.; Pauli, M.L.; Abbas, A.K.; Rosenblum, M.D. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016, 196, 2010–2014. [Google Scholar] [CrossRef]
- desJardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and Wound Healing: An Update. Regen. Med. 2018, 13, 491–495. [Google Scholar] [CrossRef]
- Wilgus, T.A. Immune Cells in the Healing Skin Wound: Influential Players at Each Stage of Repair. Pharmacol. Res. 2008, 58, 112–116. [Google Scholar] [CrossRef]
- Sun, X.; Joost, S.; Kasper, M. Plasticity of Epithelial Cells during Skin Wound Healing. Cold Spring Harb. Perspect. Biol. 2023, 15, a041232. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Glass, D.R.; Tsai, A.G.; Oliveria, J.P.; Hartmann, F.J.; Kimmey, S.C.; Calderon, A.A.; Borges, L.; Glass, M.C.; Wagar, L.E.; Davis, M.M.; et al. An Integrated Multi-Omic Single-Cell Atlas of Human B Cell Identity. Immunity 2020, 53, 217–232. [Google Scholar] [CrossRef]
- Glass, M.C.; Glass, D.R.; Oliveria, J.-P.; Mbiribindi, B.; Esquivel, C.O.; Krams, S.M.; Bendall, S.C.; Martinez, O.M. Human IL-10-Producing B Cells Have Diverse States That Are Induced from Multiple B Cell Subsets. Cell Rep. 2022, 39, 110728. [Google Scholar] [CrossRef]
- Mickael, M.-E.; Bieńkowska, I.; Sacharczuk, M. An Update on the Evolutionary History of Bregs. Genes 2022, 13, 890. [Google Scholar] [CrossRef]
- Haas, K.M. Noncanonical B Cells: Characteristics of Uncharacteristic B Cells. J. Immunol. 2023, 211, 1257–1265. [Google Scholar] [CrossRef]
- Debes, G.F.; McGettigan, S.E. Skin-Associated B Cells in Health and Inflammation. J. Immunol. 2019, 202, 1659–1666. [Google Scholar] [CrossRef]
- Neta, R.; Salvin, S.B. Specific Suppression of Delayed Hypersensitivity: The Possible Presence of a Suppressor B Cell in the Regulation of Delayed Hypersensitivity. J. Immunol. 1974, 113, 1716–1725. [Google Scholar] [CrossRef]
- Ben Nasr, M.; Usuelli, V.; Seelam, A.J.; D’Addio, F.; Abdi, R.; Markmann, J.F.; Fiorina, P. Regulatory B Cells in Autoimmune Diabetes. J. Immunol. 2021, 206, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef] [PubMed]
- Nouël, A.; Pochard, P.; Simon, Q.; Ségalen, I.; Le Meur, Y.; Pers, J.O.; Hillion, S. B-Cells Induce Regulatory T Cells through TGF-β/IDO Production in A CTLA-4 Dependent Manner. J. Autoimmun. 2015, 59, 53–60. [Google Scholar] [CrossRef]
- Shen, P.; Roch, T.; Lampropoulou, V.; O’Connor, R.A.; Stervbo, U.; Hilgenberg, E.; Ries, S.; Dang, V.D.; Jaimes, Y.; Daridon, C.; et al. IL-35-Producing B Cells Are Critical Regulators of Immunity during Autoimmune and Infectious Diseases. Nature 2014, 507, 366–370. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; Liu, H.; Zhu, L.; Zhu, H.; Zhang, J.; Ren, L.; Wang, P.; Hu, F.; Su, Y. Impairment of Granzyme B-Producing Regulatory B Cells Correlates with Exacerbated Rheumatoid Arthritis. Front. Immunol. 2017, 8, 768. [Google Scholar] [CrossRef]
- Figueiró, F.; Muller, L.; Funk, S.; Jackson, E.K.; Battastini, A.M.O.; Whiteside, T.L. Phenotypic and Functional Characteristics of CD39high Human Regulatory B Cells (Breg). Oncoimmunology 2016, 5, e1082703. [Google Scholar] [CrossRef]
- Kaku, H.; Cheng, K.F.; Al-Abed, Y.; Rothstein, T.L. A Novel Mechanism of B Cell-Mediated Immune Suppression through CD73 Expression and Adenosine Production. J. Immunol. 2014, 193, 5904–5913. [Google Scholar] [CrossRef]
