The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies
Abstract
:1. Introduction
2. Methods of Literature Search and Review Criteria
3. Genetic and Environmental Factors in Obesity
4. The Spectrum of Endocrine Disruptors
4.1. Bisphenol A
4.2. Phthalates
5. Mechanisms Linking Endocrine Disruptors to Obesity
5.1. Effects on Adipogenesis
5.2. Effects on Epigenetic Regulation
5.3. Effects on Neuroendocrine Signals of Appetite and Satiety
5.4. Effects on Proinflammatory Pathways and Oxidative Stress
5.5. Effects on Gut Microbiome
5.6. Effects on Thermogenic Adipose Tissue
6. Evidence from Mechanistic Studies Linking BPA and Phthalates to Obesity
| Authors, Year | Type of Cell Culture | Main Findings | Remarks |
|---|---|---|---|
| Bisphenol A and obesity | |||
| Riu et al., 2011 [152] | NIH3T3-L1 cell line (pre-adipocytes) | 1. ↑ adipogenesis 2. ↑ lipid accumulation 3. ↑ mRNA level of PPARγ 4. ↑ PPARγ activity | 1. Animal in vitro model 2. ED: TBBPA 3. Obesogenic effects at 10 µM |
| Valentino et al., 2013 [118] | Primary hADSCs | 1. (-) mRNA level of PPARγ, GLUT4 2. ↓ of glucose utilization 3. ↓ tyrosine phosphorylation of insulin receptor (IR) 3. ↓ of PKB/Akt phosphorylation 4. ↑ of IL-6, IFN-γ 5. ↑ of JAK/STAT, JNK 6. ↑ activity NF-kB pathway | 1. Human in vitro model 2. ED: BPA 3. Biological effects at 1 nM |
| Bastos Sales et al., 2013 [102] | Murine N2A, human SK-N-AS neuroblastoma cells and murine pre-adipocyte fibroblasts (3T3-L1) | 1. Modest ↓ in global DNA methylation in murine N2A cells 2. No changes in global DNA methylation in human SK-N-AS cells. 3. ↑ adipocyte differentiation in murine 3T3-L1 pre-adipocytes | 1. Animal and human in vitro model 2. ED: BPA and a range of several EDCs not belonging to bisphenols 3. Biological effects at ≥ 10 μΜ |
| Menale et al., 2015 [137] | Primary pre-adipocytes | 1. ↑ adipogenesis 2. ↑ lipid accumulation 3. ↑ mRNA level of ERα (10 nM, 100 nM) 4. (-) mRNA level of ERβ 5. ↑ production of IL1B, IL18, CCL20 (10 nM) | 1. Human in vitro model 2. ED: BPA 3. Obesogenic effects at 1 nM, 10 nM, 100 nM |
| Ariemma et al., 2016 [92] | 3T3-L1 Pre-adipocytes | 1. Undifferentiated cells: - ↑ proliferation - ↑ differentiation - ↑ expression of PPARγ, C/EBPα and FABP4/AP2 2. Mature adipocytes: - Hypertrophy - ↑ lipid accumulation - ↑ mRNA of leptin, IL6, IFNγ - ↓ glucose utilization | 1. Animal in vitro model 2. ED: BPA 3. Obesogenic effects at 1 nM |
| Longo et al., 2020 [103] | 3T3L1 and NIH3T3 (committed and uncommitted pre-adipocytes, respectively) | - ↓ DNA methylation at PPARγ promoter, without affecting mRNA expression in pre-adipocytes - Transient ↑ in PPARγ expression and lipid accumulation at D4 of differentiation in 3T3L1 cells - Ending BPA exposure restores the PPARγ promoter methylation and inflammatory profile of 3T3L1 cells. - Expression of PPARγ is barely detectable and its promoter is completely methylated in NIH3T3 cells - ↑ PPARγ expression is more evident both in pre-adipocytes and during the adipocyte differentiation | 1. Animal in vitro model 2. ED: BPA 3. Biological effects at low doses: 1 nM |
| Cohen et al., 2021 [153] | Primary hADSCs | 1. ↑ adipogenesis and lipid production at 0.1 nM 2. ↓ adipogenesis and lipid production at 10 nM | 1. Human in vitro model 2. ED: BPA 3. Biological effects at 0.1 nM, 10 nM |
| Yamasaki et al., 2021 [154] | ST-13 cell line (pre-adipocytes) | - Undifferentiated cells: 1. (-) lipid accumulation 2. (-) mRNA level of PPARγ 3. ↑ mRNA level of AACS, PLIN1, FAS, CIDEA, LSD-1 - Mature adipocytes: 1. (-) lipid accumulation 2. (-) mRNA level of AACS, SCOT | 1. Animal in vitro model 2. ED: TBBPA 3. Obesogenic effects at 0.5 µM, 1 µM |
| Schaffert et al., 2021 [121] | SGBSs (pre-adipocytes) | 1. ↑ binding to PPARγ (50 µM) 2. (-) PPARγ activity (10 nM, 100 nM, 1 µM, 10 µM) 3. ↓ lipid accumulation (10 nM, 100 nM, 1 µM, 10 µM) 4. ↑ leptin (10 nM) 5. ↓ cellular ROS level (10 nM, 100 nM, 1 µM, 10 µM) 6. ↓ insulin sensitivity (1 µM) | 1. Human in vitro model 2. ED: BPA 3. Obesogenic effects at 10 nM, 100 nM, 1 µM, 10 µM, 50 µM |
| Marqueno et al., 2021 [155] | ZFL cell line (primary mouse hepatocytes) | 1. ↑ lipid accumulation (5 µM, 50 µM) 2. ↑ ROS generation (20 µM, 50 µM, 70 µM, 100 µM, 150 µM, 200 µM) | 1. Animal in vitro model 2. ED: BPA 3. Biological effects at 5 µM, 20 µM, 50 µM, 70 µM, 100 µM, 150 µM, 200 µM |
| Lee et al., 2022 [156] | Huh-7 cell line (primary hepatocytes) | 1. ↓ cell viability (200 µM, 400 µM) 2. ↑ lipid accumulation (10 µM, 50 µM, 100 µM, 200 µM) 3. Fatty acid uptake ↑ (10 µM, 50 µM, 100 µM) 4. ↑ intracellular ROS formation (10 µM, 50 µM, 100 µM, 200 µM) | 1. Human in vitro model 2. ED: BPA 3. Biological effects at 10 µM, 50 µM, 100 µM, 200 µM, 400 µM |
| Phthalates and obesity | |||
| Sargis et al., 2010 [85] | 3T3-L1 cell line (pre-adipocytes) | 1. ↑ adipogenic differentiation 2. ↑ lipid accumulation (100 nM) 3. ↑ PPARγ and glucocorticoid-like activity (1 µM) 4. ↑ adiponectin and protein expression of IR-β (1 µM–100 pM) | 1. Animal in vitro model 2. Pthalate: DCHP 3. Obesogenic effects at 100 pM, 1 nM, 10 nM, 100 nM, 1 µM |
| Dimastrogiovanni et al., 2015 [150] | RTL-W1 cell line (hepatocytes) | 1. ↑ lipid accumulation 2. ↓ alteration of membrane lipids 3. ↓ mRNA level of CD36, FAS, LPL | 1. Animal in vitro model 2. Pthalate: DEHP 3. Biological effects at 5 μΜ |
| Zhang et al., 2017 [105] | C3H10T1/2 cell line (MSCs) | 1. ↑ adipogenesis 2. ↑ mRNA level of AP2, PPARγ 3. ↑ lipid accumulation 4. ↑ protein level of FOXO1 5. ↑ acetylation of FOXO1, β-catenin 6. ↓ protein level of SIRT1, SIRT3 | 1. Animal in vitro model 2. Pthalate: BBP 3. Biological effects at 50 μΜ |
| Schaedlich et al., 2018 [151] | SGBSs (pre-adipocytes) | 1. ↓ TGsaccumulation 2. ↓ adiponectin production 3. ↓ protein level of PPARα, PPARγ 4. ↓ phosphorylation of ERK1, ERK2 5. ↑ lipolysis 6. ↑ ROS formation | 1. Human in vitro model 2. Pthalate: DEHP 3. Obesogenic effects at 50 µg/mL |
| Zhang et al., 2019 [145] | BRL-3A cell line (hepatocytes) | 1. ↑ lipid accumulation (100 µM, 200 µM) 2. ↑ mRNA level of FAS, PDK4, AP2 (10 µM, 50 µM, 100 µM, 200 µM) 3. ↑ mRNA level of PPARγ (50 µM, 100 µM, 200 µM) 4. ↓ JAK2/STAT5 signaling 5. ↓ level of indicators of oxidative stress: SOD ↓, MDA ↑ (10 µM, 50 µM, 100 µM, 200 µM) | 1. Animal in vitro model 2. Pthalate: MEHP 3. Biological effects at 10 µM, 50 µM, 100 µM, 200 µM |
| Perez-Albaladejo et al., 2021 [157] | PLHC-1 cell line (hepatocytes) | - DBP: 1. ↑ TG accumulation (20 µM) 2. ↑ ROS formation (5 µM, 20 µM, 50 µM, 100 µM) - DEHP: 1. ↑ TG accumulation (5 µM, 10 µM) 2. ↑ ROS formation (100 µM) | 1. Animal in vitro model 2. Phthalates: DBP and DEHP 3. Biological effects at - DBP: 5 µM, 20 µM, 50 µM, 100 µM - DEHP: 5 µM, 10 µM, 100 µM |
| Meruvu et al., 2021 [104] | 3T3-L1 cells | - ↑ miR-34a-5p expression - ↑ adipogenesis - ↓ Nampt, Sirt1 and Sirt3 gene expression levels; ↓ Nampt protein - ↓ adipogenesis, ↑ Nampt protein and NAD+ after miR-34a-5p knockdown in the presence of BBP | 1. Animal in vitro model 2. Phthalate: BBP 2. Biological effects at various doses of BBP without exogenous adipogenic stimuli |
| Al-Abdulla et al., 2022 [158] | MIN-6 cell line (pancreatic cells) | 1. ↓ viability of cells after 24 exposure at 1 μΜ 2. ↑ mRNA level of SUR1, GLUT2 at 10 μΜ 3. ↓ GSIS (20 μΜ glucose) 4. ↓ insulin content at 1 μΜ | 1. Animal in vitro model 2. Pthalate: DEHP 3. Dose: 100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM |
