Multi-Target-Directed Cinnamic Acid Hybrids Targeting Alzheimer’s Disease
Abstract
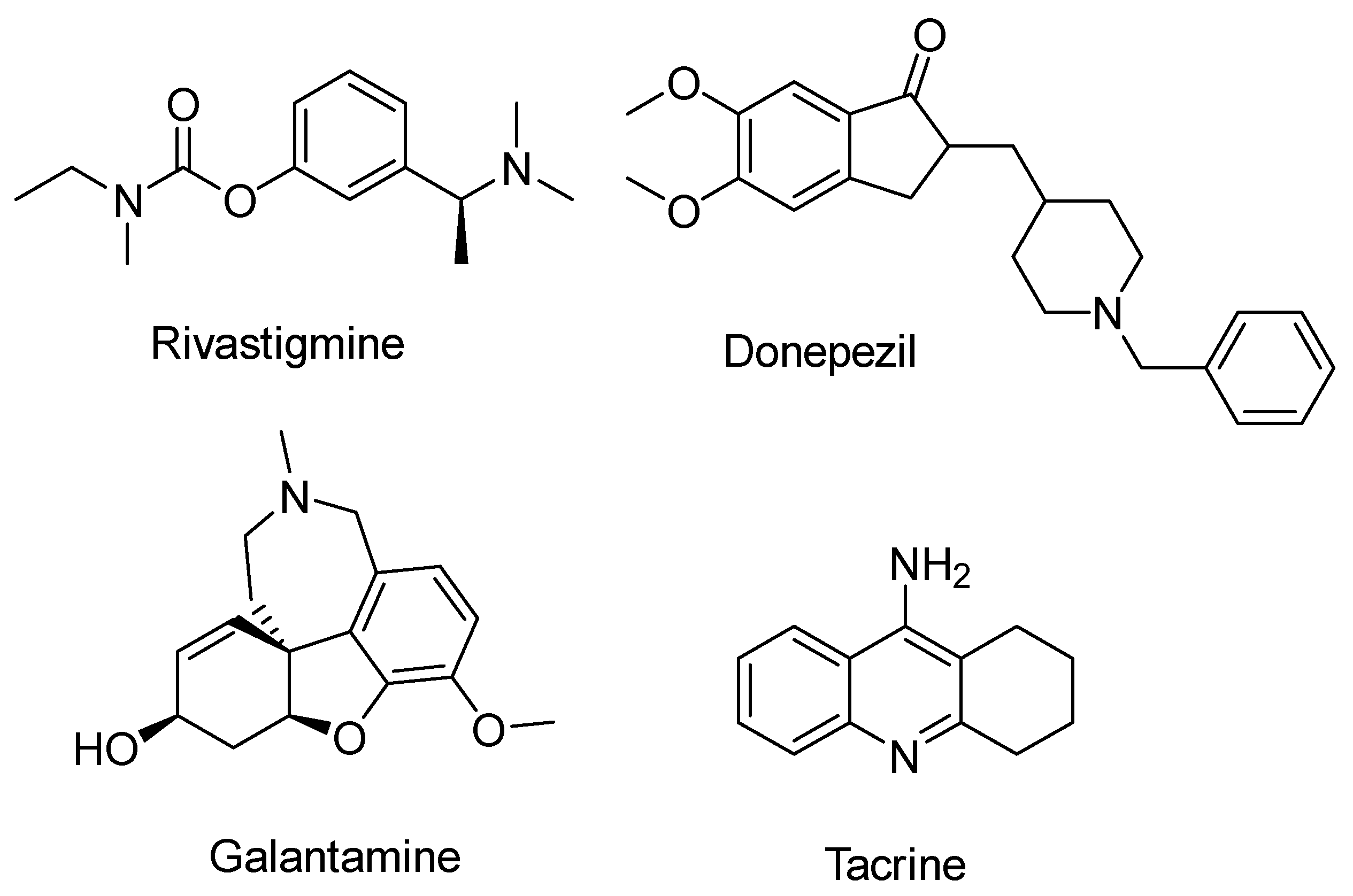
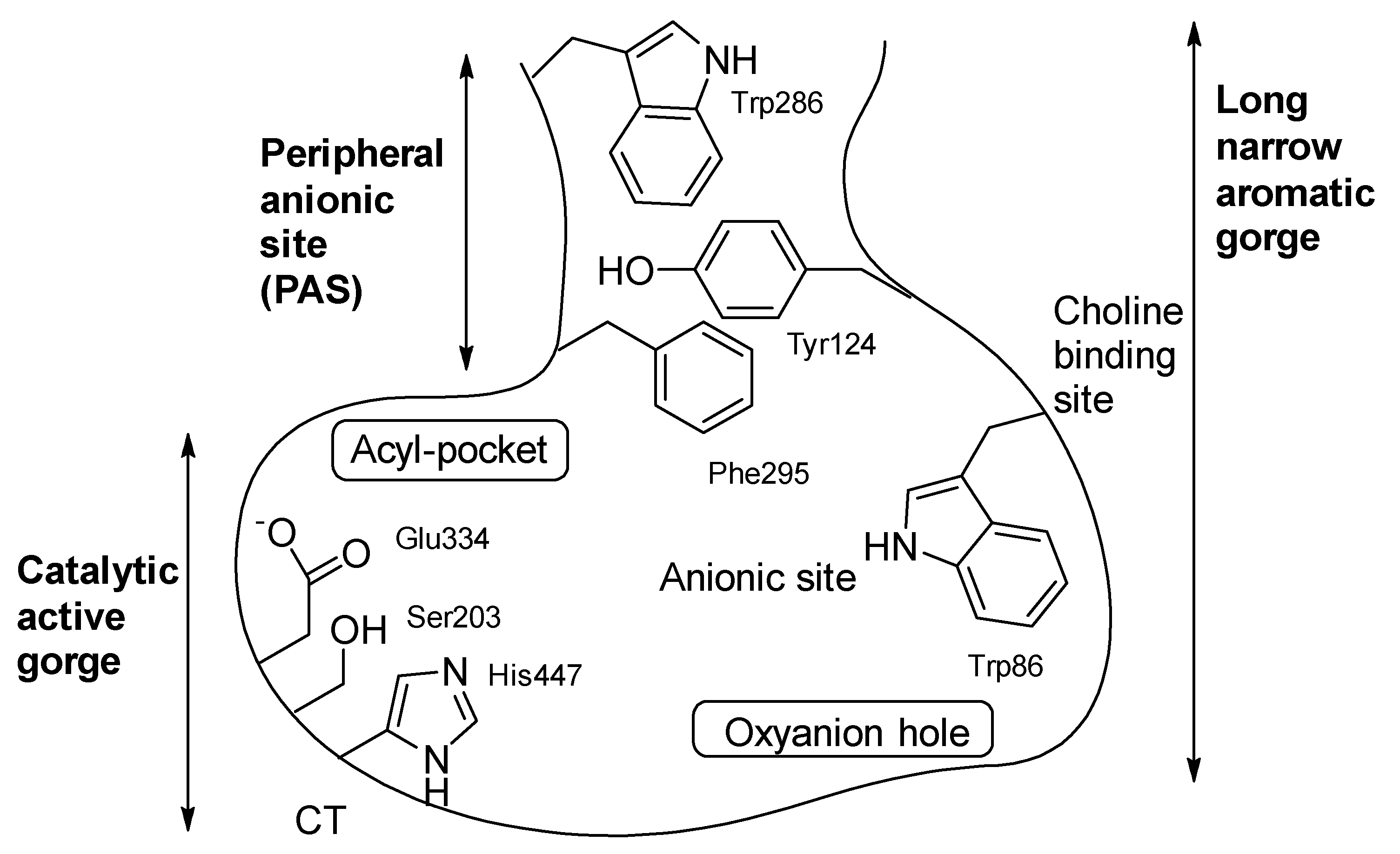
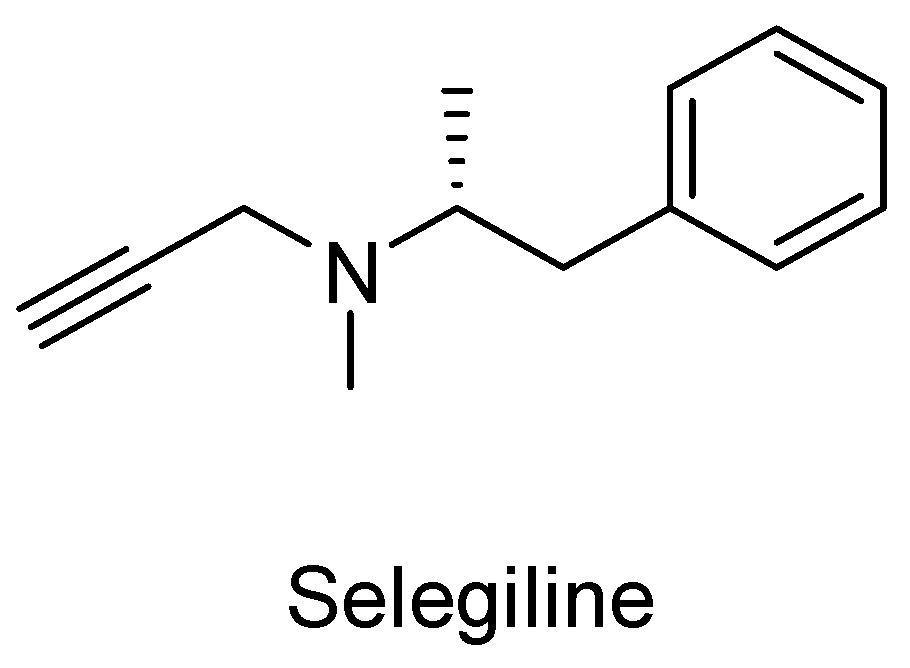
1. Introduction
2. Results
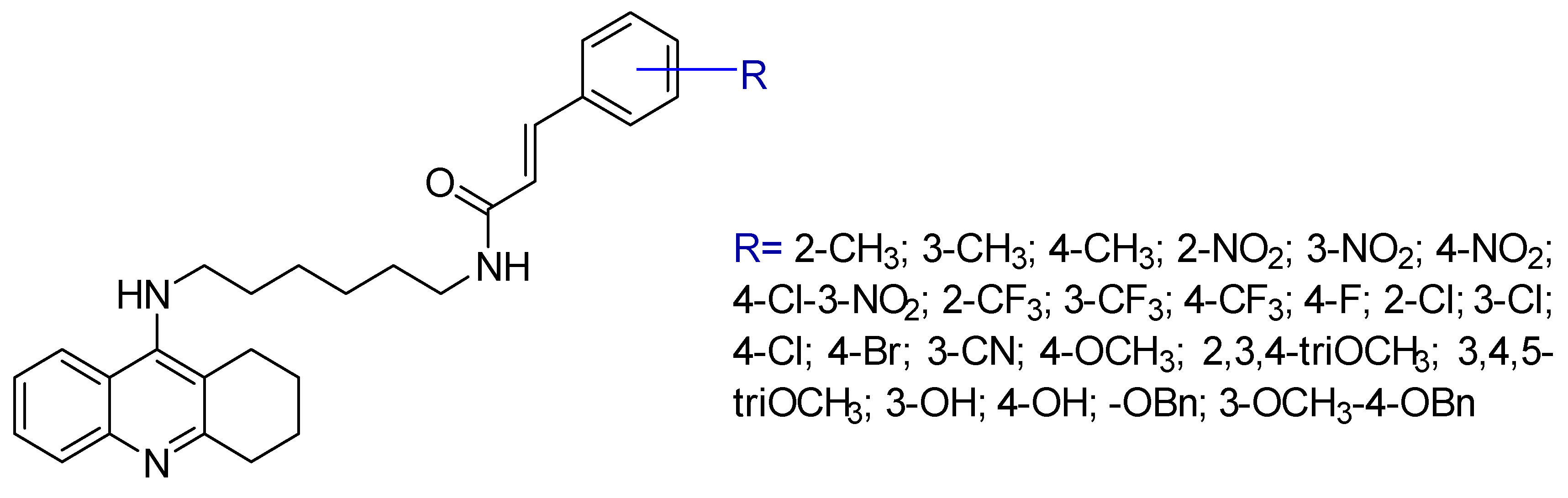
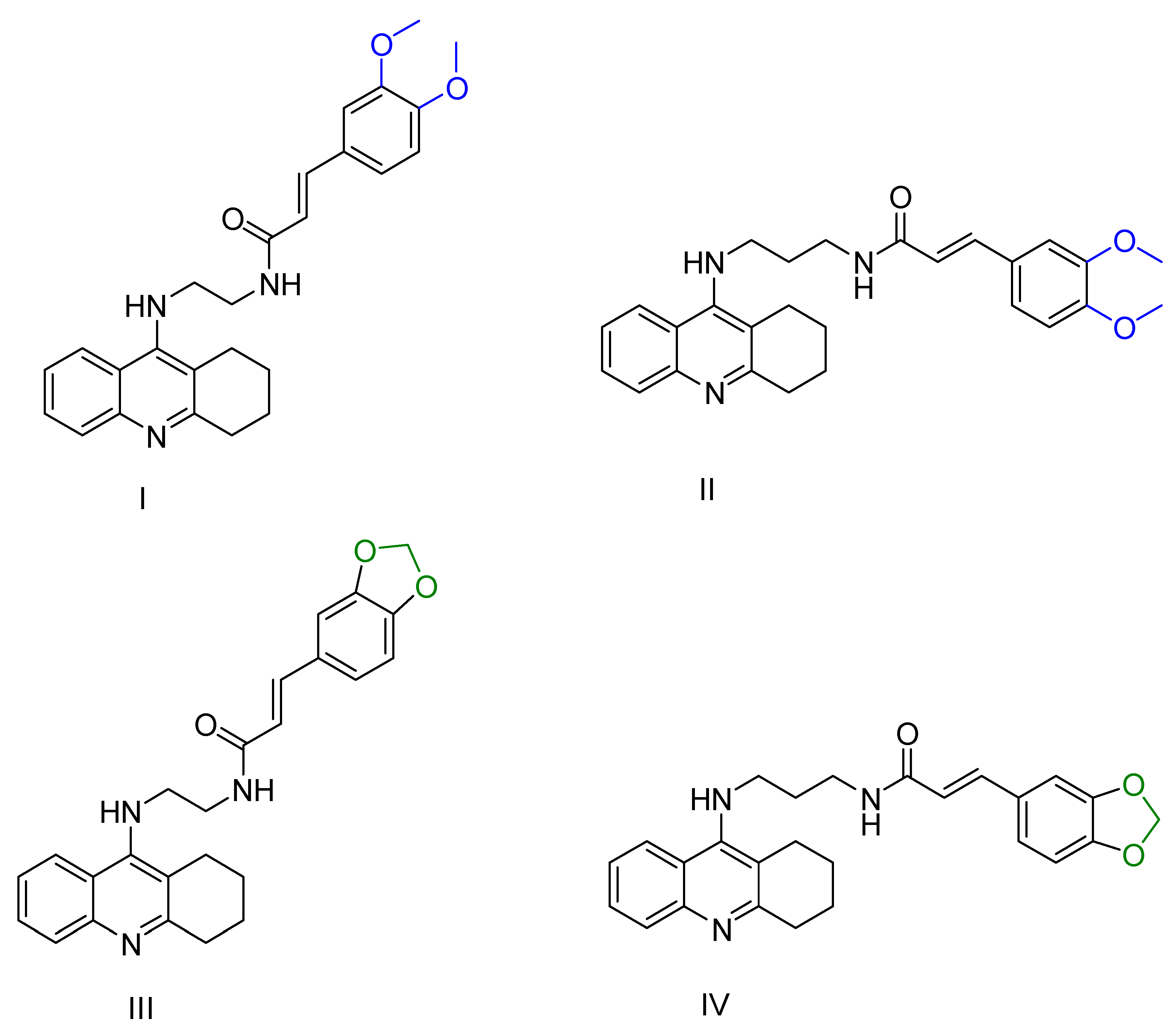