- Choi, J.K.; Yu, C.-R.; Bing, S.J.; Jittayasothorn, Y.; Mattapallil, M.J.; Kang, M.; Park, S.B.; Lee, H.-S.; Dong, L.; Shi, G.; et al. IL-27-Producing B-1a Cells Suppress Neuroinflammation and CNS Autoimmune Diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2109548118. [Google Scholar] [CrossRef]
- Zheremyan, E.A.; Ustiugova, A.S.; Radko, A.I.; Stasevich, E.M.; Uvarova, A.N.; Mitkin, N.A.; Kuprash, D.V.; Korneev, K.V. Novel Potential Mechanisms of Regulatory B Cell-Mediated Immunosuppression. Biochemistry 2023, 88, 13–21. [Google Scholar] [CrossRef]
- Jin, S.-P.; Koh, S.-J.; Yu, D.-A.; Kim, M.-W.; Yun, H.T.; Lee, D.H.; Yoon, H.-S.; Cho, S.; Park, H.-S. Imiquimod-Applied Interleukin-10 Deficient Mice Better Reflects Severe and Persistent Psoriasis with Systemic Inflammatory State. Exp. Dermatol. 2018, 27, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous Scarring: Pathophysiology, Molecular Mechanisms, and Scar Reduction Therapeutics Part I. The Molecular Basis of Scar Formation. J. Am. Acad. Dermatol. 2012, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Geherin, S.A.; Gómez, D.; Glabman, R.A.; Ruthel, G.; Hamann, A.; Debes, G.F. IL-10+ Innate-like B Cells Are Part of the Skin Immune System and Require α4β1 Integrin To Migrate between the Peritoneum and Inflamed Skin. J. Immunol. 2016, 196, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Geherin, S.A.; Fintushel, S.R.; Lee, M.H.; Wilson, R.P.; Patel, R.T.; Alt, C.; Young, A.J.; Hay, J.B.; Debes, G.F. The Skin, a Novel Niche for Recirculating B Cells. J. Immunol. 2012, 188, 6027–6035. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Newman, W.; Tanaka, Y.; Shaw, S. Lymphocyte Interactions with Endothelial Cells. Immunol. Today 1992, 13, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Aira, L.E.; Debes, G.F. Skin-Homing Regulatory B Cells Required for Suppression of Cutaneous Inflammation. J. Investig. Dermatol. 2021, 141, 1995–2005.e6. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.N.; Halliday, G.M. B Cells Activated in Lymph Nodes in Response to Ultraviolet Irradiation or by Interleukin-10 Inhibit Dendritic Cell Induction of Immunity. J. Investig. Dermatol. 2005, 124, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Quiros, M.; Nishio, H.; Neumann, P.A.; Siuda, D.; Brazil, J.C.; Azcutia, V.; Hilgarth, R.; O’Leary, M.N.; Garcia-Hernandez, V.; Leoni, G.; et al. Macrophage-Derived IL-10 Mediates Mucosal Repair by Epithelial WISP-1 Signaling. J. Clin. Investig. 2017, 127, 3510–3520. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef]
- Yanaba, K.; Kamata, M.; Ishiura, N.; Shibata, S.; Asano, Y.; Tada, Y.; Sugaya, M.; Kadono, T.; Tedder, T.F.; Sato, S. Regulatory B Cells Suppress Imiquimod-Induced, Psoriasis-like Skin Inflammation. J. Leukoc. Biol. 2013, 94, 563–573. [Google Scholar] [CrossRef]
- Matsushita, T.; Kobayashi, T.; Mizumaki, K.; Kano, M.; Sawada, T.; Tennichi, M.; Okamura, A.; Hamaguchi, Y.; Iwakura, Y.; Hasegawa, M.; et al. BAFF Inhibition Attenuates Fibrosis in Scleroderma by Modulating the Regulatory and Effector B Cell Balance. Sci. Adv. 2018, 4, eaas9944. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.-D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Sîrbulescu, R.F.; Mamidi, A.; Chan, S.-Y.C.; Jin, G.; Boukhali, M.; Sobell, D.; Ilieş, I.; Chung, J.Y.; Haas, W.; Whalen, M.J.; et al. B Cells Support the Repair of Injured Tissues by Adopting MyD88-Dependent Regulatory Functions and Phenotype. FASEB J. 2021, 35, e22019. [Google Scholar] [CrossRef]
