Regulating Pathways of Bacillus pumilus Adamalysin-like Metalloendopeptidase Expression
Abstract
1. Introduction
2. Results
2.1. Promoter Region Analysis of mprBp
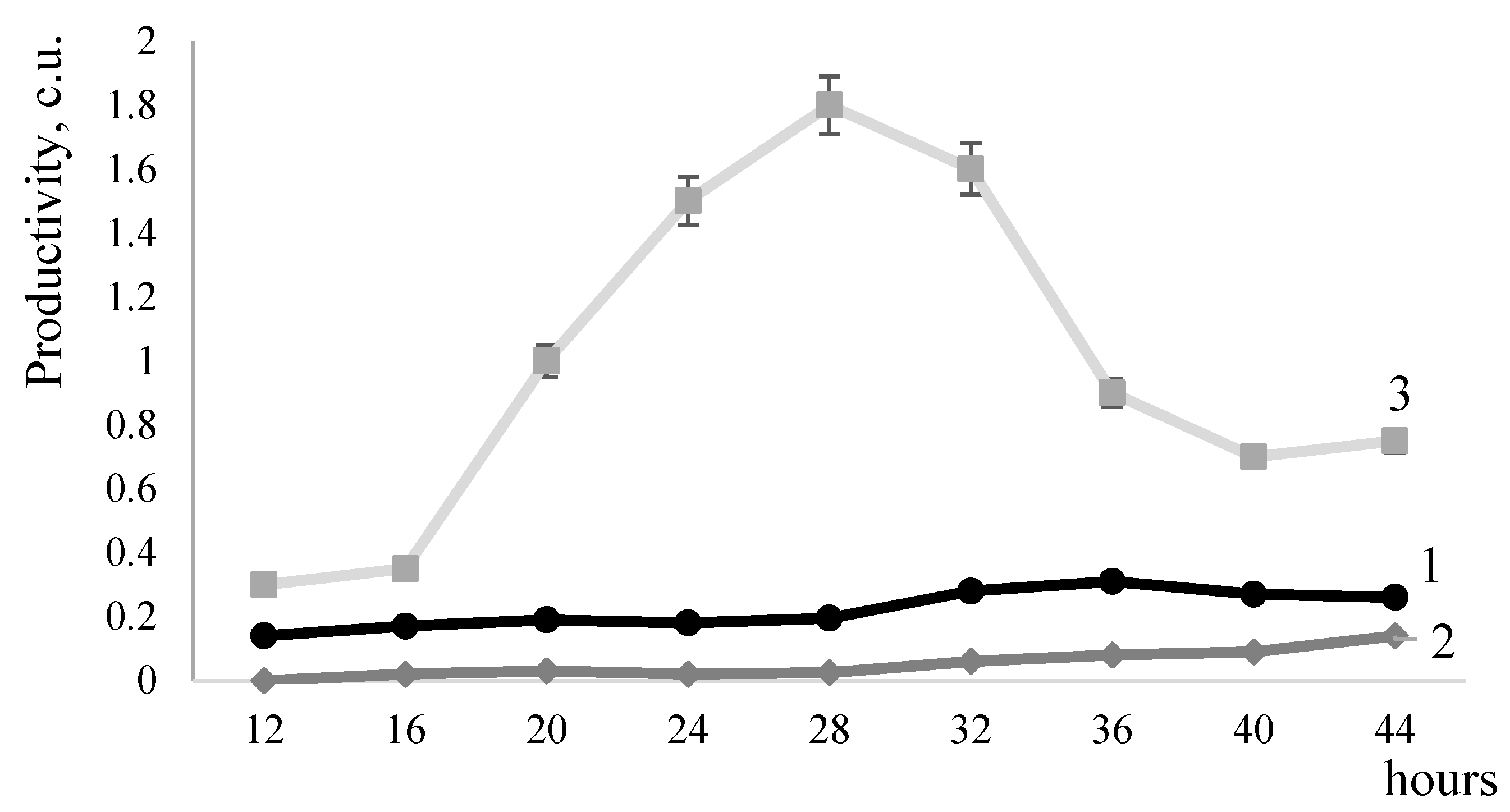
2.2. Effect of DegU Transcription Factor on Metalloendopeptidase Activity
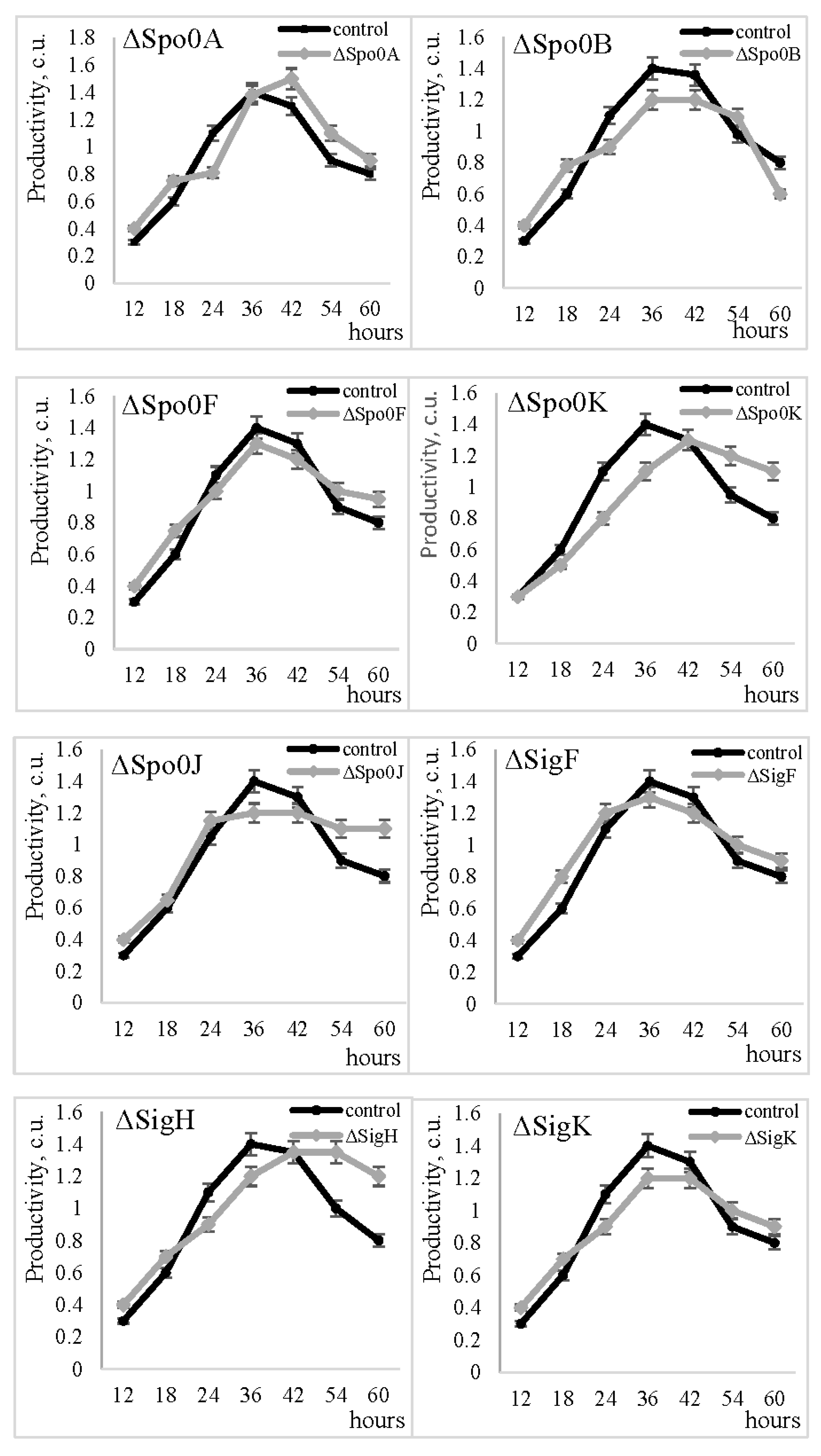
2.3. Effect of Spo- Regulatory Proteins on MprBp Activity
3. Discussion
4. Materials and Methods
4.1. Plasmids, Bacterial Strains and Media
4.2. DNA Techniques
4.3. Proteolytic Activity
4.4. Bioinformatic Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pudova, D.S.; Toymentseva, A.A.; Gogoleva, N.E.; Shagimardanova, E.I.; Mardanova, A.M.; Sharipova, M.R. Comparative Genome Analysis of Two Bacillus pumilus Strains Producing High Level of Extracellular Hydrolases. Genes 2022, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Rudakova, N.L.; Balaban, N.P.; Danilova, Y.V.; Rudenskaya, G.N.; Sharipova, M.R. Characteristics of a novel secreted zinc-dependent endopeptidase of Bacillus intermedius. Biochemistry 2010, 75, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Sabirova, A.R.; Rudakova, N.L.; Balaban, N.P.; Ilyinskaya, O.N.; Demidyuk, I.V.; Kostrov, S.V.; Rudenskaya, G.N.; Sharipova, M.R. A novel secreted metzincin metalloproteinase from Bacillus intermedius. FEBS Lett. 2010, 584, 4419–4425. [Google Scholar] [CrossRef]
- Yao, Z.; Meng, Y.; Le, H.G.; Lee, S.J.; Jeon, H.S.; Yoo, J.Y.; Kim, J.H. Increase of a Fibrinolytic Enzyme Production through Promoter Replacement of aprE3-5 from Bacillus subtilis CH3-5. J Microbiol Biotechnol. 2021, 31, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Ma, Y.; Deng, Y.; Zhou, Z.; Cao, Y.; Yang, B.; Bai, J.; Sun, Q. Enhancing Protease and Amylase Activities in Bacillus licheniformis XS-4 for Traditional Soy Sauce Fermentation Using ARTP Mutagenesis. Foods. 2023, 12, 2381. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Shi, C.; Gong, W.; Liu, J.; Meng, X.; Liu, F.; Lu, F.; Zhang, H. Heterologous expression and characterization of an M4 family extracellular metalloprotease for detergent application. J Gen Appl Microbiol. 2023. [CrossRef] [PubMed]
- Rojas-Tapias, D.F.; Helmann, J.D. Identification of Novel Spx Regulatory Pathways in Bacillus subtilis Uncovers a Close Relationship between the CtsR and Spx Regulons. J Bacteriol. 2019, 201, e00151-19. [Google Scholar] [CrossRef] [PubMed]