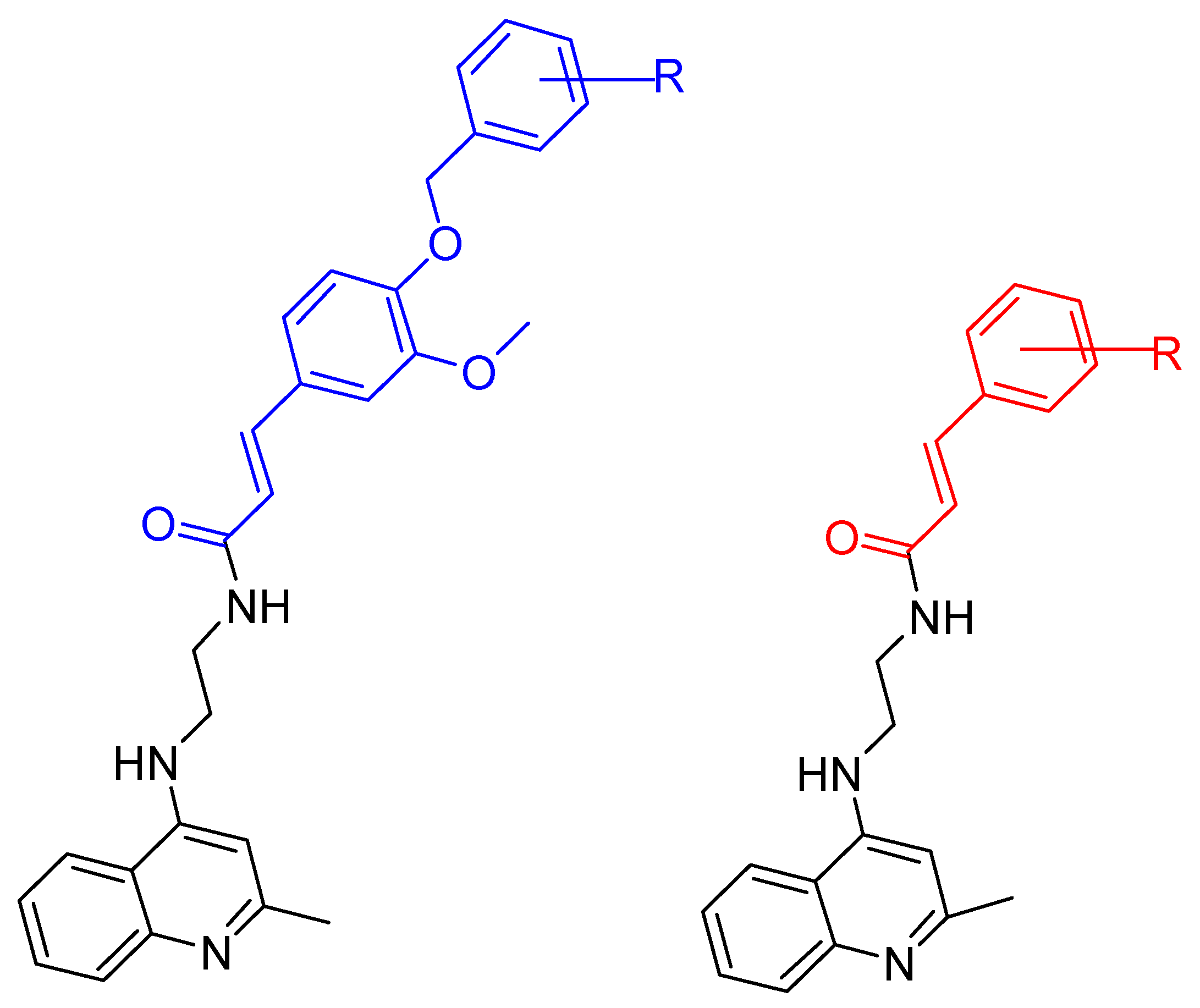
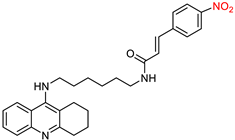
2.1. Tacrine Derivatives
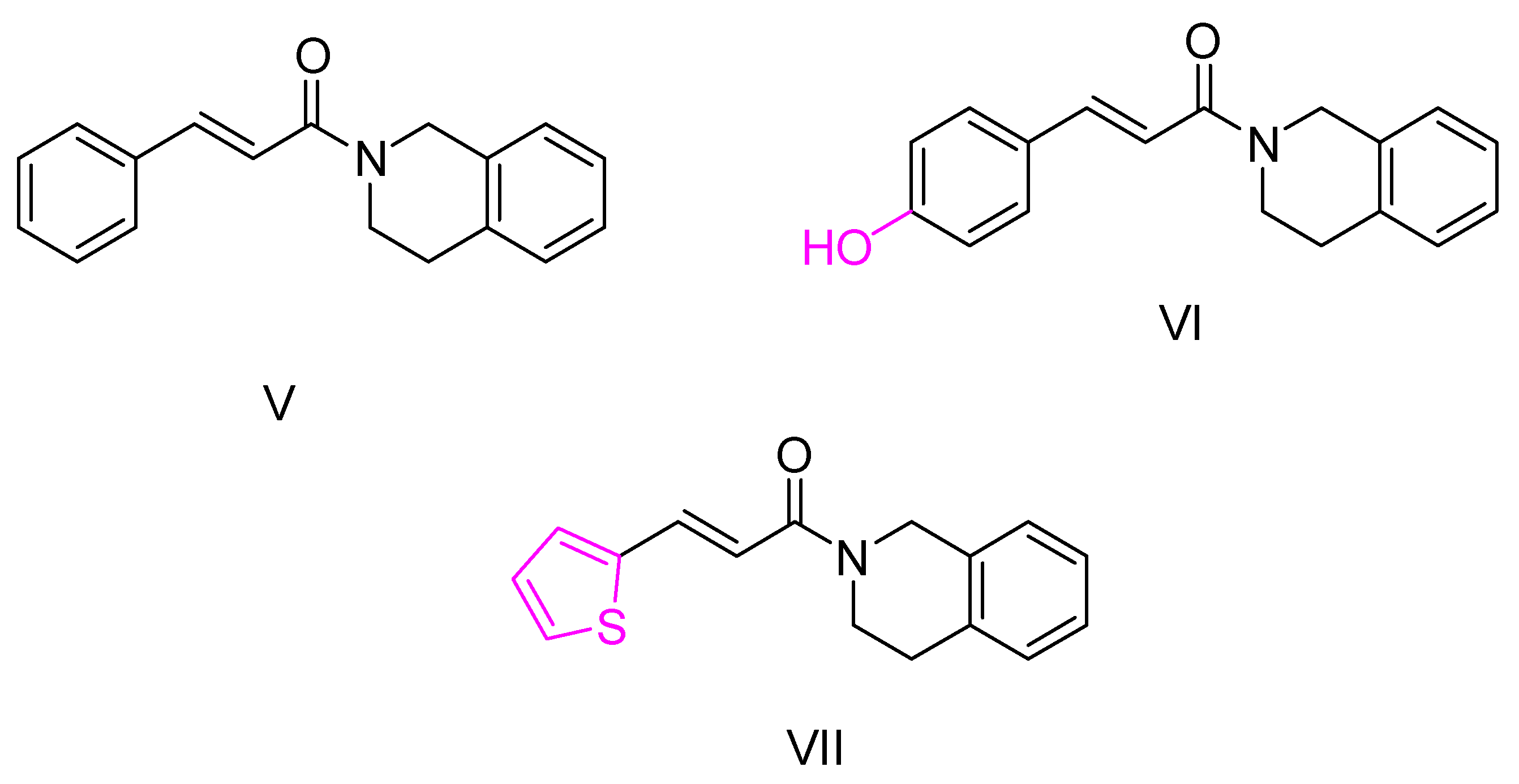
2.2. Isoquinoline and Quinoline Derivatives
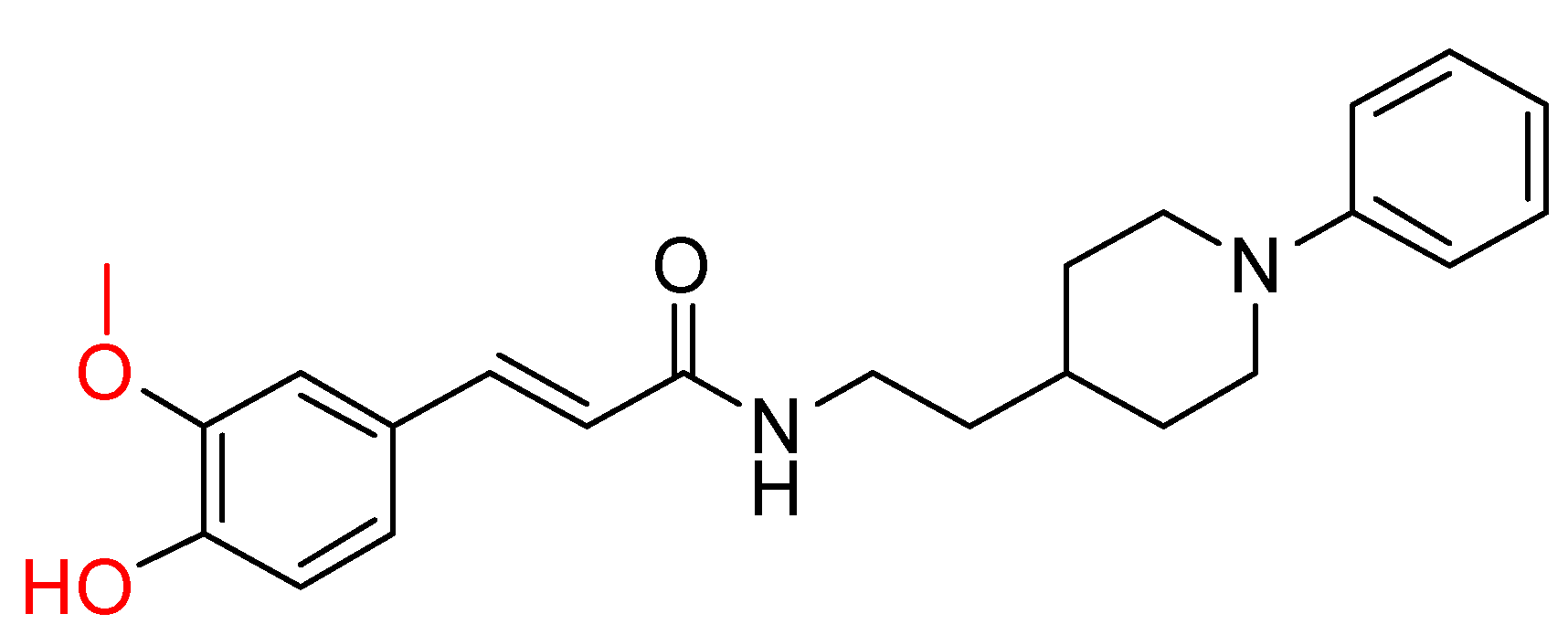
2.3. Benzylpiperidine Derivatives
2.4. β-Carboline Analogs
2.5. Tryptamine Derivatives
2.6. Rivastigmine Derivatives
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to dementia. London: Alzheimer’s Disease International. 2019. Available online: https://www.alzint.org/resource/world-alzheimer-report-2019/ (accessed on 25 December 2023).