- Varricchi, G.; Granata, F.; Loffredo, S.; Genovese, A.; Marone, G. Angiogenesis and Lymphangiogenesis in Inflammatory Skin Disorders. J. Am. Acad. Dermatol. 2015, 73, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- van de Veen, W.; Globinska, A.; Jansen, K.; Straumann, A.; Kubo, T.; Verschoor, D.; Wirz, O.F.; Castro-Giner, F.; Tan, G.; Rückert, B.; et al. A Novel Proangiogenic B Cell Subset Is Increased in Cancer and Chronic Inflammation. Sci. Adv. 2020, 6, eaaz3559. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.M.; Kireeva, M.L.; Kolesnikova, T.V.; Lau, L.F. CYR61, a Product of a Growth Factor-Inducible Immediate Early Gene, Promotes Angiogenesis and Tumor Growth. Proc. Natl. Acad. Sci. USA 1998, 95, 6355–6360. [Google Scholar] [CrossRef] [PubMed]
- Oehler, M.K.; Hague, S.; Rees, M.C.P.; Bicknell, R. Adrenomedullin Promotes Formation of Xenografted Endometrial Tumors by Stimulation of Autocrine Growth and Angiogenesis. Oncogene 2002, 21, 2815–2821. [Google Scholar] [CrossRef][Green Version]
- Choudhuri, R.; Zhang, H.T.; Donnini, S.; Ziche, M.; Bicknell, R. An Angiogenic Role for the Neurokines Midkine and Pleiotrophin in Tumorigenesis. Cancer Res. 1997, 57, 1814–1819. [Google Scholar]
- Auchampach, J.A. Adenosine Receptors and Angiogenesis. Circ. Res. 2007, 101, 1075–1077. [Google Scholar] [CrossRef]
- Yang, C.; Lee, H.; Pal, S.; Jove, V.; Deng, J.; Zhang, W.; Hoon, D.S.B.; Wakabayashi, M.; Forman, S.; Yu, H. B Cells Promote Tumor Progression via STAT3 Regulated-Angiogenesis. PLoS ONE 2013, 8, e64159. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, H.; Zhang, W.; Yazaki, P.J.; Zhang, Z.; Le, K.; Li, W.; Lee, H.; Kwak, L.; Forman, S.; et al. CD5 Binds to Interleukin-6 and Induces a Feed-Forward Loop with the Transcription Factor STAT3 in B Cells to Promote Cancer. Immunity 2016, 44, 913–923. [Google Scholar] [CrossRef]
- Kalekar, L.A.; Rosenblum, M.D. Regulatory T Cells in Inflammatory Skin Disease: From Mice to Humans. Int. Immunol. 2019, 31, 457–463. [Google Scholar] [CrossRef]
- Haertel, E.; Joshi, N.; Hiebert, P.; Kopf, M.; Werner, S. Regulatory T Cells Are Required for Normal and Activin-Promoted Wound Repair in Mice. Eur. J. Immunol. 2018, 48, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-B.; Flach, C.-F.; Czerkinsky, C.; Holmgren, J. B Lymphocytes Promote Expansion of Regulatory T Cells in Oral Tolerance: Powerful Induction by Antigen Coupled to Cholera Toxin B Subunit. J. Immunol. 2008, 181, 8278–8287. [Google Scholar] [CrossRef] [PubMed]
- Kessel, A.; Haj, T.; Peri, R.; Snir, A.; Melamed, D.; Sabo, E.; Toubi, E. Human CD19(+)CD25(high) B Regulatory Cells Suppress Proliferation of CD4(+) T Cells and Enhance Foxp3 and CTLA-4 Expression in T-Regulatory Cells. Autoimmun. Rev. 2012, 11, 670–677. [Google Scholar] [CrossRef]
- Reichardt, P.; Dornbach, B.; Rong, S.; Beissert, S.; Gueler, F.; Loser, K.; Gunzer, M. Naive B Cells Generate Regulatory T Cells in the Presence of a Mature Immunologic Synapse. Blood 2007, 110, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-H.; Chiang, B.-L. Regulatory T Cells Induced by B Cells: A Novel Subpopulation of Regulatory T Cells. J. Biomed. Sci. 2017, 24, 86. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dittel, B.N. Mechanisms of Regulatory B Cell Function in Autoimmune and Inflammatory Diseases beyond IL-10. J. Clin. Med. Res. 2017, 6, 12. [Google Scholar] [CrossRef]