| Schaffert et al., 2022 [141] | SGBSs (pre-adipocytes) | 1. DINP: - ↑ binding to PPARγ - (-) PPARγ activation - (-) lipid accumulation - ↑ adipsin (10 µM) - Mature adipocytes: * 10 µM: ↑ MCP-1, LAP3, GPX1 * 10 nM: ↑ GPX8, GSR * 10 nM, 10 µM: ↑ LEP, GPX4 * 10 nM, 10 µM: ↓ adiponectin 2. DPHP: - ↑ binding to PPARγ - (-) PPARγ activation - Undifferentiated cells: * (-) lipid accumulation * ↓ MCP-1 (10 nM, 10 µM) - Mature adipocytes: * ↓ lipid accumulation (10 µM, 25 µM, 50 µM, 100 µM) * 10 µM: ↑ LEP, MCP-1, LAP-3, GPX4, GPX8, adipsin * 10 nM: ↑ GSR * 10 nM, 10 µM: ↑ GPX1, GSTO1 * 10 nM, 10 µM: ↓ adiponectin 3. MHINP: - ↑ binding to PPARγ (100 µM, 200 µM, 400 µM) - ↑ PPARγ activation (1 µM) - Undifferentiated cells: * ↑ pre-adipocyte differentiation, lipid accumulation (10 µM, 25 µM, 50 µM, 100 µM) * 10 µM: ↑ LEP, PLIN1, GPD1, FASN, FABP4, FABP5 * 10 nM: ↓ MCP-1 * 10 nM, 10 µM: ↑ adipsin - Mature adipocytes: * 1 µM: ↑ lipid accumulation * 10 µM: ↑ LAP3, adipsin * 10 nM: ↑ GSR, GPX8 * 10 nM, 10 µM: ↑LEP, MCP-1, GPX1, GPX4, GSTO1 * 10 nM, 10 µM: ↓ adiponectin 4. OH-MPHP: - ↑ binding to PPARγ - ↑ PPARγ activation - Undifferentiated cells: * ↑ pre-adipocyte differentiation, lipid accumulation (10 µM, 25 µM, 50 µM) * ↑ LEP, GPD1, FASN, FABP4, FABP5 (10 µM) - Mature adipocytes: * 10 µM: ↑ LAP3, GPX1, GPX4, GPX8, adipsin * 10 nM, 10 µM: ↑ LEP, GSR, MCP-1, GSTO1 * 10 nM, 10 µM: ↓ adiponectin * 10 nM, 10 µM, 25 µM, 50 µM, 100 µM: ↓ lipid accumulation | 1. Human in vitro model 2. Phthalates: DINP, DPHP, MHINP, OH-MPHP 2. Obesogenic effects at - DINP: 10 nM, 10 µM, - DPHP: 10 nM, 10 µM, 25 µM, 50 µM, 100 µM - MHINP: 10 nM, 10 µM, 25 µM, 50 µM, 100 µM, 200 µM, 400 µM - OH-MPHP: 10 nM, 10 µM, 25 µM, 50 µM, 100 µM, 200 µM, 400 µM |
7. Evidence from Animal Studies Linking BPA and Phthalates to Obesity
| Author, Year | Type of Animal Used | Main Findings | Remarks |
|---|---|---|---|
| Bisphenol A and obesity | |||
| Pu et al., 2017 [161] | Primiparous female sheep | 1. ↑ differentiation rate in adipocytes 2. ↑ mRNA expression of PPARγ in fetal adipose tissue 3. ↑ expression of FABP4, GLUT4 and SOX6 in the offspring 4. ↑ gene expression of GR, ESR1, ESR2 and ERRα | 1. Type of exposure: sc 2. Exposure duration: 147 days 3. Daily BPA dose: 0.5 mg/kg |
| Desai et al., 2018 [160] | 12-week-old female Sprague–Dawley rats | 1. ↑ body weight 2. ↑ mass of adipose tissue 3. Hypertophic adipocytes in male offspring 4. ↑ expression of PPARγ 5. ↑ TNF-α and CD68 in adipose tissue | 1. Findings occurred in the offspring 2. Type of exposure: drinking water 3. Exposure duration: 2 weeks before mating up to weaning 4. Daily BPA dose: 5 mg/L |
| Stoker et al., 2019 [173] | 90-day-old female Wistar rats | 1. ↑ food intake 2. ↑ epididymal and perirenal fat deposition 3. ↑ fasting serum glucose and leptin in male mice 4. ↑ expression of hypothalamic orexigenic neuropeptides in male mice | 1. Findings occurred in the offspring 2. Type of exposure: drinking water 3. Exposure duration: pregnancy day 9 to weaning 4. Daily BPA dose: 50 µg/kg |
| Lin et al., 2019 [174] | 3-week-old male Wistar rats | 1. ↑ fat deposition (visceral, liver) 2. ↑ TCHOL, LDL, TGs 3. ↓ HDL 4. ↑ TNF-α, IL-17 5. ↑ mRNA of SREBP1 and ACC1 6. ↑ TLR4 and NF-κB in the liver 7. ↓ HSL, ERα and ZAG in the liver | 1. Type of exposure: drinking water 2. Exposure duration: 8 weeks 3. Daily BPA dose: 1 µg/mL |
| Tian et al., 2021 [113] | 5-month-old wild-type adult male Danio rerio | 1. ↑ weight gain, length, food intake 2. ↑ lipid accumulation in liver 3. Microvesicular fatty changes, hepatocyte ballooning, infiltration with inflammatory cells 4. ↑ of CB1 5. ↑ of insulin signaling pathways 6. ↓ expression of PPARα in adipose tissue and liver 7. ↓ gpr55 | 1. Type of exposure: water in static system 2. Exposure duration: 28 days 3. Daily BPA dose: 20, 100 and 500 μg/L |
| Shih et al., 2021 [175] | 15-week-old female Sprague–Dawley rats | 1. ↑ abdominal lipid weight up to 77% in female offspring 2. ↑ TCHOL, LDL, TGs 3. ↓ HDL 4. ↑ leptin 5. ↑ of Prevotella, C. perfringens, C. ruminantius in feces | 1. Type of exposure: oral gavage 2. Exposure duration: 6th-36th day after pregnancy 3. Daily BPA dose: 50 µg/kg |
| Zhuang et al., 2023 [165] | 7-week-old male and female ICR mice | 1. ↑ weight gain in the offspring 2. ↑ size of adipocytes 3. ↓ insulin sensitivity 4. No obesogenic effects via estrogen signaling pathway 5. Obesogenic effects via TGF-β signaling pathway | 1. Type of exposure: drinking water 2. Exposure duration: 7 days treatment up to delivery 3. Daily BPA dose: 0.5 μg/kg |
| Phthalates and obesity | |||
| Hao et al., 2013 [162] | C57BL/6J mice | 1. ↑ expression of PPARγ, aP2, LPL and FAS 2. ↑ expression of C/EBP, Srebf1 3. ↑ glucose, TCHOL, TGs in serum 4. Obese phenotype only at the dose of 0.25 mg/kg in female offspring 5. ↑ weight gain in male offspring | 1. Type of phthalate: DEHP 2. Ip DEHP at the dose of 0.5 mg/kg in six-week-old male mice 3. Female mice: - Type of exposure: gavage - Exposure duration: from day 12 of gestation until day 7 of lactation - Daily DEHP dose: 0.05, 0.25 or 0.5 mg/kg |
| Klöting et al., 2015 [163] | Obesity-resistant 129S6 mice | In female (but not in male) mice: 1. ↑ weight gain 2. ↑ fat mass 3. ↓ insulin tolerance 4. ↓ Pparg and adiponectin in scAT 5. ↑↑ Esr1 protein levels in SC and visceral adipose tissue 6. (-) TCHOL, TGs in serum 7. ↑ in phospholipid and carnitine | 1. Type of phthalate: DEHP 2. Type of exposure: oral 3. Exposure duration: 10 weeks 4. Daily DEHP dose: 0.05 mg/kg |
| Lv et al., 2016 [135] | Male C3H/He mice | 1. ↑ food intake, adipogenesis and weight gain in all exposure groups except for 0.05 mg/kg 2. Interruption in hypothalamic appetite-related neuropeptides: - ↑ expression of AgRP in all groups - ↑ expression of NPY at 50 and 200 mg/kg - ↓ expression of POMC at 200 mg/kg 3. Hypothalamic leptin resistance resulting in hypothyroidism 4. ↓ WAT lipid metabolism at 0.5 mg/kg 5. ↑ WAT lipid metabolism at 50 and 200 mg/kg | 1. Type of phthalate: DEHP 2. Type of exposure: gavage 3. Exposure duration: 5 weeks 4. Daily DEHP dose: 0.05, 0.5, 5, 50 and 200 mg/kg |
| Zhang et al., 2020 [176] | C57BL/6 J male and female mice | 1. Weight gain in male mice on HFD at 3 mg/kg/d 2. At the dose of 3 mg/kg/d: - ↑ activation of SREBP1 - (-) of SREBP2, PPARγ - ↑ expression of downstream regulatory genes of SREBP1 (FAS, ACC, HMGCR) 3. ↓ insulin tolerance in male mice between HFD + BBP3 and HFD groups | 1. Type of phthalate: BBP 2. Type of exposure: oral 3. Exposure duration: 16 weeks 4. Daily BBP dose: 4 μg/kg, 169 μg/kg, 3 mg/kg, 50 mg/kg |
| Guo et al., 2020 [172] | Zebrafish embryos 0.75 hpf | - ↑ expression of PPARγ due to the following: 1. Significant regional DNA demethylation 2. Upregulation of tet1 and tet2 gene transcription) - As a result, ↓ TCHOL and TGs due to ↑ expression of downstream genes involved in lipid metabolism | 1. Type of phthalate: TBPH and TBMEHP 2. Type of exposure: glass Petri dish containing 100 mL of TBPH or TBMEHP 3. Exposure duration: until 72 hpf 4. Daily TBPH and TBMEHP dose: 0.2–2000 nM |
| Buerger et al., 2020 [177] | Zebrafish (Danio rerio) | GI dysbiosis in the OF + DEHP group as a result of the following: - ↑ of Bacteroidetes - ↑ of UFAs - ↑ lipid metabolism - ↓ carbohydrate metabolism - ↓ glycerolipid metabolism - ↓ glycerophospholipid metabolism - ↓ carbohydrate, galactose, inositol phosphate, taurine and hypotaurine metabolism | 1. Type of phthalate: DEHP 2. Type of exposure: oral 3. Exposure duration: 60 days 4. Daily DEHP dose: 3 mg/kg |
8. Evidence from Human Studies Linking BPA and Phthalates to Obesity
8.1. Bisphenol A and Obesity
8.2. Phthalates and Obesity
9. Perspectives, Controversies and Challenges
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- World Obesity Federation. World Obesity Atlas 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 14 November 2023).