- Rudakova, N.L.; Sabirova, A.R.; Balaban, N.P.; Tikhonova, A.O.; Sharipova, M.R. Features of Gene Expression of Bacillus pumilus Metalloendopeptidase. Biochemistry 2016, 81, 884–891. [Google Scholar] [CrossRef]
- Murudkar, C.S.; Kodgire, P.; Rao, K.K. The carboxy terminal domain of Epr, a minor extracellular serine protease, is essential for the swarming motility of Bacillus subtilis 168. FEMS Microbiol. Lett. 2006, 257, 24–31. [Google Scholar] [CrossRef]
- Davidson, F.A.; Seon-Yi, C.; Stanley-Wall, N.R. Selective heterogeneity in exoprotease production by Bacillus subtilis. PLoS ONE 2012, 7, e38574. [Google Scholar] [CrossRef][Green Version]
- Veening, J.W.; Igoshin, O.A.; Eijlander, R.T.; Nijland, R.; Hamoen, L.W.; Kuipers, O.P. Transient heterogeneity in extracellular protease production by Bacillus subtilis. Mol. Syst. Biol. 2008, 4, 184. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, D.T.; Murray, E.J.; Stanley-Wall, N.R. DegU and Spo0A jointly control transcription of two loci required for complex colony development by Bacillus subtilis. J. Bacteriol. 2009, 191, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.J.; Kiley, T.B.; Stanley-Wall, N.R. A pivotal role for the response regulator DegU in controlling multicellular behaviour. Microbiology 2009, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.T. Bacterial differentiation via gradual activation of global regulators. Curr. Genet. 2016, 62, 125–128. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, Q.; Tian, S.; Ge, Y.; Yuan, Z.; Hu, X. Regulator DegU is required for multicellular behavior in Lysinibacillus sphaericus. Res. Microbiol. 2018, 169, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Meliawati, M.; May, T.; Eckerlin, J.; Heinrich, D.; Herold, A.; Schmid, J. Insights in the Complex DegU, DegS, and Spo0A Regulation System of Paenibacillus polymyxa by CRISPR-Cas9-Based Targeted Point Mutations. Appl. Environ. Microbiol. 2022, 88, e0016422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Li, Y.; Chen, S. Fusaricidin Biosynthesis Is Controlled via a KinB-Spo0A-AbrB Signal Pathway in Paenibacillus polymyxa WLY78. Mol. Plant Microbe Interact. 2021, 34, 1378–1389. [Google Scholar] [CrossRef]