- Romagnani, S. IL4I1: Key Immunoregulator at a Crossroads of Divergent T-Cell Functions. Eur. J. Immunol. 2016, 46, 2302–2305. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Chien, S. Macrophage Differentiation in Normal and Accelerated Wound Healing. Results Probl. Cell Differ. 2017, 62, 353–364. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Jansen, K.; Cevhertas, L.; Ma, S.; Satitsuksanoa, P.; Akdis, M.; van de Veen, W. Regulatory B Cells, A to Z. Allergy 2021, 76, 2699–2715. [Google Scholar] [CrossRef]
- Zhang, B.; Vogelzang, A.; Miyajima, M.; Sugiura, Y.; Wu, Y.; Chamoto, K.; Nakano, R.; Hatae, R.; Menzies, R.J.; Sonomura, K.; et al. B Cell-Derived GABA Elicits IL-10+ Macrophages to Limit Anti-Tumour Immunity. Nature 2021, 599, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; He, S.; Wang, Y.; Lu, Y.; Gu, M.; Li, D.; Tang, T.; Nie, S.; Zhang, M.; Lv, B.; et al. Regulatory B Cells Improve Ventricular Remodeling after Myocardial Infarction by Modulating Monocyte Migration. Basic Res. Cardiol. 2021, 116, 46. [Google Scholar] [CrossRef] [PubMed]
- Tanno, H.; Kawakami, K.; Kanno, E.; Suzuki, A.; Takagi, N.; Yamamoto, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Invariant NKT Cells Promote Skin Wound Healing by Preventing a Prolonged Neutrophilic Inflammatory Response. Wound Repair Regen. 2017, 25, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Schioppa, T.; Moore, R.; Thompson, R.G.; Rosser, E.C.; Kulbe, H.; Nedospasov, S.; Mauri, C.; Coussens, L.M.; Balkwill, F.R. B Regulatory Cells and the Tumor-Promoting Actions of TNF-α during Squamous Carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10662–10667. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jin, Y.; Tian, Y.; Zhang, H.; Wu, J.; Lu, W.; Lu, X. Regulatory B Cells Contribute to the Impaired Antitumor Immunity in Ovarian Cancer Patients. Tumour Biol. 2016, 37, 6581–6588. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Du, R.; Pang, N.; Zhang, F.; Dong, D.; Ding, J.; Ding, Y. Role of Regulatory B Cells in the Progression of Cervical Cancer. Mediat. Inflamm. 2019, 2019, 6519427. [Google Scholar] [CrossRef]
- Bodogai, M.; Moritoh, K.; Lee-Chang, C.; Hollander, C.M.; Sherman-Baust, C.A.; Wersto, R.P.; Araki, Y.; Miyoshi, I.; Yang, L.; Trinchieri, G.; et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015, 75, 3456–3465. [Google Scholar] [CrossRef]
- Parekh, V.V.; Prasad, D.V.R.; Banerjee, P.P.; Joshi, B.N.; Kumar, A.; Mishra, G.C. B Cells Activated by Lipopolysaccharide, but Not by Anti-Ig and Anti-CD40 Antibody, Induce Anergy in CD8+ T Cells: Role of TGF-Beta 1. J. Immunol. 2003, 170, 5897–5911. [Google Scholar] [CrossRef]
- Shang, J.; Zha, H.; Sun, Y. Phenotypes, Functions, and Clinical Relevance of Regulatory B Cells in Cancer. Front. Immunol. 2020, 11, 582657. [Google Scholar] [CrossRef]
- Rosser, E.C.; Blair, P.A.; Mauri, C. Cellular Targets of Regulatory B Cell-Mediated Suppression. Mol. Immunol. 2014, 62, 296–304. [Google Scholar] [CrossRef]
- Strang, H.; Kaul, A.; Parikh, U.; Masri, L.; Saravanan, S.; Li, H.; Miao, Q.; Balaji, S. Chapter 11—Role of Cytokines and Chemokines in Wound Healing. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: New York, NY, USA, 2020; pp. 197–235. ISBN 9780128164136. [Google Scholar]