- Wang, Y.; Beydoun, M.A.; Min, J.; Xue, H.; Kaminsky, L.A.; Cheskin, L.J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int. J. Epidemiol. 2020, 49, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514–527. [Google Scholar] [CrossRef] [PubMed]
- NCD-RisC. Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: A pooled analysis of 2181 population-based studies with 65 million participants. Lancet 2020, 396, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Vlami, K.; Dalamaga, M.; Giatrakou, S.; Theodoropoulos, K.; Gyftopoulos, S.; Stavrianeas, N.; Papiris, S.; Rigopoulos, D. Sleep apnea as a comorbidity in obese psoriasis patients: A cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J. Eur. Acad. Dermatol. Venereol. 2013, 27, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Papavasileiou, G.; Tsilingiris, D.; Spyrou, N.; Vallianou, N.G.; Karampela, I.; Magkos, F.; Dalamaga, M. Obesity and main urologic cancers: Current systematic evidence, novel biological mechanisms, perspectives and challenges. Semin. Cancer Biol. 2023, 91, 70–98. [Google Scholar] [CrossRef] [PubMed]
- Hroussalas, G.; Kassi, E.; Dalamaga, M.; Delimaris, I.; Zachari, A.; Dionyssiou-Asteriou, A. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas 2008, 59, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, A.; Dalamaga, M.; Kroupis, C.; Konstantoudakis, G.; Belimezi, M.; Athanasas, G.; Dimas, K. Survivin isoforms and clinicopathological characteristics in colorectal adenocarcinomas using real-time qPCR. World J. Gastroenterol. 2011, 17, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Thacharodi, A.; Priya, A.; Meenatchi, R.; Hegde, T.A.; Thangamani, R.; Nguyen, H.T.; Pugazhendhi, A. Endocrine disruptors: Unravelling the link between chemical exposure and Women’s reproductive health. Environ. Res. 2023, 241, 117385. [Google Scholar] [CrossRef]
- Emfietzoglou, R.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Could the endocrine disruptor bisphenol-A be implicated in the pathogenesis of oral and oropharyngeal cancer? Metabolic considerations and future directions. Metabolism 2019, 91, 61–69. [Google Scholar] [CrossRef]
- Heindel, J.J.; Alvarez, J.A.; Atlas, E.; Cave, M.C.; Chatzi, V.L.; Collier, D.; Corkey, B.; Fischer, D.; Goran, M.I.; Howard, S.; et al. Obesogens and Obesity: State-of-the-Science and Future Directions Summary from a Healthy Environment and Endocrine Disruptors Strategies Workshop. Am. J. Clin. Nutr. 2023, 118, 329–337. [Google Scholar] [CrossRef]
- Panuganti, K.K.; Nguyen, M.; Kshirsagar, R.K. Obesity. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 January. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459357/ (accessed on 2 December 2023).
- WHO. Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 26 December 2023).
- Liu, J.; Tsilingiris, D.; Dalamaga, M. The non-linear relationship between muscle mass and BMI calls into question the use of BMI as a major criterion for eligibility for bariatric surgery. Metabol. Open 2022, 13, 100164. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- Larson, E.A.; Dalamaga, M.; Magkos, F. The role of exercise in obesity-related cancers: Current evidence and biological mechanisms. Semin. Cancer Biol. 2023, 91, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Christodoulatos, G.S.; Karampela, I.; Vallianou, N.; Apovian, C.M. Understanding the Co-Epidemic of Obesity and COVID-19: Current Evidence, Comparison with Previous Epidemics, Mechanisms, and Preventive and Therapeutic Perspectives. Curr. Obes. Rep. 2021, 10, 214–243. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Pérusse, L.; Rao, D.C.; Bouchard, C. Familial risk of overweight and obesity in the Canadian population using the WHO/NIH criteria. Obes. Res. 2000, 8, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Pietiläinen, K.H.; Kaprio, J.; Rissanen, A.; Winter, T.; Rimpelä, A.; Viken, R.J.; Rose, R.J. Distribution and heritability of BMI in Finnish adolescents aged 16y and 17y: A study of 4884 twins and 2509 singletons. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Sørensen, T.I.; Hanis, C.; Teasdale, T.W.; Chakraborty, R.; Schull, W.J.; Schulsinger, F. An adoption study of human obesity. N. Engl. J. Med. 1986, 314, 193–198. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Keller, M.; Svensson, S.I.A.; Rohde-Zimmermann, K.; Kovacs, P.; Böttcher, Y. Genetics and Epigenetics in Obesity: What Do We Know so Far? Curr. Obes. Rep. 2023, 12, 482–501. [Google Scholar] [CrossRef]
- Kim, R.; Lippert, A.M.; Wedow, R.; Jimenez, M.P.; Subramanian, S.V. The Relative Contributions of Socioeconomic and Genetic Factors to Variations in Body Mass Index Among Young Adults. Am. J. Epidemiol. 2020, 189, 1333–1341. [Google Scholar] [CrossRef]
- Wang, Z.; Emmerich, A.; Pillon, N.J.; Moore, T.; Hemerich, D.; Cornelis, M.C.; Mazzaferro, E.; Broos, S.; Ahluwalia, T.S.; Bartz, T.M.; et al. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat. Genet. 2022, 54, 1332–1344. [Google Scholar] [CrossRef]
- Yengo, L.; Sidorenko, J.; Kemper, K.E.; Zheng, Z.; Wood, A.R.; Weedon, M.N.; Frayling, T.M.; Hirschhorn, J.; Yang, J.; Visscher, P.M. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 2018, 27, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Argyrakopoulou, G.; Fountouli, N.; Dalamaga, M.; Kokkinos, A. Revisiting Resting Metabolic Rate: What is the Relation to Weight Fluctuations? Curr. Obes. Rep. 2023, 12, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, A.; Gonzalez, K.; Dalamaga, M.; Magkos, F. The Impact of the Rate of Weight Loss on Body Composition and Metabolism. Curr. Obes. Rep. 2022, 11, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kiess, W.; Haeusler, G. Endocrine-disrupting chemicals. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101566. [Google Scholar] [CrossRef]
- WHO. Global Assessment on the State of the Science of Endocrine Disruptors. Available online: https://www.who.int/publications/i/item/WHO-PSC-EDC-02.2 (accessed on 28 November 2023).
- Nidens, N.; Vogel, M.; Körner, A.; Kiess, W. Prenatal exposure to phthalate esters and its impact on child development. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101478. [Google Scholar] [CrossRef]
- National Institute of Environmental Health Sciences. Endocrine Disruptors. Available online: https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm (accessed on 28 November 2023).
- van der Meer, T.P.; van Faassen, M.; van Beek, A.P.; Snieder, H.; Kema, I.P.; Wolffenbuttel, B.H.R.; van Vliet-Ostaptchouk, J.V. Exposure to Endocrine Disrupting Chemicals in the Dutch general population is associated with adiposity-related traits. Sci. Rep. 2020, 10, 9311. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jensen, T.K.; Jørgensen, N.; Kyhl, H.B.; Husby, S.; Skakkebæk, N.E.; Main, K.M.; Juul, A.; Andersson, A.M. Human urinary excretion of non-persistent environmental chemicals: An overview of Danish data collected between 2006 and 2012. Reproduction 2014, 147, 555–565. [Google Scholar] [CrossRef]
- Kiess, W.; Häussler, G.; Vogel, M. Endocrine-disrupting chemicals and child health. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101516. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Plastics—The Facts 2022. Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 28 November 2023).