- Tang, H.; Zhao, H.-T.; Zhong, S.-M.; Wang, Z.-Y.; Chen, Z.-F.; Liang, H. Novel oxoisoaporphine-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. Bioorg. Med. Chem. Lett. 2012, 22, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Finder, V.H. Alzheimer’s Disease: A General Introduction and Pathomechanism. J. Alzheimer’s Dis. 2010, 22, S5–S19. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Tumiatti, V.; Minarini, A.; Bolognesi, M.; Milelli, A.; Rosini, M.; Melchiorre, C. Tacrine Derivatives and Alzheimers Disease. Curr. Med. Chem. 2010, 17, 1825–1838. [Google Scholar] [CrossRef]
- Mo, S.; Gomathi, V.; Uma Maheswari, D.; Justin, A.; Venkateswarlu, B.S. Examining Novel Treatment Approaches and Problems in Alzheimer’s: An Overview. Lat. Am. J. Pharm. 2023, 42, 554–564. [Google Scholar]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Su, B.; Wang, X.; Nunomura, A.; Moreira, P.I.; Lee, H.-G.; Perry, G.; Smith, M.A.; Zhu, X. Oxidative Stress Signaling in Alzheimers Disease. Curr. Alzheimer Res. 2008, 5, 525–532. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.-Y.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Perrin, R.J.; Fagan, A.M.; Holtzman, D.M. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature 2009, 461, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, C.A.; Vilella, A.; Grabrucker, A.M. The Role of Trace Metals in Alzheimer’s Disease. In Alzheimer’s Disease; Codon Publications: New York, NY, USA, 2019; pp. 85–106. [Google Scholar]
- Devi, L.; Anandatheerthavarada, H.K. Mitochondrial trafficking of APP and alpha synuclein: Relevance to mitochondrial dysfunction in Alzheimer’s and Parkinson’s diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Arancibia, L.; Aliaga, E.; Silhol, M.; Arancibia, S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res. Rev. 2008, 59, 201–220. [Google Scholar] [CrossRef]
- Webber, K.M.; Raina, A.K.; Marlatt, M.W.; Zhu, X.; Prat, M.I.; Morelli, L.; Casadesus, G.; Perry, G.; Smith, M.A. The cell cycle in Alzheimer disease: A unique target for neuropharmacology. Mech. Ageing Dev. 2005, 126, 1019–1025. [Google Scholar] [CrossRef]
- Hamzeh, O.; Rabiei, F.; Shakeri, M.; Parsian, H.; Saadat, P.; Rostami-Mansoor, S. Mitochondrial dysfunction and inflammasome activation in neurodegenerative diseases: Mechanisms and therapeutic implications. Mitochondrion 2023, 73, 72–83. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 11, 243–256. [Google Scholar]
- Ramberg, V. Neuroinflammation in Alzheimer’s Disease: Focus on NF-κB and C/EBP Transcription Factors. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2011. [Google Scholar]
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef]
- Wenk, G.L.; Quack, G.; Moebius, H.-J.; Danysz, W. No interaction of memantine with acetylcholinesterase inhibitors approved for clinical use. Life Sci. 2000, 66, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Stancu, I.-C.; Cremers, N.; Vanrusselt, H.; Couturier, J.; Vanoosthuyse, A.; Kessels, S.; Lodder, C.; Brône, B.; Huaux, F.; Octave, J.-N.; et al. Aggregated Tau activates NLRP3–ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019, 137, 599–617. [Google Scholar] [CrossRef]
- Madav, Y.; Wairkar, S.; Prabhakar, B. Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res. Bull. 2019, 146, 171–184. [Google Scholar] [CrossRef]
- Berk, C.; Sabbagh, M.N. Successes and Failures for Drugs in Late-Stage Development for Alzheimer’s Disease. Drugs Aging 2013, 30, 783–792. [Google Scholar] [CrossRef]
- De Strooper, B. Lessons from a Failed γ-Secretase Alzheimer Trial. Cell 2014, 159, 721–726. [Google Scholar] [CrossRef]
- Yu, Y.J.; Watts, R.J. Developing Therapeutic Antibodies for Neurodegenerative Disease. Neurotherapeutics 2013, 10, 459–472. [Google Scholar] [CrossRef]
- Munoz-Torrero, D. Acetylcholinesterase Inhibitors as Disease-Modifying Therapies for Alzheimers Disease. Curr. Med. Chem. 2008, 15, 2433–2455. [Google Scholar] [CrossRef]
- Lemes, L.F.N.; Ramos, G.d.A.; de Oliveira, A.S.; da Silva, F.M.R.; Couto, G.d.C.; Boni, M.d.S.; Guimarães, M.J.R.; Souza, I.N.O.; Bartolini, M.; Andrisano, V.; et al. Cardanol-derived AChE inhibitors: Towards the development of dual binding derivatives for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 108, 687–700. [Google Scholar] [CrossRef]
- Zemek, F.; Drtinova, L.; Nepovimova, E.; Sepsova, V.; Korabecny, J.; Klimes, J.; Kuca, K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014, 13, 759–774. [Google Scholar] [CrossRef]
- De Jaeger, X.; Cammarota, M.; Prado, M.A.; Izquierdo, I.; Prado, V.F.; Pereira, G.S. Decreased acetylcholine release delays the consolidation of object recognition memory. Behav. Brain Res. 2013, 238, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Sanz-Ros, J. Alzheimer’s disease: Only prevention makes sense. Eur. J. Clin. Investig. 2018, 48, e13005. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Mortsdorf, T.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 367–384. [Google Scholar] [CrossRef]
- Desmarais, J.E.; Gauthier, S. Clinical use of cholinergic drugs in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 418–420. [Google Scholar] [CrossRef]
- Terry, A.V.; Buccafusco, J.J. The Cholinergic Hypothesis of Age and Alzheimer’s Disease-Related Cognitive Deficits: Recent Challenges and Their Implications for Novel Drug Development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Buss, S.S.; Fried, P.J.; Pascual-Leone, A. Therapeutic noninvasive brain stimulation in Alzheimer’s disease and related dementias. Curr. Opin. Neurol. 2019, 32, 292–304. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Shevtsova, E.F.; Kovaleva, N.V.; Rudakova, E.V.; Neganova, M.E.; Dubova, L.G.; Bachurin, S.O. Aminoadamantane conjugates with carbazole derivatives as potential multitarget agents for the treatment of Alzheimer’s disease. Effect of the spacer structure. Russ. Chem. Bull. 2018, 67, 2121–2126. [Google Scholar] [CrossRef]
- Liu, W.; Wu, L.; Li, D.; Huang, Y.; Liu, M.; Liu, W.; Tian, C.; Liu, X.; Jiang, X.; Hu, X.; et al. Discovery of novel tacrine derivatives as potent antiproliferative agents with CDKs inhibitory property. Bioorg. Chem. 2022, 126, 105875. [Google Scholar] [CrossRef]