- Bjarnadóttir, K.; Benkhoucha, M.; Merkler, D.; Weber, M.S.; Payne, N.L.; Bernard, C.C.A.; Molnarfi, N.; Lalive, P.H. B Cell-Derived Transforming Growth Factor-β1 Expression Limits the Induction Phase of Autoimmune Neuroinflammation. Sci. Rep. 2016, 6, 34594. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Gray, D.; Mushtaq, N.; Londei, M. Prevention of Arthritis by Interleukin 10-Producing B Cells. J. Exp. Med. 2003, 197, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zekzer, D.; Hanssen, L.; Lu, Y.; Olcott, A.; Kaufman, D.L. Lipopolysaccharide-Activated B Cells down-Regulate Th1 Immunity and Prevent Autoimmune Diabetes in Nonobese Diabetic Mice. J. Immunol. 2001, 167, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Leitner, W.W.; Golding, B.; Scott, D. Inhibitory Effects of B Cells on Antitumor Immunity. Cancer Res. 2006, 66, 7741–7747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tai, Y.-T.; Ho, M.; Xing, L.; Chauhan, D.; Gang, A.; Qiu, L.; Anderson, K.C. Regulatory B Cell-Myeloma Cell Interaction Confers Immunosuppression and Promotes Their Survival in the Bone Marrow Milieu. Blood Cancer J. 2017, 7, e547. [Google Scholar] [CrossRef] [PubMed]
- Zheremyan, E.A.; Ustiugova, A.S.; Uvarova, A.N.; Karamushka, N.M.; Stasevich, E.M.; Gogoleva, V.S.; Bogolyubova, A.V.; Mitkin, N.A.; Kuprash, D.V.; Korneev, K.V. Differentially Activated B Cells Develop Regulatory Phenotype and Show Varying Immunosuppressive Features: A Comparative Study. Front. Immunol. 2023, 14, 1178445. [Google Scholar] [CrossRef]
- Tomasini, D.; Mentzel, T.; Hantschke, M.; Cerri, A.; Paredes, B.; Rütten, A.; Schärer, L.; Kutzner, H. Plasmacytoid Dendritic Cells: An Overview of Their Presence and Distribution in Different Inflammatory Skin Diseases, with Special Emphasis on Jessner’s Lymphocytic Infiltrate of the Skin and Cutaneous Lupus Erythematosus. J. Cutan. Pathol. 2010, 37, 1132–1139. [Google Scholar] [CrossRef]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-Producing Plasmablasts Exert Regulatory Function in Autoimmune Inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef]
- Kruger, P.; Saffarzadeh, M.; Weber, A.N.R.; Rieber, N.; Radsak, M.; von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PLoS Pathog. 2015, 11, e1004651. [Google Scholar] [CrossRef] [PubMed]
- Maseda, D.; Candando, K.M.; Smith, S.H.; Kalampokis, I.; Weaver, C.T.; Plevy, S.E.; Poe, J.C.; Tedder, T.F. Peritoneal Cavity Regulatory B Cells (B10 Cells) Modulate IFN-γ+CD4+ T Cell Numbers during Colitis Development in Mice. J. Immunol. 2013, 191, 2780–2795. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.; Lampropoulou, V.; Calderon-Gomez, E.; Roch, T.; Stervbo, U.; Shen, P.; Kühl, A.A.; Loddenkemper, C.; Haury, M.; Nedospasov, S.A.; et al. Signaling via the MyD88 Adaptor Protein in B Cells Suppresses Protective Immunity during Salmonella Typhimurium Infection. Immunity 2010, 33, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Podstawka, J.; Sinha, S.; Hiroki, C.H.; Sarden, N.; Granton, E.; Labit, E.; Kim, J.H.; Andonegui, G.; Lou, Y.; Snarr, B.D.; et al. Marginating Transitional B Cells Modulate Neutrophils in the Lung during Inflammation and Pneumonia. J. Exp. Med. 2021, 218, e20210409. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S. Regulatory Plasma Cells. Curr. Opin. Pharmacol. 2015, 23, 1–5. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, N.; Martínez-Jiménez, I.; García-Ojalvo, A.; Mendoza-Mari, Y.; Guillén-Nieto, G.; Armstrong, D.G.; Berlanga-Acosta, J. Wound Chronicity, Impaired Immunity and Infection in Diabetic Patients. MEDICC Rev. 2022, 24, 44–58. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic Insight into Diabetic Wounds: Pathogenesis, Molecular Targets and Treatment Strategies to Pace Wound Healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Mendez-Frausto, G.; Romero-Aguilera, G.; Sanchez-Gutierrez, R.; García-Jacobo, R.E.; Lara-Ramírez, E.E.; Uresti-Rivera, E.E.; Gonzalez-Amaro, R.; Enciso-Moreno, J.A.; García-Hernández, M.H. B Regulatory Cells Associated with Changes in Biochemical and Inflammatory Parameters in Normal-Glycemic Individuals, Pre-Diabetes and T2DM Patients. Diabetes Res. Clin. Pract. 2021, 173, 108692. [Google Scholar] [CrossRef]
- Sîrbulescu, R.F.; Boehm, C.K.; Soon, E.; Wilks, M.Q.; Ilieş, I.; Yuan, H.; Maxner, B.; Chronos, N.; Kaittanis, C.; Normandin, M.D.; et al. Mature B Cells Accelerate Wound Healing after Acute and Chronic Diabetic Skin Lesions. Wound Repair Regen. 2017, 25, 774–791. [Google Scholar] [CrossRef]
- Goldman, M.P. Treatment of Varicose and Telangiectatic Leg Veins: Double-Blind Prospective Comparative Trial between Aethoxyskerol and Sotradecol. Dermatol. Surg. 2002, 28, 52–55. [Google Scholar] [CrossRef]
- Grigorieva, E.; Khailov, E.; Deignan, P. Optimal Treatment Strategies for Control Model of Psoriasis. In Proceedings of the 2017 Proceedings of the Conference on Control and its Applications (CT), Philadelphia, PA, USA, 10–12 July 2017; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2017; pp. 86–93. [Google Scholar]
- Hayashi, M.; Yanaba, K.; Umezawa, Y.; Yoshihara, Y.; Kikuchi, S.; Ishiuji, Y.; Saeki, H.; Nakagawa, H. IL-10-Producing Regulatory B Cells Are Decreased in Patients with Psoriasis. J. Dermatol. Sci. 2016, 81, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mavropoulos, A.; Varna, A.; Zafiriou, E.; Liaskos, C.; Alexiou, I.; Roussaki-Schulze, A.; Vlychou, M.; Katsiari, C.; Bogdanos, D.P.; Sakkas, L.I. IL-10 Producing Bregs Are Impaired in Psoriatic Arthritis and Psoriasis and Inversely Correlate with IL-17- and IFNγ-Producing T Cells. Clin. Immunol. 2017, 184, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mizumaki, K.; Horii, M.; Kano, M.; Komuro, A.; Matsushita, T. Suppression of IL-23-Mediated Psoriasis-like Inflammation by Regulatory B Cells. Sci. Rep. 2021, 11, 2106. [Google Scholar] [CrossRef] [PubMed]
- Kersh, A.E.; Feldman, R.J. Autoimmune Sequelae Following Rituximab Therapy: A Review of the Literature and Potential Immunologic Mechanisms. J. Clin. Rheumatol. 2018, 24, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Grabbe, S.; Schwarz, T. Immunoregulatory Mechanisms Involved in Elicitation of Allergic Contact Hypersensitivity. Immunol. Today 1998, 19, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Saint-Mezard, P.; Berard, F.; Dubois, B.; Kaiserlian, D.; Nicolas, J.-F. The Role of CD4+ and CD8+ T Cells in Contact Hypersensitivity and Allergic Contact Dermatitis. Eur. J. Dermatol. 2004, 14, 131–138. [Google Scholar] [PubMed]