- Ogura, M.; Tsukahara, K. SwrA regulates assembly of Bacillus subtilis DegU via its interaction with N-terminal domain of DegU. J. Biochem. 2012, 151, 643–655. [Google Scholar] [CrossRef]
- Mäder, U.; Antelmann, H.; Buder, T.; Dahl, M.K.; Hecker, M.; Homuth, G. Bacillus subtilis functional genomics: Genome-wide analysis of the DegS-DegU regulon by transcriptomics and proteomics. Mol. Genet. Genomics. 2002, 268, 455–467. [Google Scholar] [CrossRef]
- Msadek, T. When the going gets tough: Survival strategies and environmental signaling networks in Bacillus subtilis. Trends Microbiol. 1999, 7, 201–207. [Google Scholar] [CrossRef]
- von Bodman, S.B.; Willey, J.M.; Diggle, S.P. Cell-cell communication in bacteria: United we stand. J. Bacteriol. 2008, 190, 4377–4391. [Google Scholar] [CrossRef]
- Nakano, M.M.; Zuber, P. The primary role of comA in establishment of the competent state in Bacillus subtilis is to activate expression of srfA. J. Bacteriol. 1991, 173, 7269–7274. [Google Scholar] [CrossRef][Green Version]
- Mysliwiec, T.H.; Errington, J.; Vaidya, A.B.; Bramucci, M.G. The Bacillus subtilis spo0J gene: Evidence for involvement in catabolite repression of sporulation. J Bacteriol. 1991, 173, 1911–1919. [Google Scholar] [CrossRef][Green Version]
- Piggot, P.J.; Hilbert, D.W. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 2004, 7, 579–586. [Google Scholar] [CrossRef]
- Fimlaid, K.A.; Shen, A. Diverse mechanisms regulate sporulation sigma factor activity in the Firmicutes. Curr. Opin. Microbiol. 2015, 24, 88–95. [Google Scholar] [CrossRef]
- Sun, G.; Yang, M.; Jiang, L.; Huang, M. Regulation of pro-σK activation: A key checkpoint in Bacillus subtilis sporulation. Environ. Microbiol. 2021, 23, 2366–2373. [Google Scholar] [CrossRef]
- Ogura, M.; Kawata-Mukai, M.; Itaya, M.; Takio, K.; Tanaka, T. Multiple copies of the proB gene enhance degS-dependent extracellular protease production in Bacillus subtilis. J. Bacteriol. 1994, 176, 5673–5680. [Google Scholar] [CrossRef][Green Version]
- Verhamme, D.T.; Kiley, T.B.; Stanley-Wall, N.R. DegU co-ordinates multicellular behaviour exhibited by Bacillus subtilis. Mol. Microbiol. 2007, 65, 554–568. [Google Scholar] [CrossRef]