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J. Bisphenol A--sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, Y.J. Bisphenols and Thyroid Hormone. Endocrinol. Metab. 2019, 34, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Mas, M.J.; Ballesteros-Gómez, A.; Rubio, S. Supramolecular solvent-based microextraction of emerging bisphenol A replacements (colour developers) in indoor dust from public environments. Chemosphere 2019, 222, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Chemical components of plastics as endocrine disruptors: Overview and commentary. Birth Defects Res. 2020, 112, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Seachrist, D.D.; Bonk, K.W.; Ho, S.M.; Prins, G.S.; Soto, A.M.; Keri, R.A. A review of the carcinogenic potential of bisphenol A. Reprod. Toxicol. 2016, 59, 167–182. [Google Scholar] [CrossRef]
- Robinson, L.; Miller, R. The Impact of Bisphenol A and Phthalates on Allergy, Asthma, and Immune Function: A Review of Latest Findings. Curr. Environ. Health Rep. 2015, 2, 379–387. [Google Scholar] [CrossRef]
- Biemann, R.; Blüher, M.; Isermann, B. Exposure to endocrine-disrupting compounds such as phthalates and bisphenol A is associated with an increased risk for obesity. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101546. [Google Scholar] [CrossRef]
- Hoekstra, E.J.; Simoneau, C. Release of bisphenol A from polycarbonate: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Guo, T.L. Developmental Bisphenol A Exposure Modulates Immune-Related Diseases. Toxics 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Di Credico, A.; Gaggi, G.; Bucci, I.; Ghinassi, B.; Di Baldassarre, A. The Effects of Combined Exposure to Bisphenols and Perfluoroalkyls on Human Perinatal Stem Cells and the Potential Implications for Health Outcomes. Int. J. Mol. Sci. 2023, 24, 15018. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Aslam Khan, M.U.; Abdullah, A.M.; Abd Razak, S.I. A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials. Polymers 2021, 13, 1409. [Google Scholar] [CrossRef]
- Liu, J.; Martin, J.W. Prolonged Exposure to Bisphenol A from Single Dermal Contact Events. Environ. Sci. Technol. 2017, 51, 9940–9949. [Google Scholar] [CrossRef]
- Christensen, K.L.; Lorber, M.; Koslitz, S.; Brüning, T.; Koch, H.M. The contribution of diet to total bisphenol A body burden in humans: Results of a 48 h fasting study. Environ. Int. 2012, 50, 7–14. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Olsén, L.; Lampa, E.; Birkholz, D.A.; Lind, L.; Lind, P.M. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Ecotoxicol. Environ. Saf. 2012, 75, 242–248. [Google Scholar] [CrossRef]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef]
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues. Chemosphere 2021, 268, 129273. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency, 2022. The use of bisphenol A and its alternatives in thermal paper in the EU during 2014–2022. Echa. Available online: https://echa.europa.eu/documents/10162/2564887/bpa_thermal_paper_report_2020_en.pdf/59eca269-c788-7942-5c17-3bd822d9cba0 (accessed on 2 December 2023).
- Bisphenol, A. Available online: https://www.efsa.europa.eu/en/topics/topic/bisphenol (accessed on 28 November 2023).
- Kodila, A.; Franko, N.; Sollner Dolenc, M. A review on immunomodulatory effects of BPA analogues. Arch. Toxicol. 2023, 97, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Blanco, J.; Rovira, J.; Kumar, V.; Domingo, J.L.; Schuhmacher, M. Bisphenol A analogues (BPS and BPF) present a greater obesogenic capacity in 3T3-L1 cell line. Food Chem. Toxicol. 2020, 140, 111298. [Google Scholar] [CrossRef]
- Ramskov Tetzlaff, C.N.; Svingen, T.; Vinggaard, A.M.; Rosenmai, A.K.; Taxvig, C. Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A. Environ. Toxicol. 2020, 35, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.M.; Xu, H.S.; Guo, T.L. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: Insights from a multi-species comparison. Crit. Rev. Toxicol. 2021, 51, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Ahmad, M. Computational study suggesting reconsideration of BPA analogues based on their endocrine disrupting potential estimated by binding affinities to nuclear receptors. Ecotoxicol. Environ. Saf. 2019, 171, 154–161. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Phthalates. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/phthalates (accessed on 1 December 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Barnes, S.J. Understanding plastics pollution: The role of economic development and technological research. Environ. Pollut. 2019, 249, 812–821. [Google Scholar] [CrossRef]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbühler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Lange, R.; Schmidt, P.; Rodriguez Martin, L.; Remy, S.; Springer, A.; Puklová, V.; Černá, M.; Rudnai, P.; Középesy, S.; et al. Exposure to Phthalates in European Children, Adolescents and Adults since 2005: A Harmonized Approach Based on Existing HBM Data in the HBM4EU Initiative. Toxics 2023, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhu, Q.; Zhao, Y.; Ge, W.; Zhao, Y.; Song, Q.; Zhou, Y.; Shi, H.; Zhang, Y. Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence. Environ. Int. 2018, 121, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Govarts, E.; Gilles, L.; Rodriguez Martin, L.; Santonen, T.; Apel, P.; Alvito, P.; Anastasi, E.; Andersen, H.R.; Andersson, A.M.; Andryskova, L.; et al. Harmonized human biomonitoring in European children, teenagers and adults: EU-wide exposure data of 11 chemical substance groups from the HBM4EU Aligned Studies (2014–2021). Int. J. Hyg. Environ. Health 2023, 249, 114119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, N.; Liu, M.; Liu, Y.; He, A.; Wang, L.; Luo, H.; Yao, Y.; Sun, H. Dysregulation of steroid metabolome in follicular fluid links phthalate exposure to diminished ovarian reserve of childbearing-age women. Environ. Pollut. 2023, 330, 121730. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, M.; Huang, K.; Cui, J.; Liu, H.; Yu, Y.; Ma, S.; Liu, X.; Lin, M. Phthalate metabolites in breast milk from mothers in Southern China: Occurrence, temporal trends, daily intake, and risk assessment. J. Hazard. Mater. 2023, 464, 132895. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Rosen, E.M.; Rosario, Z.; Feric, Z.; Calafat, A.M.; McElrath, T.F.; Vélez Vega, C.; Cordero, J.F.; Alshawabkeh, A.; Meeker, J.D. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ. Int. 2019, 132, 105099. [Google Scholar] [CrossRef]
- Choi, G.; Villanger, G.D.; Drover, S.S.M.; Sakhi, A.K.; Thomsen, C.; Nethery, R.C.; Zeiner, P.; Knudsen, G.P.; Reichborn-Kjennerud, T.; Øvergaard, K.R.; et al. Prenatal phthalate exposures and executive function in preschool children. Environ. Int. 2021, 149, 106403. [Google Scholar] [CrossRef]
- Stahlhut, R.W.; van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2018/2005 of 17 December 2018 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards bis(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBP) and diisobutyl phthalate (DIBP) (Text with EEA relevance.). Available online: https://eur-lex.europa.eu/eli/reg/2018/2005/oj (accessed on 1 December 2023).
- van der Meer, T.P.; Chung, M.K.; van Faassen, M.; Makris, K.C.; van Beek, A.P.; Kema, I.P.; Wolffenbuttel, B.H.R.; van Vliet-Ostaptchouk, J.V.; Patel, C.J. Temporal exposure and consistency of endocrine disrupting chemicals in a longitudinal study of individuals with impaired fasting glucose. Environ. Res. 2021, 197, 110901. [Google Scholar] [CrossRef]
- Karampela, I.; Christodoulatos, G.S.; Dalamaga, M. The Role of Adipose Tissue and Adipokines in Sepsis: Inflammatory and Metabolic Considerations, and the Obesity Paradox. Curr. Obes. Rep. 2019, 8, 434–457. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ashari, S. Mechanistic insight into toxicity of phthalates, the involved receptors, and the role of Nrf2, NF-κB, and PI3K/AKT signaling pathways. Environ. Sci. Pollut. Res. Int. 2021, 28, 35488–35527. [Google Scholar] [CrossRef] [PubMed]
- González-Casanova, J.E.; Bermúdez, V.; Caro Fuentes, N.J.; Angarita, L.C.; Caicedo, N.H.; Rivas Muñoz, J.; Rojas-Gómez, D.M. New Evidence on BPA’s Role in Adipose Tissue Development of Proinflammatory Processes and Its Relationship with Obesity. Int. J. Mol. Sci. 2023, 24, 8231. [Google Scholar] [CrossRef] [PubMed]
- Sargis, R.M.; Johnson, D.N.; Choudhury, R.A.; Brady, M.J. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity 2010, 18, 1283–1288. [Google Scholar] [CrossRef]
- Feige, J.N.; Gelman, L.; Rossi, D.; Zoete, V.; Métivier, R.; Tudor, C.; Anghel, S.I.; Grosdidier, A.; Lathion, C.; Engelborghs, Y.; et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J. Biol. Chem. 2007, 282, 19152–19166. [Google Scholar] [CrossRef]
- Esteban, J.; Serrano-Maciá, M.; Sánchez-Pérez, I.; Alonso-Magdalena, P.; Pellín, M.C.; García-Arévalo, M.; Nadal, Á.; Barril, J. In utero exposure to bisphenol-A disrupts key elements of retinoid system in male mice offspring. Food Chem. Toxicol. 2019, 126, 142–151. [Google Scholar] [CrossRef]