- Watkins, P.B. Hepatotoxic Effects of Tacrine Administration in Patients With Alzheimer’s Disease. Jama 1994, 271, 992. [Google Scholar] [CrossRef] [PubMed]
- Qizilbash, N.; Birks, J.; López Arrieta, J.; Lewington, S.; Szeto, S. Tacrine for Alzheimer’s Disease; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Parvathy Dharshini, S.A.; Sneha, N.P.; Yesudhas, D.; Kulandaisamy, A.; Rangaswamy, U.; Shanmugam, A.; Gromiha, M.M. Exploring Plausible Therapeutic Targets for Alzheimer’s Disease using Multi-omics Approach, Machine Learning and Docking. Curr. Top. Med. Chem. 2022, 22, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular Pathogenesis of Alzheimer’s Disease: An Update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef]
- Khan, S.S.; Khatik, G.L.; Datusalia, A.K. Strategies for Treatment of Disease-Associated Dementia Beyond Alzheimer’s Disease: An Update. Curr. Neuropharmacol. 2022, 21, 309–339. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An Important New Target in Alzheimer’s Disease Therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinesterases: New Roles in Brain Function and in Alzheimer’s Disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Perry, E.K.; Perry, R.H.; Blessed, G.; Tomlinson, B.E. Changes in Brain Cholinesterases in Senile Dementia of Alzheimer Type. Neuropathol. Appl. Neurobiol. 1978, 4, 273–277. [Google Scholar] [CrossRef]
- Shao, Z.; Liu, J. Spatio-temporal Hybrid Automata for Cyber-Physical Systems. In Proceedings of the Theoretical Aspects of Computing–ICTAC 2013: 10th International Colloquium, Shanghai, China, 4–6 September 2013; pp. 337–354. [Google Scholar]
- Khoobi, M.; Alipour, M.; Sakhteman, A.; Nadri, H.; Moradi, A.; Ghandi, M.; Shafiee, A. Design, synthesis, biological evaluation and docking study of 5-oxo-4,5-dihydropyrano[3,2-c]chromene derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. Eur. J. Med. Chem. 2013, 68, 260–269. [Google Scholar] [CrossRef]
- Bullock, R.; Lane, R. Executive Dyscontrol in Dementia, with Emphasis on Subcortical Pathology and the Role of Butyrylcholinesterase. Curr. Alzheimer Res. 2007, 4, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A Review of Butyrylcholinesterase as a Therapeutic Target in the Treatment of Alzheimer’s Disease. Prim. Care Companion J. Clin. Psychiatry 2013, 15, 26731. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Taylor, P.; Radić, Z.; Marchot, P. Structural insights into ligand interactions at the acetylcholinesterase peripheral anionic site. EMBO J. 2003, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Peitzika, S.-C.; Pontiki, E. A Review on Recent Approaches on Molecular Docking Studies of Novel Compounds Targeting Acetylcholinesterase in Alzheimer Disease. Molecules 2023, 28, 1084. [Google Scholar] [CrossRef] [PubMed]
- Santin, Y.; Resta, J.; Parini, A.; Mialet-Perez, J. Monoamine oxidases in age-associated diseases: New perspectives for old enzymes. Ageing Res. Rev. 2021, 66, 101256. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H. A critical appraisal of MAO-B inhibitors in the treatment of Parkinson’s disease. J. Neural Transm. 2022, 129, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Schedin-Weiss, S.; Inoue, M.; Hromadkova, L.; Teranishi, Y.; Yamamoto, N.G.; Wiehager, B.; Tjernberg, L.O. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimer’s Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef]
- Good, P.F.; Werner, P.; Hsu, A.; Olanow, C.W.; Perl, D.P. Evidence of neuronal oxidative damage in Alzheimer’s disease. Am. J. Pathol. 1996, 149, 21–28. [Google Scholar]
- Masters, C.L.; Cappai, R.; Barnham, K.J.; Villemagne, V.L. Molecular mechanisms for Alzheimer’s disease: Implications for neuroimaging and therapeutics. J. Neurochem. 2006, 97, 1700–1725. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Mullard, A. Alzheimer amyloid hypothesis lives on. Nat. Rev. Drug Discov. 2017, 16, 3–5. [Google Scholar] [CrossRef]
- Butini, S.; Brindisi, M.; Brogi, S.; Maramai, S.; Guarino, E.; Panico, A.; Saxena, A.; Chauhan, V.; Colombo, R.; Verga, L.; et al. Multifunctional Cholinesterase and Amyloid Beta Fibrillization Modulators. Synthesis and Biological Investigation. ACS Med. Chem. Lett. 2013, 4, 1178–1182. [Google Scholar] [CrossRef][Green Version]
- Bajda, M.; Guzior, N.; Ignasik, M.; Malawska, B. Multi-Target-Directed Ligands in Alzheimer’s Disease Treatment. Curr. Med. Chem. 2011, 18, 4949–4975. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Rosini, M.; Andrisano, V.; Bartolini, M.; Minarini, A.; Tumiatti, V.; Melchiorre, C. MTDL Design Strategy in the Context of Alzheimers Disease: From Lipocrine to Memoquin and Beyond. Curr. Pharm. Des. 2009, 15, 601–613. [Google Scholar] [CrossRef]
- Spilovska, K.; Korabecny, J.; Sepsova, V.; Jun, D.; Hrabinova, M.; Jost, P.; Muckova, L.; Soukup, O.; Janockova, J.; Kucera, T.; et al. Novel Tacrine-Scutellarin Hybrids as Multipotent Anti-Alzheimer’s Agents: Design, Synthesis and Biological Evaluation. Molecules 2017, 22, 1006. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Melchiorre, C. From dual binding site acetylcholinesterase inhibitors to multi-target-directed ligands (MTDLs): A step forward in the treatment of Alzheimer’s disease. Mini-Rev. Med. Chem. 2008, 8, 960–967. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-Directed Ligands To Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Chen, H. AChE Inhibition-based Multi-target-directed Ligands, a Novel Pharmacological Approach for the Symptomatic and Disease-modifying Therapy of Alzheimer’s Disease. Curr. Neuropharmacol. 2016, 14, 364–375. [Google Scholar] [CrossRef]
- Benek, O.; Korabecny, J.; Soukup, O. A Perspective on Multi-target Drugs for Alzheimer’s Disease. Trends Pharmacol. Sci. 2020, 41, 434–445. [Google Scholar] [CrossRef]
- Knez, D.; Sova, M.; Košak, U.; Gobec, S. Dual inhibitors of cholinesterases and monoamine oxidases for Alzheimer’s disease. Futur. Med. Chem. 2017, 9, 811–832. [Google Scholar] [CrossRef]
- Lu, J.; Pan, W.; Hu, Y.-J.; Wang, Y.-T. Multi-Target Drugs: The Trend of Drug Research and Development. PLoS ONE 2012, 7, e40262. [Google Scholar] [CrossRef]
- Prati, F.; Uliassi, E.; Bolognesi, M.L. Two diseases, one approach: Multitarget drug discovery in Alzheimer’s and neglected tropical diseases. MedChemComm 2014, 5, 853–861. [Google Scholar] [CrossRef]