- Watanabe, R.; Fujimoto, M.; Ishiura, N.; Kuwano, Y.; Nakashima, H.; Yazawa, N.; Okochi, H.; Sato, S.; Tedder, T.F.; Tamaki, K. CD19 Expression in B Cells Is Important for Suppression of Contact Hypersensitivity. Am. J. Pathol. 2007, 171, 560–570. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, M.B.; Lee, D.; Min, K.Y.; Koo, J.; Kim, H.W.; Park, Y.H.; Kim, S.J.; Ikutani, M.; Takaki, S.; et al. The Regulatory B Cell-Mediated Peripheral Tolerance Maintained by Mast Cell IL-5 Suppresses Oxazolone-Induced Contact Hypersensitivity. Sci. Adv. 2019, 5, eaav8152. [Google Scholar] [CrossRef]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of Immune Events in the Murine Contact Hypersensitivity Model: Toward the Understanding of Allergic Contact Dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef]
- Rosendahl, A.-H.; Schönborn, K.; Krieg, T. Pathophysiology of Systemic Sclerosis (scleroderma). Kaohsiung J. Med. Sci. 2022, 38, 187–195. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, J.; Wu, H.; Sawalha, A.H.; Lu, Q. Clinical Treatment Options in Scleroderma: Recommendations and Comprehensive Review. Clin. Rev. Allergy Immunol. 2022, 62, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Le Huu, D.; Matsushita, T.; Jin, G.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Tedder, T.F.; Fujimoto, M. Donor-Derived Regulatory B Cells Are Important for Suppression of Murine Sclerodermatous Chronic Graft-versus-Host Disease. Blood 2013, 121, 3274–3283. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Fujimoto, M. Decreased Levels of Regulatory B Cells in Patients with Systemic Sclerosis: Association with Autoantibody Production and Disease Activity. Rheumatology 2016, 55, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Fukasawa, T.; Ebata, S.; Yoshizaki-Ogawa, A.; Sato, S. Involvement of B Cells in the Development of Systemic Sclerosis. Front. Immunol. 2022, 13, 938785. [Google Scholar] [CrossRef] [PubMed]
- Rothfield, N.; Sontheimer, R.D.; Bernstein, M. Lupus Erythematosus: Systemic and Cutaneous Manifestations. Clin. Dermatol. 2006, 24, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Maz, M.P.; Martens, J.W.S.; Hannoudi, A.; Reddy, A.L.; Hile, G.A.; Kahlenberg, J.M. Recent Advances in Cutaneous Lupus. J. Autoimmun. 2022, 132, 102865. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Chu, Y.; Xue, Y.; Xuan, D.; Zheng, S.; Zou, H. T Follicular Helper Cells and Regulatory B Cells Dynamics in Systemic Lupus Erythematosus. PLoS ONE 2014, 9, e88441. [Google Scholar] [CrossRef]
- Méndez-Flores, S.; Hernández-Molina, G.; Enríquez, A.B.; Faz-Muñoz, D.; Esquivel, Y.; Pacheco-Molina, C.; Furuzawa-Carballeda, J. Cytokines and Effector/Regulatory Cells Characterization in the Physiopathology of Cutaneous Lupus Erythematous: A Cross-Sectional Study. Mediat. Inflamm. 2016, 2016, 7074829. [Google Scholar] [CrossRef]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+)CD24(hi)CD38(hi) B Cells Exhibit Regulatory Capacity in Healthy Individuals but Are Functionally Impaired in Systemic Lupus Erythematosus Patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Vital, E.M.; Wittmann, M.; Edward, S.; Md Yusof, M.Y.; MacIver, H.; Pease, C.T.; Goodfield, M.; Emery, P. Brief Report: Responses to Rituximab Suggest B Cell-Independent Inflammation in Cutaneous Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 67, 1586–1591. [Google Scholar] [CrossRef]