- Nettore, I.C.; Franchini, F.; Palatucci, G.; Macchia, P.E.; Ungaro, P. Epigenetic Mechanisms of Endocrine-Disrupting Chemicals in Obesity. Biomedicines 2021, 9, 1716. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, D.; Franssen, D.; Heger, S.; Parent, A.S. Endocrine-disrupting chemicals and their effects on puberty. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101579. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. PPARgamma: A nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef] [PubMed]
- Ariemma, F.; D’Esposito, V.; Liguoro, D.; Oriente, F.; Cabaro, S.; Liotti, A.; Cimmino, I.; Longo, M.; Beguinot, F.; Formisano, P.; et al. Low-Dose Bisphenol-A Impairs Adipogenesis and Generates Dysfunctional 3T3-L1 Adipocytes. PLoS ONE 2016, 11, e0150762. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yao, X.; Liu, S.; Yin, N.; Faiola, F. Non-cytotoxic nanomolar concentrations of bisphenol A induce human mesenchymal stem cell adipogenesis and osteogenesis. Ecotoxicol. Environ. Saf. 2018, 164, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Reina-Pérez, I.; Olivas-Martínez, A.; Mustieles, V.; Ruiz-Ojeda, F.J.; Molina-Molina, J.M.; Olea, N.; Fernández, M.F. Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells. Food Chem. Toxicol. 2021, 152, 112216. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.H.; Waxman, D.J. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. 2003, 74, 297–308. [Google Scholar] [CrossRef]
- Yin, L.; Yu, K.S.; Lu, K.; Yu, X. Benzyl butyl phthalate promotes adipogenesis in 3T3-L1 preadipocytes: A High Content Cellomics and metabolomic analysis. Toxicol. Vitro 2016, 32, 297–309. [Google Scholar] [CrossRef]
- Yang, Q.; Nagano, T.; Shah, Y.; Cheung, C.; Ito, S.; Gonzalez, F.J. The PPAR alpha-humanized mouse: A model to investigate species differences in liver toxicity mediated by PPAR alpha. Toxicol. Sci. 2008, 101, 132–139. [Google Scholar] [CrossRef]
- Schmidt, J.S.; Schaedlich, K.; Fiandanese, N.; Pocar, P.; Fischer, B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ. Health Perspect. 2012, 120, 1123–1129. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Rivera, F.J.; Guerrero-Bosagna, C. Bisphenol-A and metabolic diseases: Epigenetic, developmental and transgenerational basis. Environ. Epigenet 2016, 2, dvw022. [Google Scholar] [CrossRef]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. Jama 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Teppala, S.; Madhavan, S.; Shankar, A. Bisphenol A and Metabolic Syndrome: Results from NHANES. Int. J. Endocrinol. 2012, 2012, 598180. [Google Scholar] [CrossRef]
- Bastos Sales, L.; Kamstra, J.H.; Cenijn, P.H.; van Rijt, L.S.; Hamers, T.; Legler, J. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol. Vitro 2013, 27, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Nigro, C.; Oriente, F.; Formisano, P.; Miele, C.; Beguinot, F. Low-dose Bisphenol-A Promotes Epigenetic Changes at Pparγ Promoter in Adipose Precursor Cells. Nutrients 2020, 12, 3498. [Google Scholar] [CrossRef] [PubMed]
- Meruvu, S.; Zhang, J.; Choudhury, M. Butyl Benzyl Phthalate Promotes Adipogenesis in 3T3-L1 Cells via the miRNA-34a-5p Signaling Pathway in the Absence of Exogenous Adipogenic Stimuli. Chem. Res. Toxicol. 2021, 34, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Choudhury, M. The plasticizer BBP selectively inhibits epigenetic regulator sirtuin during differentiation of C3H10T1/2 stem cell line. Toxicol. Vitro 2017, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Strakovsky, R.S.; Wang, H.; Engeseth, N.J.; Flaws, J.A.; Helferich, W.G.; Pan, Y.X.; Lezmi, S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol. Appl. Pharmacol. 2015, 284, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Ikeda-Araki, A.; Ishihara, T.; Miyake, K.; Miyashita, C.; Nakajima, T.; Kobayashi, S.; Ishizuka, M.; Kubota, T.; Kishi, R. Effect of prenatal exposure to phthalates on epigenome-wide DNA methylations in cord blood and implications for fetal growth: The Hokkaido Study on Environment and Children’s Health. Sci. Total Environ. 2021, 783, 147035. [Google Scholar] [CrossRef] [PubMed]
- Charisiadis, P.; Andrianou, X.D.; van der Meer, T.P.; den Dunnen, W.F.A.; Swaab, D.F.; Wolffenbuttel, B.H.R.; Makris, K.C.; van Vliet-Ostaptchouk, J.V. Possible Obesogenic Effects of Bisphenols Accumulation in the Human Brain. Sci. Rep. 2018, 8, 8186. [Google Scholar] [CrossRef]
- Naomi, R.; Yazid, M.D.; Bahari, H.; Keong, Y.Y.; Rajandram, R.; Embong, H.; Teoh, S.H.; Halim, S.; Othman, F. Bisphenol A (BPA) Leading to Obesity and Cardiovascular Complications: A Compilation of Current In Vivo Study. Int. J. Mol. Sci. 2022, 23, 2969. [Google Scholar] [CrossRef]
- McIlwraith, E.K.; Lieu, C.V.; Belsham, D.D. Bisphenol A induces miR-708-5p through an ER stress-mediated mechanism altering neuronatin and neuropeptide Y expression in hypothalamic neuronal models. Mol. Cell Endocrinol. 2022, 539, 111480. [Google Scholar] [CrossRef]
- Loganathan, N.; McIlwraith, E.K.; Belsham, D.D. Bisphenol A Induces Agrp Gene Expression in Hypothalamic Neurons through a Mechanism Involving ATF3. Neuroendocrinology 2021, 111, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Li, H.; Wu, Z.; Yan, J.; Liu, Q.; Ou, C.; Chen, M. A Promising Therapeutic Target for Metabolic Diseases: Neuropeptide Y Receptors in Humans. Cell Physiol. Biochem. 2018, 45, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yan, S.; Meng, Z.; Huang, S.; Sun, W.; Jia, M.; Teng, M.; Zhou, Z.; Zhu, W. New insights into bisphenols induced obesity in zebrafish (Danio rerio): Activation of cannabinoid receptor CB1. J. Hazard. Mater. 2021, 418, 126100. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.; Fayed, R.H.; Sedik, A.A.; Khalil, H.M.A. Dose-dependent toxic effects of di-(2-ethylhexyl) phthalate in male rats: Focus on behavioral alterations and inducing TLR4/NF-κB signaling pathway. Toxicol. Appl. Pharmacol. 2023, 468, 116515. [Google Scholar] [CrossRef] [PubMed]
- Rönn, M.; Lind, L.; Örberg, J.; Kullberg, J.; Söderberg, S.; Larsson, A.; Johansson, L.; Ahlström, H.; Lind, P.M. Bisphenol A is related to circulating levels of adiponectin, leptin and ghrelin, but not to fat mass or fat distribution in humans. Chemosphere 2014, 112, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Basu, S.; Ghosh, S.; Guria, S.; Mukherjee, S. Diethyl phthalate, a plasticizer, induces adipocyte inflammation and apoptosis in mice after long-term dietary administration. J. Biochem. Mol. Toxicol. 2023, e23561. [Google Scholar] [CrossRef] [PubMed]
- Schaedlich, K.; Beier, L.S.; Kolbe, J.; Wabitsch, M.; Ernst, J. Pro-inflammatory effects of DEHP in SGBS-derived adipocytes and THP-1 macrophages. Sci. Rep. 2021, 11, 7928. [Google Scholar] [CrossRef] [PubMed]
- Valentino, R.; D’Esposito, V.; Passaretti, F.; Liotti, A.; Cabaro, S.; Longo, M.; Perruolo, G.; Oriente, F.; Beguinot, F.; Formisano, P. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS ONE 2013, 8, e82099. [Google Scholar] [CrossRef]
- Shi, M.; Lin, Z.; Ye, L.; Chen, X.; Zhang, W.; Zhang, Z.; Luo, F.; Liu, Y.; Shi, M. Estrogen receptor-regulated SOCS3 modulation via JAK2/STAT3 pathway is involved in BPF-induced M1 polarization of macrophages. Toxicology 2020, 433–434, 152404. [Google Scholar] [CrossRef]
- Piao, X.; Liu, Z.; Li, Y.; Yao, D.; Sun, L.; Wang, B.; Ma, Y.; Wang, L.; Zhang, Y. Investigation of the effect for bisphenol A on oxidative stress in human hepatocytes and its interaction with catalase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 221, 117149. [Google Scholar] [CrossRef]
- Schaffert, A.; Arnold, J.; Karkossa, I.; Blüher, M.; von Bergen, M.; Schubert, K. The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages. Cells 2021, 10, 2367. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Zhou, Y.; Zhu, Z.; Li, Y.; Li, Z.; Zhang, Y.; Hu, X.; Zhu, F.; Wang, Y.; Fang, M.; et al. Environmental endocrine disruptor Bisphenol A induces metabolic derailment and obesity via upregulating IL-17A in adipocytes. Environ. Int. 2023, 172, 107759. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Gupta, N.; Mathur, R.; Nimesh, S.; Mathur, S.K. A Study on Impact of BPA in the Adipose Tissue Dysfunction (Adiposopathy) in Asian Indian Type 2 Diabetes Mellitus Subjects. Indian. J. Clin. Biochem. 2020, 35, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Hong, Y.C.; Oh, S.Y.; Park, M.S.; Kim, H.; Leem, J.H.; Ha, E.H. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ. Res. 2009, 109, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Veeramachaneni, D.N.R.; Walters, W.A.; Lozupone, C.; Palmer, J.; Hewage, M.K.K.; Bhatnagar, R.; Amir, A.; Kennett, M.J.; Knight, R.; et al. Perinatal Bisphenol A Exposure Induces Chronic Inflammation in Rabbit Offspring via Modulation of Gut Bacteria and Their Metabolites. mSystems 2017, 2, e00093-17. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Ontiveros, Y.; Páez, S.; Monteagudo, C.; Rivas, A. Endocrine Disruptors in Food: Impact on Gut Microbiota and Metabolic Diseases. Nutrients 2020, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Diamante, G.; Cely, I.; Zamora, Z.; Ding, J.; Blencowe, M.; Lang, J.; Bline, A.; Singh, M.; Lusis, A.J.; Yang, X. Systems toxicogenomics of prenatal low-dose BPA exposure on liver metabolic pathways, gut microbiota, and metabolic health in mice. Environ. Int. 2021, 146, 106260. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Wen, L.; Song, Y.; He, X.; Yue, J.; Wu, J.; Chen, X.; Cai, Z.; Qi, Z. DEHP exposure elevated cardiovascular risk in obese mice by disturbing the arachidonic acid metabolism of gut microbiota. Sci. Total Environ. 2023, 875, 162615. [Google Scholar] [CrossRef]