- Gupta, M.; Rao, K.K. Phosphorylation of DegU is essential for activation of amyE expression in Bacillus subtilis. J. Biosci. 2014, 39, 747–752. [Google Scholar] [CrossRef]
- Kayumov, A.R.; Sharipova, M.R. The mechanisms of regulation of bacterial subtilases synthesis. Uch. Zap. Kaz. Uni. S. Estestv. Nauki. 2005, 147, 89–98. [Google Scholar]
- Kunst, F.; Rapoport, G. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J. Bacteriol. 1995, 177, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.W.; Smits, W.K.; Kuipers, O.P. Bistability, epigenetics, and bet-hedging in bacteria. Annu. Rev. Microbiol. 2008, 62, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Engelberg-Kulka, H.; Amitai, S.; Kolodkin-Gal, I.; Hazan, R. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2006, 2, e135. [Google Scholar] [CrossRef] [PubMed]
- Tjalsma, H.; van den Dolder, J.; Meijer, W.J.; Venema, G.; Bron, S.; van Dijl, J.M. The plasmid-encoded signal peptidase SipP can functionally replace the major signal peptidases SipS and SipT of Bacillus subtilis. J. Bacteriol. 1999, 181, 2448–2454. [Google Scholar] [CrossRef]
- Altenbuchner, J. Editing of the Bacillus subtilis Genome by the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2016, 82, 5421–5427. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Khazak, V.E. Ekspressionnaia edinitsa v oblasti initsiatsii replikatsii plazmidy pSM19035 streptokokkov [Expression unit in the region of replication initiation in the streptococcal plasmid pSM19035]. Mol. Biol. 1990, 24, 993–1000. (In Russian) [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Anagnostopoulos, C.; Spizizen, J. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 1961, 81, 741–746. [Google Scholar] [CrossRef]
- Demidyuk, I.V.; Kalashnikov, A.E.; Gromova, T.Y.; Gasanov, E.V.; Safina, D.R.; Zabolotskaya, M.V.; Rudenskaya, G.N.; Kostrov, S.V. Cloning, sequencing, expression, and characterization of protealysin, a novel neutral proteinase from Serratia proteamaculans representing a new group of thermolysin-like proteases with short N-terminal region of precursor. Protein Expr. Purif. 2006, 47, 551–561. [Google Scholar] [CrossRef]

| Regulatory Protein | Conservative Sequence | Number of Sites | Homology, % |
|---|---|---|---|
| Spo0A | TGTCGAA [17] | 4 | 71–86 |
| DegU | GTCATTAN7TAAATATC [18] | 0 | - |