- Melchiorre, C.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V. Polyamines in Drug Discovery: From the Universal Template Approach to the Multitarget-Directed Ligand Design Strategy. J. Med. Chem. 2010, 53, 5906–5914. [Google Scholar] [CrossRef]
- Sang, Z.; Li, Y.; Qiang, X.; Xiao, G.; Liu, Q.; Tan, Z.; Deng, Y. Multifunctional scutellarin–rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 668–680. [Google Scholar] [CrossRef]
- Xie, S.S.; Lan, J.S.; Wang, X.; Wang, Z.M.; Jiang, N.; Li, F.; Kong, L.Y. Design, synthesis and biological evaluation of novel donepezil–coumarin hybrids as multi-target agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2016, 24, 1528–1539. [Google Scholar] [CrossRef]
- Dias, K.S.T.; Viegas, C. Multi-Target Directed Drugs: A Modern Approach for Design of New Drugs for the treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2014, 12, 239–255. [Google Scholar] [CrossRef]
- Keri, R.S.; Quintanova, C.; Chaves, S.; Silva, D.F.; Cardoso, S.M.; Santos, M.A. New Tacrine Hybrids with Natural-Based Cysteine Derivatives as Multitargeted Drugs for Potential Treatment of Alzheimer’s Disease. Chem. Biol. Drug Des. 2016, 87, 101–111. [Google Scholar] [CrossRef]
- Leon, R.; Marco-Contelles, J. A Step Further Towards Multitarget Drugs for Alzheimer and Neuronal Vascular Diseases: Targeting the Cholinergic System, Amyloid-β Aggregation and Ca2++ Dyshomeostasis. Curr. Med. Chem. 2011, 18, 552–576. [Google Scholar] [CrossRef]
- Simoni, E.; Daniele, S.; Bottegoni, G.; Pizzirani, D.; Trincavelli, M.L.; Goldoni, L.; Cavalli, A. Combining galantamine and memantine in multitargeted, new chemical entities potentially useful in Alzheimer’s disease. J. Med. Chem. 2012, 55, 9708–9721. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Capsoni, S.; Andrisano, V.; Bartolini, M.; Margotti, E.; Cattaneo, A.; Recanatini, M.; Melchiorre, C. A Small Molecule Targeting the Multifactorial Nature of Alzheimer’s Disease. Angew. Chem. Int. Ed. 2007, 46, 3689–3692. [Google Scholar] [CrossRef]
- Cummings, J.; Ritter, A.; Zhong, K. Clinical Trials for Disease-Modifying Therapies in Alzheimer’s Disease: A Primer, Lessons Learned, and a Blueprint for the Future. J. Alzheimer’s Dis. 2018, 64, S3-22. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef]
- Al Maruf, A.; Lip, H.; Wong, H.; O’brien, P.J. Protective effects of ferulic acid and related polyphenols against glyoxal- or methylglyoxal-induced cytotoxicity and oxidative stress in isolated rat hepatocytes. Chem. Interact. 2015, 234, 96–104. [Google Scholar] [CrossRef]
- Villareal, M.O.; Sasaki, K.; Margout, D.; Savry, C.; Almaksour, Z.; Larroque, M.; Isoda, H. Neuroprotective effect of Picholine virgin olive oil and its hydroxycinnamic acids component against β-amyloid-induced toxicity in SH-SY5Y neurotypic cells. Cytotechnology 2016, 68, 2567–2578. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Chen, Q.; Lu, J.; Rapposelli, S.; Pi, R. A review on the hybrids of hydroxycinnamic acid as multi-target-directed ligands against Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 543–550. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Bar-Am, O.; Youdim, M.B. Induction of Neurotrophic Factors GDNF and BDNF Associated with the Mechanism of Neurorescue Action of Rasagiline and Ladostigil. Ann. N. Y. Acad. Sci. 2007, 1122, 155–168. [Google Scholar] [CrossRef]
- Luo, X.-T.; Wang, C.-M.; Liu, Y.; Huang, Z.-G. New multifunctional melatonin-derived benzylpyridinium bromides with potent cholinergic, antioxidant, and neuroprotective properties as innovative drugs for Alzheimer’s disease. Eur. J. Med. Chem. 2015, 103, 302–311. [Google Scholar] [CrossRef]
- Xie, S.S.; Lan, J.S.; Wang, X.B.; Jiang, N.; Dong, G.; Li, Z.R.; Kong, L.Y. Multifunctional tacrine–trolox hybrids for the treatment of Alzheimer’s disease with cholinergic, antioxidant, neuroprotective and hepatoprotective properties. Eur. J. Med. Chem. 2015, 93, 42–50. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 631–638. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Goncalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular Hybridization as a Tool for Designing Multitarget Drug Candidates for Complex Diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.-S.; Hou, J.-W.; Liu, Y.; Ding, Y.; Zhang, Y.; Li, L.; Zhang, T. Design, synthesis and evaluation of novel cinnamic acid derivatives bearing N-benzyl pyridinium moiety as multifunctional cholinesterase inhibitors for Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2017, 32, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.; Mo, J.; Yang, H.; Jiang, X.; Lin, H.; Gu, K.; Pei, Y.; Wu, L.; Tan, R.; et al. Synthesis and bioevaluation of new tacrine-cinnamic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2018, 33, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, H.; Yang, H.; Tan, R.; Bian, Y.; Fu, T.; Li, W.; Wu, L.; Pei, Y.; Sun, H. Discovery of new acetylcholinesterase and butyrylcholinesterase inhibitors through structure-based virtual screening. RSC Adv. 2017, 7, 3429–3438. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Zhu, J.; Gu, K.; Li, Q.; He, S.; Sun, H. Design, synthesis, in vitro and in vivo evaluation of tacrine–cinnamic acid hybrids as multi-target acetyl- and butyrylcholinesterase inhibitors against Alzheimer’s disease. RSC Adv. 2017, 7, 33851–33867. [Google Scholar] [CrossRef]
- Quintanova, C.; Keri, R.S.; Marques, S.M.; G-Fernandes, M.; Cardoso, S.M.; Serralheiro, M.L.; Santos, M.A. Design, synthesis and bioevaluation of tacrine hybrids with cinnamate and cinnamylidene acetate derivatives as potential anti-Alzheimer drugs. MedChemComm 2015, 6, 1969–1977. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Wang, K.; Shi, J.; Zhou, Y.; He, Y.; Mi, J.; Yang, J.; Liu, S.; Tang, X.; Liu, W.; Tan, Z.; et al. Design, synthesis and evaluation of cinnamic acid hybrids as multi-target-directed agents for the treatment of Alzheimer’s disease. Bioorg. Chem. 2021, 112, 104879. [Google Scholar] [CrossRef]