- Hébert, V.; Petit, M.; Maho-Vaillant, M.; Golinski, M.-L.; Riou, G.; Derambure, C.; Boyer, O.; Joly, P.; Calbo, S. Modifications of the Transcriptomic Profile of Autoreactive B Cells From Pemphigus Patients After Treatment With Rituximab or a Standard Corticosteroid Regimen. Front. Immunol. 2019, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Q.; Xu, R.-C.; Chen, Y.-Y.; Yuan, H.-J.; Cao, H.; Zhao, X.-Q.; Zheng, J.; Wang, Y.; Pan, M. Impaired Function of CD19+CD24hiCD38hi Regulatory B Cells in Patients with Pemphigus. Br. J. Dermatol. 2015, 172, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpour, S. Interleukin 21 as a New Possible Player in Pemphigus: Is It a Suitable Target? Int. Immunopharmacol. 2016, 34, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Colliou, N.; Picard, D.; Caillot, F.; Calbo, S.; Corre, S.L.; Lim, A.; Lemercier, B.; Mauff, B.L.; Maho-Vaillant, M.; Jacquot, S.; et al. Long-Term Remissions of Severe Pemphigus After Rituximab Therapy Are Associated with Prolonged Failure of Desmoglein B Cell Response. Sci. Transl. Med. 2013, 5, 175ra30. [Google Scholar] [CrossRef]
- Zong, M.; Lundberg, I.E. Pathogenesis, Classification and Treatment of Inflammatory Myopathies. Nat. Rev. Rheumatol. 2011, 7, 297–306. [Google Scholar] [CrossRef]
- Magro, C.M.; Segal, J.P.; Crowson, A.N.; Chadwick, P. The Phenotypic Profile of Dermatomyositis and Lupus Erythematosus: A Comparative Analysis. J. Cutan. Pathol. 2010, 37, 659–671. [Google Scholar] [CrossRef]
- Li, W.; Tian, X.; Lu, X.; Peng, Q.; Shu, X.; Yang, H.; Li, Y.; Wang, Y.; Zhang, X.; Liu, Q.; et al. Significant Decrease in Peripheral Regulatory B Cells Is an Immunopathogenic Feature of Dermatomyositis. Sci. Rep. 2016, 6, 27479. [Google Scholar] [CrossRef]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B Cell Depletion Therapies in Autoimmune Disease: Advances and Mechanistic Insights. Nat. Rev. Drug Discov. 2021, 20, 179–199. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Q.; Huang, L. B Cell Depletion Therapies in Autoimmune Diseases: Monoclonal Antibodies or Chimeric Antigen Receptor-Based Therapy? Front. Immunol. 2023, 14, 1126421. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Brook, M.O.; Shankar, S.; Hester, J.; Issa, F. Towards Regulatory Cellular Therapies in Solid Organ Transplantation. Trends Immunol. 2022, 43, 8–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheremyan, E.A.; Ustiugova, A.S.; Karamushka, N.M.; Uvarova, A.N.; Stasevich, E.M.; Bogolyubova, A.V.; Kuprash, D.V.; Korneev, K.V. Breg-Mediated Immunoregulation in the Skin. Int. J. Mol. Sci. 2024, 25, 583. https://doi.org/10.3390/ijms25010583
Zheremyan EA, Ustiugova AS, Karamushka NM, Uvarova AN, Stasevich EM, Bogolyubova AV, Kuprash DV, Korneev KV. Breg-Mediated Immunoregulation in the Skin. International Journal of Molecular Sciences. 2024; 25(1):583. https://doi.org/10.3390/ijms25010583
Chicago/Turabian StyleZheremyan, Elina A., Alina S. Ustiugova, Nina M. Karamushka, Aksinya N. Uvarova, Ekaterina M. Stasevich, Apollinariya V. Bogolyubova, Dmitry V. Kuprash, and Kirill V. Korneev. 2024. "Breg-Mediated Immunoregulation in the Skin" International Journal of Molecular Sciences 25, no. 1: 583. https://doi.org/10.3390/ijms25010583
APA StyleZheremyan, E. A., Ustiugova, A. S., Karamushka, N. M., Uvarova, A. N., Stasevich, E. M., Bogolyubova, A. V., Kuprash, D. V., & Korneev, K. V. (2024). Breg-Mediated Immunoregulation in the Skin. International Journal of Molecular Sciences, 25(1), 583. https://doi.org/10.3390/ijms25010583