| DegU~P | GTCATTA [18] | 4 | 60–65 |
| Strains/Plasmids | Description | Source |
|---|---|---|
| Bacillus pumilus 3-19 | StrR [1] | Laboratory collection (“Agrobioengineering” research laboratory, KFU, Kazan, Russia) |
| B. subtilis 8g5 | trpC2; tyr; his; nic; ura; rib; met; ade; sip [34] | Professor Dr. Jan Maarten van Dijl University of Groningen. Department of Medical Microbiology, Netherlands |
| B. subtilis 8g5 ΔdegSΔdegU | ΔdegS; ΔdegU KmR | |
| B. subtilis 8g5 degU32(Hy) | Hy- phenotype KmR | |
| B. subtilis 168 (trpC2) | [35] | Professor D. Zeigler, Bacillus Genetic Stock Center (BGSC), The Ohio State University, USA |
| B. subtilis 168 (trpC2) Δspo0A | Δspo0A | |
| B. subtilis 168 (trpC2) Δspo0B | Δspo0B | |
| B. subtilis 168 (trpC2) Δspo0E | Δspo0E | |
| B. subtilis 168 (trpC2) Δspo0K | Δspo0K | |
| B. subtilis 168 (trpC2) Δspo0J | Δspo0J | |
| B. subtilis 168 (trpC2) ΔsigF | ΔsigF | |
| B. subtilis 168 (trpC2) ΔsigH | ΔsigH | |
| B. subtilis 168 (trpC2) ΔsigK | ΔsigK | |
| pCB22 | Expression vector EU19035, AmpR, EmR [36] | Kostrov S.V., IMG RAS, Moscow, Russia |
| pSA1 | With mprBp 1,1 kb, EmR | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudakova, N.L.; Sabirova, A.R.; Khasanov, D.I.; Danilova, I.V.; Sharipova, M.R. Regulating Pathways of Bacillus pumilus Adamalysin-like Metalloendopeptidase Expression. Int. J. Mol. Sci. 2024, 25, 62. https://doi.org/10.3390/ijms25010062
Rudakova NL, Sabirova AR, Khasanov DI, Danilova IV, Sharipova MR. Regulating Pathways of Bacillus pumilus Adamalysin-like Metalloendopeptidase Expression. International Journal of Molecular Sciences. 2024; 25(1):62. https://doi.org/10.3390/ijms25010062
Chicago/Turabian StyleRudakova, Natalia L., Albina R. Sabirova, Damir I. Khasanov, Iuliia V. Danilova, and Margarita R. Sharipova. 2024. "Regulating Pathways of Bacillus pumilus Adamalysin-like Metalloendopeptidase Expression" International Journal of Molecular Sciences 25, no. 1: 62. https://doi.org/10.3390/ijms25010062
APA StyleRudakova, N. L., Sabirova, A. R., Khasanov, D. I., Danilova, I. V., & Sharipova, M. R. (2024). Regulating Pathways of Bacillus pumilus Adamalysin-like Metalloendopeptidase Expression. International Journal of Molecular Sciences, 25(1), 62. https://doi.org/10.3390/ijms25010062