- Su, H.; Yuan, P.; Lei, H.; Zhang, L.; Deng, D.; Zhang, L.; Chen, X. Long-term chronic exposure to di-(2-ethylhexyl)-phthalate induces obesity via disruption of host lipid metabolism and gut microbiota in mice. Chemosphere 2022, 287, 132414. [Google Scholar] [CrossRef]
- Francis, C.E.; Allee, L.; Nguyen, H.; Grindstaff, R.D.; Miller, C.N.; Rayalam, S. Endocrine disrupting chemicals: Friend or foe to brown and beige adipose tissue? Toxicology 2021, 463, 152972. [Google Scholar] [CrossRef]
- Himms-Hagen, J. Brown adipose tissue thermogenesis: Interdisciplinary studies. Faseb J. 1990, 4, 2890–2898. [Google Scholar] [CrossRef] [PubMed]
- Nunez, A.A.; Kannan, K.; Giesy, J.P.; Fang, J.; Clemens, L.G. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 2001, 42, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Chiang, C.W.; Lin, H.C.; Zhao, J.F.; Li, C.T.; Shyue, S.K.; Lee, T.S. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch. Toxicol. 2016, 90, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- van Esterik, J.C.; Dollé, M.E.; Lamoree, M.H.; van Leeuwen, S.P.; Hamers, T.; Legler, J.; van der Ven, L.T. Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation. Toxicology 2014, 321, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Cheng, J.; Huang, S.; Zhang, Y.; Wu, S.; Qiu, Y.; Geng, Y.; Zhang, Q.; Huang, G.; Ma, Q.; et al. DEHP induces obesity and hypothyroidism through both central and peripheral pathways in C3H/He mice. Obesity 2016, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Stratigou, T.; Dalamaga, M.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Christodoulatos, G.S.; Karampela, I.; Papavassiliou, A.G. Hyperirisinemia is independently associated with subclinical hypothyroidism: Correlations with cardiometabolic biomarkers and risk factors. Endocrine 2018, 61, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Menale, C.; Piccolo, M.T.; Cirillo, G.; Calogero, R.A.; Papparella, A.; Mita, L.; Del Giudice, E.M.; Diano, N.; Crispi, S.; Mita, D.G. Bisphenol A effects on gene expression in adipocytes from children: Association with metabolic disorders. J. Mol. Endocrinol. 2015, 54, 289–303. [Google Scholar] [CrossRef]
- Campioli, E.; Batarseh, A.; Li, J.; Papadopoulos, V. The endocrine disruptor mono-(2-ethylhexyl) phthalate affects the differentiation of human liposarcoma cells (SW 872). PLoS ONE 2011, 6, e28750. [Google Scholar] [CrossRef]
- Biemann, R.; Navarrete Santos, A.; Navarrete Santos, A.; Riemann, D.; Knelangen, J.; Blüher, M.; Koch, H.; Fischer, B. Endocrine disrupting chemicals affect the adipogenic differentiation of mesenchymal stem cells in distinct ontogenetic windows. Biochem. Biophys. Res. Commun. 2012, 417, 747–752. [Google Scholar] [CrossRef]
- Ohlstein, J.F.; Strong, A.L.; McLachlan, J.A.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J. Mol. Endocrinol. 2014, 53, 345–353. [Google Scholar] [CrossRef]
- Schaffert, A.; Karkossa, I.; Ueberham, E.; Schlichting, R.; Walter, K.; Arnold, J.; Blüher, M.; Heiker, J.T.; Lehmann, J.; Wabitsch, M.; et al. Di-(2-ethylhexyl) phthalate substitutes accelerate human adipogenesis through PPARγ activation and cause oxidative stress and impaired metabolic homeostasis in mature adipocytes. Environ. Int. 2022, 164, 107279. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Yasuda, K.; Mori, C.; Yoshinaga, M.; Aoki, N.; Tsujimoto, G.; Tsuda, K. Promoting insulin secretion in pancreatic islets by means of bisphenol A and nonylphenol via intracellular estrogen receptors. Food Chem. Toxicol. 2005, 43, 713–719. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, E.; Li, T.; Rosen, E.D. Exposure of adipocytes to bisphenol-A in vitro interferes with insulin action without enhancing adipogenesis. PLoS ONE 2018, 13, e0201122. [Google Scholar] [CrossRef]
- Boucher, J.G.; Husain, M.; Rowan-Carroll, A.; Williams, A.; Yauk, C.L.; Atlas, E. Identification of mechanisms of action of bisphenol a-induced human preadipocyte differentiation by transcriptional profiling. Obesity 2014, 22, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Zhao, T.; Yang, L.; Guo, S.; Shi, Y.; Zhang, X.; Zhou, L.; Ye, L. Mono-2-ethylhexyl phthalate (MEHP) promoted lipid accumulation via JAK2/STAT5 and aggravated oxidative stress in BRL-3A cells. Ecotoxicol. Environ. Saf. 2019, 184, 109611. [Google Scholar] [CrossRef] [PubMed]
- Bucher, S.; Jalili, P.; Le Guillou, D.; Begriche, K.; Rondel, K.; Martinais, S.; Zalko, D.; Corlu, A.; Robin, M.A.; Fromenty, B. Bisphenol a induces steatosis in HepaRG cells using a model of perinatal exposure. Environ. Toxicol. 2017, 32, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Martella, A.; Silvestri, C.; Maradonna, F.; Gioacchini, G.; Allarà, M.; Radaelli, G.; Overby, D.R.; Di Marzo, V.; Carnevali, O. Bisphenol A Induces Fatty Liver by an Endocannabinoid-Mediated Positive Feedback Loop. Endocrinology 2016, 157, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Hou, M.; Pan, X.; Li, X. The environmental obesogen bisphenol A promotes adipogenesis by increasing the amount of 11β-hydroxysteroid dehydrogenase type 1 in the adipose tissue of children. Int. J. Obes 2013, 37, 999–1005. [Google Scholar] [CrossRef]
- Grasselli, E.; Cortese, K.; Voci, A.; Vergani, L.; Fabbri, R.; Barmo, C.; Gallo, G.; Canesi, L. Direct effects of Bisphenol A on lipid homeostasis in rat hepatoma cells. Chemosphere 2013, 91, 1123–1129. [Google Scholar] [CrossRef]
- Dimastrogiovanni, G.; Córdoba, M.; Navarro, I.; Jáuregui, O.; Porte, C. Alteration of cellular lipids and lipid metabolism markers in RTL-W1 cells exposed to model endocrine disrupters. Aquat. Toxicol. 2015, 165, 277–285. [Google Scholar] [CrossRef]
- Schaedlich, K.; Gebauer, S.; Hunger, L.; Beier, L.S.; Koch, H.M.; Wabitsch, M.; Fischer, B.; Ernst, J. DEHP deregulates adipokine levels and impairs fatty acid storage in human SGBS-adipocytes. Sci. Rep. 2018, 8, 3447. [Google Scholar] [CrossRef] [PubMed]
- Riu, A.; Grimaldi, M.; le Maire, A.; Bey, G.; Phillips, K.; Boulahtouf, A.; Perdu, E.; Zalko, D.; Bourguet, W.; Balaguer, P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011, 119, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.C.; Cohenour, E.R.; Harnett, K.G.; Schuh, S.M. BPA, BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity. Int. J. Mol. Sci. 2021, 22, 5363. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Hasegawa, S.; Imai, M.; Fukui, T.; Takahashi, N. Browning Effect of Brominated Flame Retardant, TBBP-A, on Undifferentiated Adipocytes. BPB Rep. 2021, 4, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Marqueño, A.; Pérez-Albaladejo, E.; Denslow, N.D.; Bowden, J.A.; Porte, C. Untargeted lipidomics reveals the toxicity of bisphenol A bis(3-chloro-2- hydroxypropyl) ether and bisphenols A and F in zebrafish liver cells. Ecotoxicol. Environ. Saf. 2021, 219, 112311. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Wang, Y.C.; Hsu, Y.A.; Chen, C.S.; Weng, R.C.; Lu, Y.P.; Chuang, C.Y.; Wan, L. Bisphenol A Coupled with a High-Fat Diet Promotes Hepatosteatosis through Reactive-Oxygen-Species-Induced CD36 Overexpression. Toxics 2022, 10, 208. [Google Scholar] [CrossRef]
- Pérez-Albaladejo, E.; Solís, A.; Bani, I.; Porte, C. PLHC-1 topminnow liver cells: An alternative model to investigate the toxicity of plastic additives in the aquatic environment. Ecotoxicol. Environ. Saf. 2021, 208, 111746. [Google Scholar] [CrossRef]
- Al-Abdulla, R.; Ferrero, H.; Soriano, S.; Boronat-Belda, T.; Alonso-Magdalena, P. Screening of Relevant Metabolism-Disrupting Chemicals on Pancreatic β-Cells: Evaluation of Murine and Human In Vitro Models. Int. J. Mol. Sci. 2022, 23, 4182. [Google Scholar] [CrossRef]
- Wei, J.; Lin, Y.; Li, Y.; Ying, C.; Chen, J.; Song, L.; Zhou, Z.; Lv, Z.; Xia, W.; Chen, X.; et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 2011, 152, 3049–3061. [Google Scholar] [CrossRef]
- Desai, M.; Ferrini, M.G.; Jellyman, J.K.; Han, G.; Ross, M.G. In vivo and in vitro bisphenol A exposure effects on adiposity. J. Dev. Orig. Health Dis. 2018, 9, 678–687. [Google Scholar] [CrossRef]