- Li, T.; Pan, W.; Wang, K.; Liu, W.; Ma, Q.; Sang, Z. Novel Ferulic Acid-donepezil Hybrids as Multifunctional Agents for th e Treatment of Alzheimer’s Disease with Butyrylcholinesterase, Amyloid-β, Antioxidant and Neuroprotective Properties. Lett. Drug Des. Discov. 2017, 14, 918–929. [Google Scholar] [CrossRef]
- Sang, Z.; Wang, K.; Han, X.; Cao, M.; Tan, Z.; Liu, W. Design, Synthesis, and Evaluation of Novel Ferulic Acid Derivatives as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 1008–1024. [Google Scholar] [CrossRef]
- Sang, Z.; Pan, W.; Wang, K.; Ma, Q.; Yu, L.; Yang, Y.; Bai, P.; Leng, C.; Xu, Q.; Li, X.; et al. Design, synthesis and evaluation of novel ferulic acid- O -alkylamine derivatives as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 130, 379–392. [Google Scholar] [CrossRef]
- Sang, Z.; Wang, K.; Shi, J.; Cheng, X.; Zhu, G.; Wei, R.; Ma, Q.; Yu, L.; Zhao, Y.; Tan, Z.; et al. Apigenin-rivastigmine hybrids as multi-target-directed liagnds for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 187, 111958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Y.; Xu, L.; Sha, C.; Zhang, H.; Xu, W. Resveratrol alleviates lipopolysaccharide-induced inflammation in PC-12 cells and in rat model. BMC Biotechnol. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Huang, C.; Wu, J.; Sun, L.; Hao, W.; Leung, L.K.; Huang, J. The neuroprotective effects of ipriflavone against H2O2 and amyloid beta induced toxicity in human neuroblastoma SH-SY5Y cells. Eur. J. Pharmacol. 2013, 721, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Umar, T.; Shalini, S.; Raza, M.K.; Gusain, S.; Kumar, J.; Ahmed, W.; Hoda, N. New amyloid beta-disaggregating agents: Synthesis, pharmacological evaluation, crystal structure and molecular docking of N-(4-((7-chloroquinolin-4-yl)oxy)-3-ethoxybenzyl)amines. MedChemComm 2018, 9, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N.; Kofinas, A.; Papanikolaou, M.G.; Miras, H.N.; Drouza, C.; Kalampounias, A.G.; Leondaritis, G. Synthesis, characterization and pharmacological evaluation of quinoline derivatives and their complexes with copper(ΙΙ) in in vitro cell models of Alzheimer’s disease. J. Inorg. Biochem. 2021, 217, 111393. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-X.; Cheng, Z.-Q.; Zhou, L.; Xie, H.-X.; Wang, Y.-Y.; Zhu, K.; Jiao, Y.; Liu, G.; Jiang, C.-S. Synthesis and biological evaluation of quinoline/cinnamic acid hybrids as amyloid-beta aggregation inhibitors. Monatshefte Für Chem. Chem. Mon. 2020, 151, 845–852. [Google Scholar] [CrossRef]
- Guzior, N.; Wieckowska, A.; Panek, D.; Malawska, B. Recent Development of Multifunctional Agents as Potential Drug Candidates for the Treatment of Alzheimer’s Disease. Curr. Med. Chem. 2014, 22, 373–404. [Google Scholar] [CrossRef]
- Jiang, C.-S.; Ge, Y.-X.; Cheng, Z.-Q.; Wang, Y.-Y.; Tao, H.-R.; Zhu, K.; Zhang, H. Discovery of New Selective Butyrylcholinesterase (BChE) Inhibitors with Anti-Aβ Aggregation Activity: Structure-Based Virtual Screening, Hit Optimization and Biological Evaluation. Molecules 2019, 24, 2568. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, H.; Chen, Y.; Lin, H.; Li, Q.; Mo, J.; Bian, Y.; Pei, Y.; Sun, H. Synthesis, pharmacology and molecular docking on multifunctional tacrine-ferulic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2018, 33, 496–506. [Google Scholar] [CrossRef]
- Mo, J.; Yang, H.; Chen, T.; Li, Q.; Lin, H.; Feng, F.; Sun, H. Design, synthesis, biological evaluation, and molecular modeling studies of quinoline-ferulic acid hybrids as cholinesterase inhibitors. Bioorg. Chem. 2019, 93, 103310. [Google Scholar] [CrossRef]
- Pourabdi, L.; Khoobi, M.; Nadri, H.; Moradi, A.; Moghadam, F.H.; Emami, S.; Mojtahedi, M.M.; Haririan, I.; Forootanfar, H.; Ameri, A.; et al. Synthesis and structure-activity relationship study of tacrine-based pyrano[2,3-c]pyrazoles targeting AChE/BuChE and 15-LOX. Eur. J. Med. Chem. 2016, 123, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-S.; Wang, X.-B.; Li, J.-Y.; Yang, L.; Kong, L.-Y. Design, synthesis and evaluation of novel tacrine–coumarin hybrids as multifunctional cholinesterase inhibitors against Alzheimer’s disease. Eur. J. Med. Chem. 2013, 64, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Montanari, S.; Bartolini, M.; Neviani, P.; Belluti, F.; Gobbi, S.; Pruccoli, L.; Rampa, A. Multitarget Strategy to Address Alzheimer’s Disease: Design, Synthesis, Biological Evaluation, and Computational Studies of Coumarin-Based Derivatives. ChemMedChem 2016, 11, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Bartolini, M.; Piazzi, L.; Valgimigli, L.; Amorati, R.; Bolondi, C.; Rampa, A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharmacol. Res. Perspect. 2014, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Estrada, M.; Herrera-Arozamena, C.; Pérez, C.; Viña, D.; Romero, A.; Morales-García, J.A.; Pérez-Castillo, A.; Rodríguez-Franco, M.I. New cinnamic–N-benzylpiperidine and cinnamic–N,N-dibenzyl(N-methyl)amine hybrids as Alzheimer-directed multitarget drugs with antioxidant, cholinergic, neuroprotective and neurogenic properties. Eur. J. Med. Chem. 2016, 121, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.; Sood, A.; Peerannawar, S.; Kugyela, N.; Kulkarni, A.; Tulsan, R.; Török, M. Synthesis and application of β-carbolines as novel multi-functional anti-Alzheimer’s disease agents. Bioorg. Med. Chem. Lett. 2017, 27, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Hamann, J.; Wernicke, C.; Lehmann, J.; Reichmann, H.; Rommelspacher, H.; Gille, G. 9-Methyl-β-carboline up-regulates the appearance of differentiated dopaminergic neurones in primary mesencephalic culture. Neurochem. Int. 2008, 52, 688–700. [Google Scholar] [CrossRef]
- Gruss, M.; Appenroth, D.; Flubacher, A.; Enzensperger, C.; Bock, J.; Fleck, C.; Braun, K. 9-Methyl-β-carboline-induced cognitive enhancement is associated with elevated hippocampal dopamine levels and dendritic and synaptic proliferation. J. Neurochem. 2012, 121, 924–931. [Google Scholar] [CrossRef]
- Francik, R.; Kazek, G.; Cegła, M.; Stepniewski, M. Antioxidant activity of beta-carboline derivatives. Acta Pol. Pharmaceut. Drug Res. 2011, 68, 185–189. [Google Scholar]
- Sanchez-Ramos, J.R. Banisterine and Parkinson’s Disease. Clin. Neuropharmacol. 1991, 14, 391–402. [Google Scholar] [CrossRef]