- Pu, Y.; Gingrich, J.D.; Steibel, J.P.; Veiga-Lopez, A. Sex-Specific Modulation of Fetal Adipogenesis by Gestational Bisphenol A and Bisphenol S Exposure. Endocrinology 2017, 158, 3844–3858. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Cheng, X.; Guo, J.; Xia, H.; Ma, X. Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Front. Biosci. 2013, 5, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Hesselbarth, N.; Gericke, M.; Kunath, A.; Biemann, R.; Chakaroun, R.; Kosacka, J.; Kovacs, P.; Kern, M.; Stumvoll, M.; et al. Di-(2-Ethylhexyl)-Phthalate (DEHP) Causes Impaired Adipocyte Function and Alters Serum Metabolites. PLoS ONE 2015, 10, e0143190. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Cheng, X.; Xia, H.; Ma, X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. 2012, 32, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Sun, X.; Deng, S.; Wen, Y.; Xu, Q.; Guan, Q. In vivo effects of low dose prenatal bisphenol A exposure on adiposity in male and female ICR offspring. Ecotoxicol. Environ. Saf. 2023, 257, 114946. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Marmugi, A.; Viguié, C.; Gayrard, V.; Picard-Hagen, N.; Mselli-Lakhal, L. Are BPA Substitutes as Obesogenic as BPA? Int. J. Mol. Sci. 2022, 23, 4238. [Google Scholar] [CrossRef]
- Huff, M.; da Silveira, W.A.; Carnevali, O.; Renaud, L.; Hardiman, G. Systems Analysis of the Liver Transcriptome in Adult Male Zebrafish Exposed to the Plasticizer (2-Ethylhexyl) Phthalate (DEHP). Sci. Rep. 2018, 8, 2118. [Google Scholar] [CrossRef]
- Amara, I.; Timoumi, R.; Annabi, E.; Neffati, F.; Najjar, M.F.; Bouaziz, C.; Abid-Essefi, S. Di (2-ethylhexyl) phthalate induces cardiac disorders in BALB/c mice. Environ. Sci. Pollut. Res. Int. 2019, 26, 7540–7549. [Google Scholar] [CrossRef]
- Tête, A.; Gallais, I.; Imran, M.; Legoff, L.; Martin-Chouly, C.; Sparfel, L.; Bescher, M.; Sergent, O.; Podechard, N.; Lagadic-Gossmann, D. MEHP/ethanol co-exposure favors the death of steatotic hepatocytes, possibly through CYP4A and ADH involvement. Food Chem. Toxicol. 2020, 146, 111798. [Google Scholar] [CrossRef]
- Jia, P.P.; Junaid, M.; Xin, G.Y.; Wang, Y.; Ma, Y.B.; Pei, D.S. Disruption of Intestinal Homeostasis Through Altered Responses of the Microbial Community, Energy Metabolites, and Immune System in Zebrafish after Chronic Exposure to DEHP. Front. Microbiol. 2021, 12, 729530. [Google Scholar] [CrossRef]
- Susiarjo, M.; Xin, F.; Bansal, A.; Stefaniak, M.; Li, C.; Simmons, R.A.; Bartolomei, M.S. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 2015, 156, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Han, J.; Wu, S.; Shi, X.; Wang, Q.; Zhou, B. Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate Affects Lipid Metabolism in Zebrafish Larvae via DNA Methylation Modification. Environ. Sci. Technol. 2020, 54, 355–363. [Google Scholar] [CrossRef]
- Stoker, C.; Andreoli, M.F.; Kass, L.; Bosquiazzo, V.L.; Rossetti, M.F.; Canesini, G.; Luque, E.H.; Ramos, J.G. Perinatal exposure to bisphenol A (BPA) impairs neuroendocrine mechanisms regulating food intake and kisspetin system in adult male rats. Evidences of metabolic disruptor hypothesis. Mol. Cell Endocrinol. 2020, 499, 110614. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Jia, Y.; Wu, F.; Meng, Y.; Sun, Q.; Jia, L. Combined Exposure to Fructose and Bisphenol A Exacerbates Abnormal Lipid Metabolism in Liver of Developmental Male Rats. Int. J. Environ. Res. Public. Health 2019, 16, 4152. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.K.; Tain, Y.L.; Chen, Y.W.; Hsu, W.H.; Yeh, Y.T.; Chang, S.K.C.; Liao, J.X.; Hou, C.Y. Resveratrol Butyrate Esters Inhibit Obesity Caused by Perinatal Exposure to Bisphenol A in Female Offspring Rats. Molecules 2021, 26, 4010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Powell, C.A.; Kay, M.K.; Park, M.H.; Meruvu, S.; Sonkar, R.; Choudhury, M. A moderate physiological dose of benzyl butyl phthalate exacerbates the high fat diet-induced diabesity in male mice. Toxicol. Res. 2020, 9, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Buerger, A.N.; Dillon, D.T.; Schmidt, J.; Yang, T.; Zubcevic, J.; Martyniuk, C.J.; Bisesi, J.H., Jr. Gastrointestinal dysbiosis following diethylhexyl phthalate exposure in zebrafish (Danio rerio): Altered microbial diversity, functionality, and network connectivity. Environ. Pollut. 2020, 265, 114496. [Google Scholar] [CrossRef]
- Lin, M.H.; Lee, C.Y.; Chuang, Y.S.; Shih, C.L. Exposure to bisphenol A associated with multiple health-related outcomes in humans: An umbrella review of systematic reviews with meta-analyses. Environ. Res. 2023, 237, 116900. [Google Scholar] [CrossRef]
- Deodati, A.; Bottaro, G.; Germani, D.; Carli, F.; Tait, S.; Busani, L.; Della Latta, V.; Pala, A.P.; Maranghi, F.; Tassinari, R.; et al. Urinary Bisphenol-A (BPA) and Bis(2-ethylhexyl)phthalate (DEHP) metabolite concentrations in children with obesity: A case-control study. Horm. Res. Paediatr. 2023. [Google Scholar] [CrossRef]
- Chen, M.; Lv, C.; Zhang, S.; Tse, L.A.; Hong, X.; Liu, X.; Ding, Y.; Xiao, P.; Tian, Y.; Gao, Y. Bisphenol A substitutes and childhood obesity at 7 years: A cross-sectional study in Shandong, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 73174–73184. [Google Scholar] [CrossRef]
- Bi, J.; Wang, F.; Wei, Y.; Zhang, Y.; Jia, C.; He, J.; Yao, J.; Zhang, Z.; Li, Z.; Li, P.; et al. Association of serum bisphenol A levels with incident overweight and obesity risk and the mediating effect of adiponectin. Chemosphere 2022, 308, 136287. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, J.; Huh, D.A.; Moon, K.W. Urinary bisphenol concentrations and its association with metabolic disorders in the US and Korean populations. Environ. Pollut. 2022, 295, 118679. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, P.; Liu, Y.; Li, N.; Buckley, J.P.; Chen, A.; Lanphear, B.P.; Kalkwarf, H.J.; Cecil, K.M.; Yolton, K.; Braun, J.M. Associations of mid-childhood bisphenol A and bisphenol S exposure with mid-childhood and adolescent obesity. Environ. Epidemiol. 2022, 6, e187. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Jiang, Y.; Jin, X.; He, L. Using three statistical methods to analyze the association between exposure to 9 compounds and obesity in children and adolescents: NHANES 2005-2010. Environ. Health 2020, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.; Beserra, B.T.S.; Silva, N.G.; Lima, C.L.; Rocha, P.R.S.; Coelho, M.S.; Neves, F.A.R.; Amato, A.A. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: A systematic review and meta-analysis. BMJ Open 2020, 10, e033509. [Google Scholar] [CrossRef]
- Wu, W.; Li, M.; Liu, A.; Wu, C.; Li, D.; Deng, Q.; Zhang, B.; Du, J.; Gao, X.; Hong, Y. Bisphenol A and the Risk of Obesity a Systematic Review With Meta-Analysis of the Epidemiological Evidence. Dose Response 2020, 18, 1559325820916949. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Woodward, M.; Bao, W.; Liu, B.; Trasande, L. Urinary Bisphenols and Obesity Prevalence Among U.S. Children and Adolescents. J. Endocr. Soc. 2019, 3, 1715–1726. [Google Scholar] [CrossRef]
- Liu, B.; Lehmler, H.J.; Sun, Y.; Xu, G.; Sun, Q.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab. J. 2019, 43, 59–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef]
- Hao, M.; Ding, L.; Xuan, L.; Wang, T.; Li, M.; Zhao, Z.; Lu, J.; Xu, Y.; Chen, Y.; Wang, W.; et al. Urinary bisphenol A concentration and the risk of central obesity in Chinese adults: A prospective study. J. Diabetes 2018, 10, 442–448. [Google Scholar] [CrossRef]
- Do, M.T.; Chang, V.C.; Mendez, M.A.; de Groh, M. Urinary bisphenol A and obesity in adults: Results from the Canadian Health Measures Survey. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hauser, R.; Hu, F.B.; Franke, A.A.; Liu, S.; Sun, Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: A prospective investigation in US women. Int. J. Obes. 2014, 38, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Xiao, J.; Shankar, A. Urinary bisphenol A and obesity in U.S. children. Am. J. Epidemiol. 2013, 177, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Urinary bisphenol a levels and measures of obesity: Results from the national health and nutrition examination survey 2003-2008. ISRN Endocrinol. 2012, 2012, 965243. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Attina, T.M.; Blustein, J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. Jama 2012, 308, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, M.; Chen, B.; Xu, M.; Xu, Y.; Huang, Y.; Lu, J.; Chen, Y.; Wang, W.; Li, X.; et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2012, 97, E223–E227. [Google Scholar] [CrossRef] [PubMed]
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011, 111, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.R.; Starling, A.P.; Bommarito, P.A.; Keil, A.P.; Nakiwala, D.; Calafat, A.M.; Adgate, J.L.; Dabelea, D.; Ferguson, K.K. Midpregnancy Phthalate and Phenol Biomarkers in Relation to Infant Body Composition: The Healthy Start Prospective Cohort. Environ. Health Perspect. 2023, 131, 87017. [Google Scholar] [CrossRef]