- Liao, Q.; Li, Q.; Zhao, Y.; Jiang, P.; Yan, Y.; Sun, H.; Liu, W.; Feng, F.; Qu, W. Design, synthesis and biological evaluation of novel carboline-cinnamic acid hybrids as multifunctional agents for treatment of Aslzheimer’s disease. Bioorg. Chem. 2020, 99, 103844. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Guo, Y.; Yan, J.; Luo, Z.; Luo, H.B.; Yan, M.; Li, X. Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer’s disease. J. Med. Chem. 2013, 56, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M.; Simoni, E.; Bartolini, M.; Cavalli, A.; Ceccarini, L.; Pascu, N.; Melchiorre, C. Inhibition of acetylcholinesterase, β-amyloid aggregation, and NMDA receptors in Alzheimer’s disease: A promising direction for the multi-target-directed ligands gold rush. J. Med. Chem. 2008, 51, 4381–4384. [Google Scholar] [CrossRef] [PubMed]
- Ghanei-Nasab, S.; Khoobi, M.; Hadizadeh, F.; Marjani, A.; Moradi, A.; Nadri, H.; Emami, S.; Foroumadi, A.; Shafiee, A. Synthesis and anticholinesterase activity of coumarin-3-carboxamides bearing tryptamine moiety. Eur. J. Med. Chem. 2016, 121, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ghafary, S.; Ghobadian, R.; Mahdavi, M.; Nadri, H.; Moradi, A.; Akbarzadeh, T.; Najafi, Z.; Sharifzadeh, M.; Edraki, N.; Moghadam, F.H.; et al. Design, synthesis, and evaluation of novel cinnamic acid-tryptamine hybrid for inhibition of acetylcholinesterase and butyrylcholinesterase. DARU J. Pharm. Sci. 2020, 28, 463–477. [Google Scholar] [CrossRef]
- Chen, Z.; Digiacomo, M.; Tu, Y.; Gu, Q.; Wang, S.; Yang, X.; Chu, J.; Chen, Q.; Han, Y.; Chen, J.; et al. Discovery of novel rivastigmine-hydroxycinnamic acid hybrids as multi-targeted agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 125, 784–792. [Google Scholar] [CrossRef]


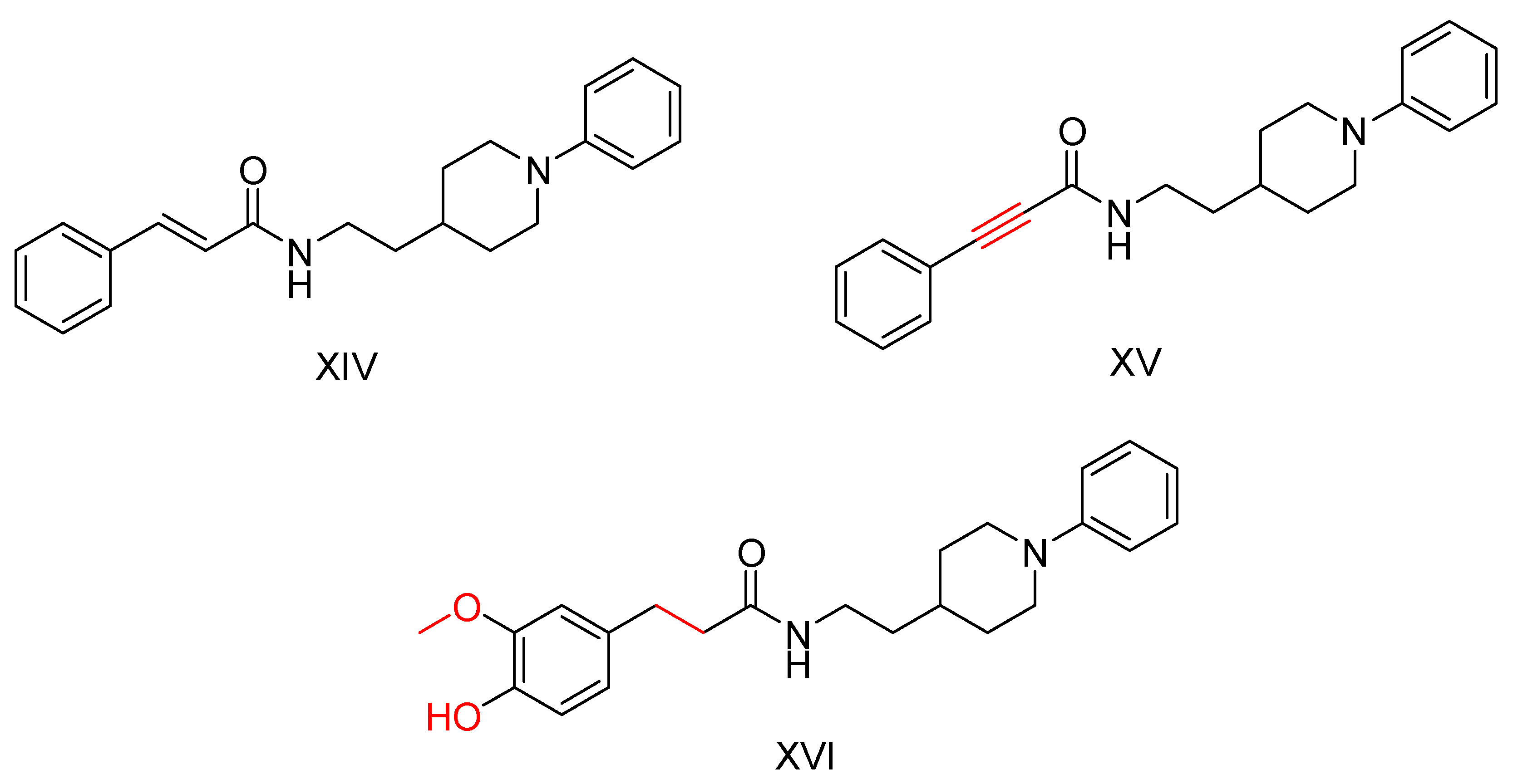
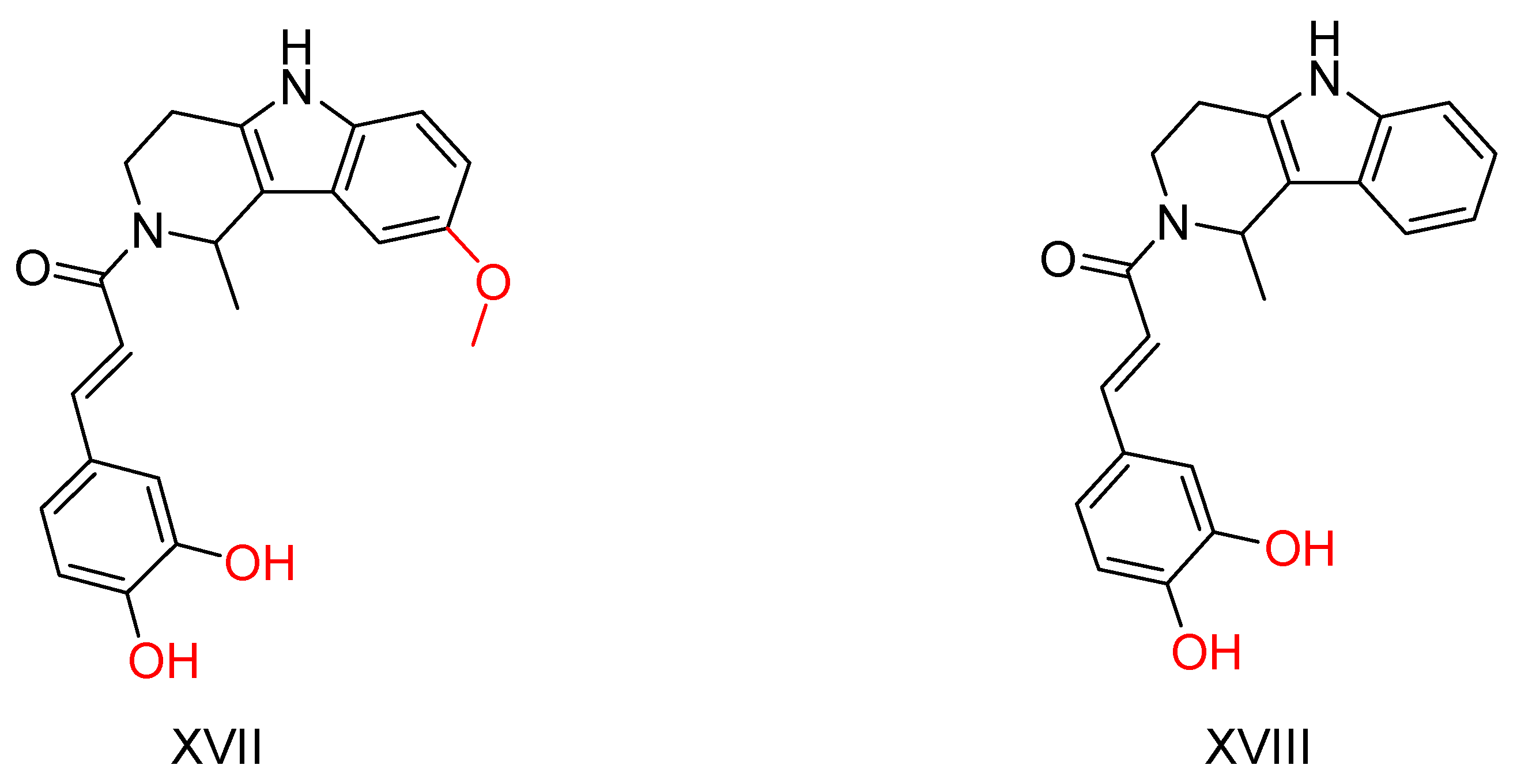
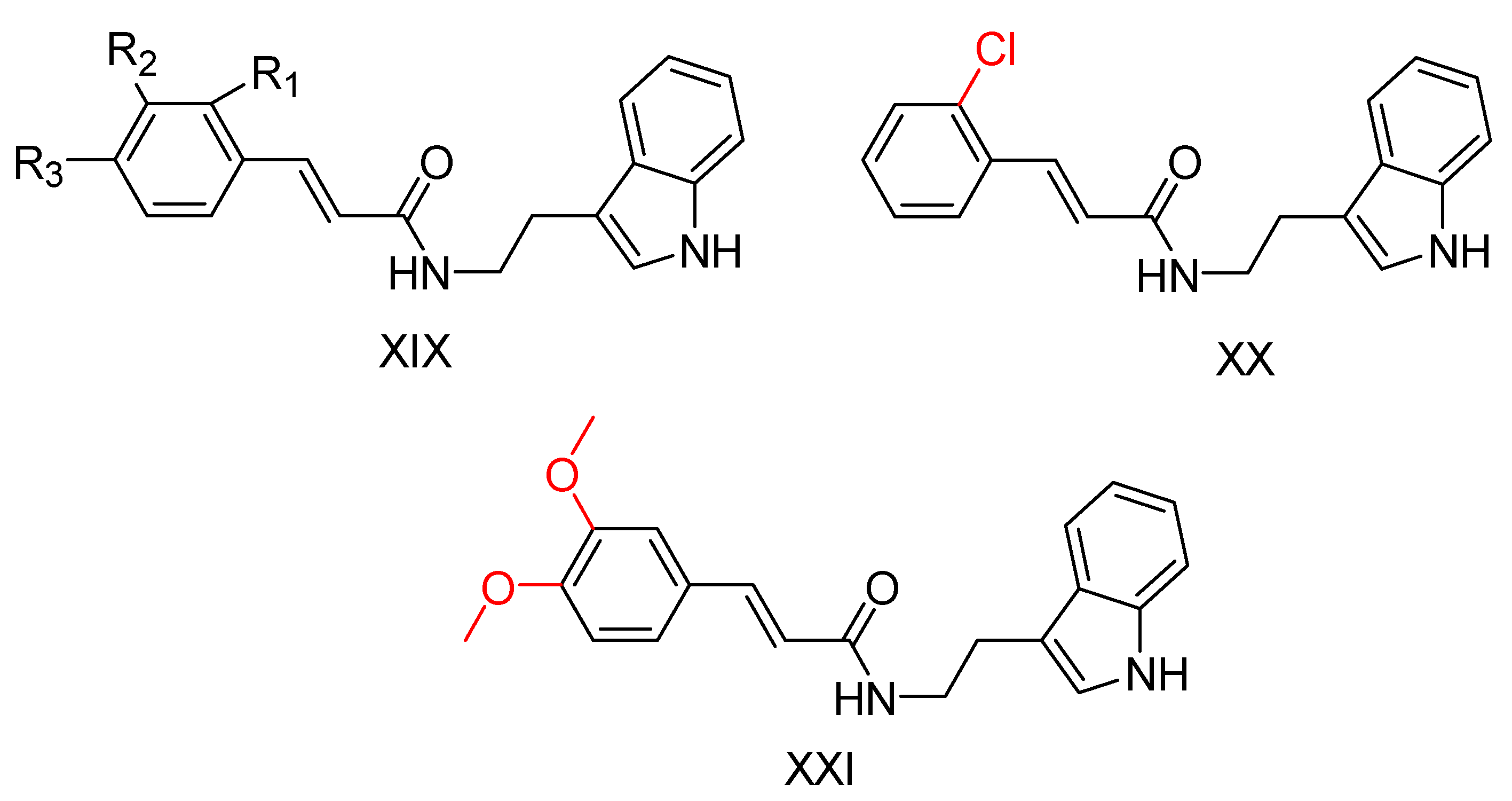

| Compound | Synthesized by | a AChE-IC50 (μM) or % | b huAChE- IC50(μM) | c BuChE-IC50 (μM) or % | d hu-BuChE-IC50 (μM) | e % Inhibition of Self-Induced Aβ | Other |
|---|---|---|---|---|---|---|---|
 | Chen, Y. et al. [96] | 2.7 ± 0.4 nM | 6.5 ± 0.6 nM | 6.3 ± 0.3 nM | 10.2 ± 1.2 nM | Under the concentration of 25 μM: 31.82 | In vivo active (Morris water maze). Preliminary safe in hepatotoxicity studies. |
 | Chen, Y. et al. [96] | 6.9 ± 1.2 nM | 16.5 ± 1.7 nM | 12.9 ± 1.7 nM | 12.9 ± 1.7 nM | Under the concentration of 25 μM: 42.22 | In vivo active (Morris water maze). Preliminary safe in hepatotoxicity studies. |
 | Chen, Y. et al. [96] | 3.7 ± 1.5 nM | 15.3 ± 1.8 nM | 22.5 ± 5.9 nM | 8.0 ± 1.1 nM | Under the concentration of 25 μM: 34.57 | In vivo active (Morris water maze). Preliminary safe in hepatotoxicity studies. |
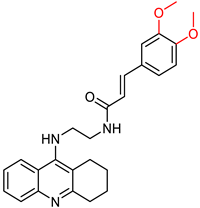 | Quintanova, C. et al. [100] | 0.09 ± 0.02 | f n.t. | n.t | n.t | Under the concentration of 80 μM: 19.6 | Percentage of inhibition of antioxidant activity for 1 mM concentration (DPPH assay): 8.8%. 2.5μΜ of the compound showed protection against Aβ and H2O2 induced toxicity in neuroblastoma cells. |
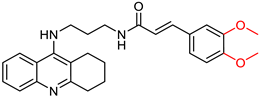 | Quintanova, C. et al. [100] | 0.09 ± 0.01 | n.t. | n.t | n.t | Under the concentration of 80 μM: 56.5 | - |
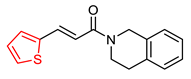 | Wang, K. et al. [102] | 19.4 ± 1.5 | n.t. | 2.1 ± 0.08 | 2.5 ± 0.09 | Under the concentration of 25 μM: 65.2 | Neuroprotective effects against Aβ-induced SH-SY5Y cell toxicity. Anti-inflammatory property (MTT assay). g MAO-B inhibition activity (IC50 = 1.3 μM). The compound improved dyskinesia recovery rate and memory impairment. |
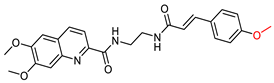 | Ge, Y-X. et al. [111] | n.t. | n.t. | n.t. | n.t. | Under the concentration of 100 μM: 76.3 ± 2.6 | Dose-dependent neuroprotective effect (evaluation) against Aβ42-induced neurotoxicity in SH-SY5Y cells. |
 | Mo, J. et al. [115] | 3.54 ± 1.12 | n.t. | 1.51 ± 0.28 | n.t. | No action | Neuroprotective effect against H2O2-induced cell death in PC12 neurons. |
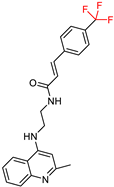 | Mo, J. et al. [115] | 3.28 ± 1.32 | n.t | 0.93 ± 0.40 | n.t. | No action | Lower hepatotoxicity than tacrine. |
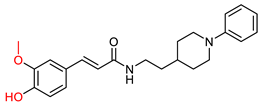 | Estrada, M. et al. [120] | n.t. | 0.39 ± 0.05 | n.t. | 0.076 ± 0.01 | n.t. | Antioxidant properties (ORAC assay). Neuroprotective properties. (Protection of cell line SH-SY5Y against damage by free radicals in the mitochondria.) |
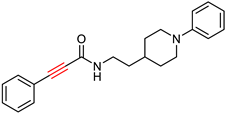 | Wang, K. et al. [102] | 16.5 ± 1.2 | n.t. | 3.3 ± 0.2 | 5.9 ± 0.4 | Under the concentration of 25 μM: 70.3 ± 5.3 | Anti-inflammatory properties. IC50 values against huMAO-B: 9.1 ± 0.2. |
 | Liao, Q. et al. [126] | 21.29 ± 3.63 | n.t. | 1.32 ± 0.85 | n.t. | Under the concentration of 20 μM: 72.51 ± 0.84 | Capability of restoring learning and memory function to AD model mice. |
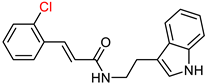 | Ghafary, S. et al. [130] | 21.11 | n.t. | 1.95 | n.t. | n.t. | Selectivity over BuChE. |
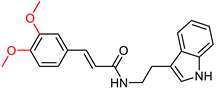 | Ghafary, S. et al. [130] | 11.51 | n.t. | >100 | n.t. | Under the concentration of 50 μM: 10.12 ± 1.23% | Mild neuroprotective activity. |
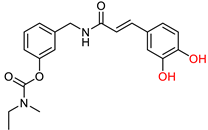 | Chen, Z. et al. [131] | Under the concentration of 5 μΜ: 23.3% | n.t. | Under the concentration of 5 μΜ: 98.34% | n.t. | Under the concentration of 10 μM: 77.6% | Antioxidant properties (DPPH assay). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drakontaeidi, A.; Pontiki, E. Multi-Target-Directed Cinnamic Acid Hybrids Targeting Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 582. https://doi.org/10.3390/ijms25010582
Drakontaeidi A, Pontiki E. Multi-Target-Directed Cinnamic Acid Hybrids Targeting Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(1):582. https://doi.org/10.3390/ijms25010582
Chicago/Turabian StyleDrakontaeidi, Aliki, and Eleni Pontiki. 2024. "Multi-Target-Directed Cinnamic Acid Hybrids Targeting Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 1: 582. https://doi.org/10.3390/ijms25010582
APA StyleDrakontaeidi, A., & Pontiki, E. (2024). Multi-Target-Directed Cinnamic Acid Hybrids Targeting Alzheimer’s Disease. International Journal of Molecular Sciences, 25(1), 582. https://doi.org/10.3390/ijms25010582