- Li, D.; Yao, Y.; Chen, D.; Wu, Y.; Liao, Y.; Zhou, L. Phthalates, physical activity, and diet, which are the most strongly associated with obesity? A case-control study of Chinese children. Endocrine 2023, 82, 69–77. [Google Scholar] [CrossRef]
- Wu, Q.; Li, G.; Zhao, C.Y.; Na, X.L.; Zhang, Y.B. Association between phthalate exposure and obesity risk: A meta-analysis of observational studies. Environ. Toxicol. Pharmacol. 2023, 102, 104240. [Google Scholar] [CrossRef]
- Boyer, T.M.; Bommarito, P.A.; Welch, B.M.; Meeker, J.D.; James-Todd, T.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Maternal exposure to phthalates and total gestational weight gain in the LIFECODES birth cohort. Reprod. Toxicol. 2023, 117, 108354. [Google Scholar] [CrossRef] [PubMed]
- Vieyra, G.; Hankinson, S.E.; Oulhote, Y.; Vandenberg, L.N.; Tinker, L.; Manson, J.E.; Shadyab, A.H.; Thomson, C.A.; Bao, W.; Allison, M.; et al. Association between urinary phthalate biomarker concentrations and adiposity among postmenopausal women. Environ. Res. 2023, 222, 115356. [Google Scholar] [CrossRef]
- Milankov, A.; Milanović, M.; Milošević, N.; Sudji, J.; Pejaković, S.; Milić, N.; Bjelica, A.; Medić Stojanoska, M. The effects of phthalate exposure on metabolic parameters in polycystic ovary syndrome. Clin. Chim. Acta 2023, 540, 117225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Gao, D.; Zou, Z.Y. The association of phthalate metabolites with childhood waist circumference and abdominal obesity. Eur. J. Pediatr. 2023, 182, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.Q.; Karvonen-Gutierrez, C.A.; Herman, W.H.; Mukherjee, B.; Park, S.K. Phthalate exposure is associated with more rapid body fat gain in midlife women: The Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study. Environ. Res. 2023, 216, 114685. [Google Scholar] [CrossRef] [PubMed]
- Kupsco, A.; Wu, H.; Calafat, A.M.; Kioumourtzoglou, M.A.; Cantoral, A.; Tamayo-Ortiz, M.; Pantic, I.; Pizano-Zárate, M.L.; Oken, E.; Braun, J.M.; et al. Prenatal maternal phthalate exposures and trajectories of childhood adiposity from four to twelve years. Environ. Res. 2022, 204, 112111. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Mendes, V.; Peleteiro, B.; Delgado, I.; Araújo, J.; Aggerbeck, M.; Annesi-Maesano, I.; Sarigiannis, D.; Ramos, E. Association between the exposure to phthalates and adiposity: A meta-analysis in children and adults. Environ. Res. 2019, 179, 108780. [Google Scholar] [CrossRef]
- Díaz Santana, M.V.; Hankinson, S.E.; Bigelow, C.; Sturgeon, S.R.; Zoeller, R.T.; Tinker, L.; Manson, J.A.E.; Calafat, A.M.; Meliker, J.R.; Reeves, K.W. Urinary concentrations of phthalate biomarkers and weight change among postmenopausal women: A prospective cohort study. Environ. Health 2019, 18, 20. [Google Scholar] [CrossRef]
- Rodríguez-Carmona, Y.; Cantoral, A.; Trejo-Valdivia, B.; Téllez-Rojo, M.M.; Svensson, K.; Peterson, K.E.; Meeker, J.D.; Schnaas, L.; Solano, M.; Watkins, D.J. Phthalate exposure during pregnancy and long-term weight gain in women. Environ. Res. 2019, 169, 26–32. [Google Scholar] [CrossRef]
- Buckley, J.P.; Engel, S.M.; Mendez, M.A.; Richardson, D.B.; Daniels, J.L.; Calafat, A.M.; Wolff, M.S.; Herring, A.H. Prenatal Phthalate Exposures and Childhood Fat Mass in a New York City Cohort. Environ. Health Perspect. 2016, 124, 507–513. [Google Scholar] [CrossRef]
- Valvi, D.; Casas, M.; Romaguera, D.; Monfort, N.; Ventura, R.; Martinez, D.; Sunyer, J.; Vrijheid, M. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ. Health Perspect. 2015, 123, 1022–1029. [Google Scholar] [CrossRef]
- Yaghjyan, L.; Sites, S.; Ruan, Y.; Chang, S.H. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999-2004. Int. J. Obes. 2015, 39, 994–1000. [Google Scholar] [CrossRef]
- Lind, P.M.; Roos, V.; Rönn, M.; Johansson, L.; Ahlström, H.; Kullberg, J.; Lind, L. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ. Health 2012, 11, 21. [Google Scholar] [CrossRef]
- Hatch, E.E.; Nelson, J.W.; Qureshi, M.M.; Weinberg, J.; Moore, L.L.; Singer, M.; Webster, T.F. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: A cross-sectional study of NHANES data, 1999–2002. Environ. Health 2008, 7, 27. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, E.; Kim, Y. The Association between Bisphenol A Exposure and Obesity in Children-A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public. Health 2019, 16, 2521. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, Y.; Wei, W.; Zhao, Y.; Jiang, Y.; Wang, N.; Li, X.; Chen, X. Association between prenatal exposure to bisphenol a and birth outcomes: A systematic review with meta-analysis. Medicine 2019, 98, e17672. [Google Scholar] [CrossRef]
- Hu, C.Y.; Li, F.L.; Hua, X.G.; Jiang, W.; Mao, C.; Zhang, X.J. The association between prenatal bisphenol A exposure and birth weight: A meta-analysis. Reprod. Toxicol. 2018, 79, 21–31. [Google Scholar] [CrossRef]
- Vrachnis, N.; Loukas, N.; Vrachnis, D.; Antonakopoulos, N.; Zygouris, D.; Kοlialexi, A.; Pergaliotis, V.; Iavazzo, C.; Mastorakos, G.; Iliodromiti, Z. A Systematic Review of Bisphenol A from Dietary and Non-Dietary Sources during Pregnancy and Its Possible Connection with Fetal Growth Restriction: Investigating Its Potential Effects and the Window of Fetal Vulnerability. Nutrients 2021, 13, 2426. [Google Scholar] [CrossRef]
- Soundararajan, A.; Prabu, P.; Mohan, V.; Gibert, Y.; Balasubramanyam, M. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol. Cell Biochem. 2019, 458, 171–183. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S. Relationship between urinary bisphenol A levels and diabetes mellitus. J. Clin. Endocrinol. Metab. 2011, 96, 3822–3826. [Google Scholar] [CrossRef] [PubMed]
- Kawa, I.A.; Masood, A.; Ganie, M.A.; Fatima, Q.; Jeelani, H.; Manzoor, S.; Rizvi, S.M.; Muzamil, M.; Rashid, F. Bisphenol A (BPA) acts as an endocrine disruptor in women with Polycystic Ovary Syndrome: Hormonal and metabolic evaluation. Obes. Med. 2019, 14, 100090. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Zhou, S.; Zhang, X.; Peng, C.; Zhou, H.; Tong, Y.; Lu, Q. Association of bisphenol A and its alternatives bisphenol S and F exposure with hypertension and blood pressure: A cross-sectional study in China. Environ. Pollut. 2020, 257, 113639. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, S.; Zhao, Z.; Chen, Y.; Xu, Y.; Li, M.; Xu, M.; Wang, W.; Ning, G.; Bi, Y.; et al. Bisphenol A exposure in relation to altered lipid profile and dyslipidemia among Chinese adults: A repeated measures study. Environ. Res. 2020, 184, 109382. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, H.S.; Samarghandian, S.; Farkhondeh, T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol. Mech. Methods 2015, 25, 507–513. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- van der Meer, T.P.; Thio, C.H.L.; van Faassen, M.; van Beek, A.P.; Snieder, H.; van Berkum, F.N.R.; Kema, I.P.; Makris, K.C.; Wolffenbuttel, B.H.R.; van Vliet-Ostaptchouk, J.V. Endocrine disrupting chemicals during diet-induced weight loss-A post-hoc analysis of the LOWER study. Environ. Res. 2021, 192, 110262. [Google Scholar] [CrossRef]
- Lee, D.W.; Lim, H.M.; Lee, J.Y.; Min, K.B.; Shin, C.H.; Lee, Y.A.; Hong, Y.C. Prenatal exposure to phthalate and decreased body mass index of children: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 8961. [Google Scholar] [CrossRef]
- LaKind, J.S.; Idri, F.; Naiman, D.Q.; Verner, M.A. Biomonitoring and Nonpersistent Chemicals-Understanding and Addressing Variability and Exposure Misclassification. Curr. Environ. Health Rep. 2019, 6, 16–21. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. Challenges encountered in the analysis of phthalate esters in foodstuffs and other biological matrices. Anal. Bioanal. Chem. 2012, 404, 2539–2554. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.; Hennings, R.; Kramer, J.; Calafat, A.M. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environ. Health Perspect. 2013, 121, 283–286. [Google Scholar] [CrossRef]
- Cerkvenik-Flajs, V. Bisphenol A background contamination encountered during advanced blood sampling and laboratory analysis. Int. J. Environ. Anal. Chem. 2022, 102, 6602–6612. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Welshons, W.V. Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol. Cell Endocrinol. 2014, 398, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Phillips, C.A.; Mitro, S.D. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003-2010. Environ. Health Perspect. 2016, 124, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.E.; Kauri, L.M.; Cakmak, S. The associations between phthalate exposure and insulin resistance, β-cell function and blood glucose control in a population-based sample. Sci. Total Environ. 2018, 612, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.Y.; Lo, Y.C.; Huang, P.C.; Huang, Y.C.; Chang, J.L.; Huang, H.B. Changes in insulin resistance mediate the associations between phthalate exposure and metabolic syndrome. Environ. Res. 2019, 175, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, F.; Aquilina, A.; Vassallo, J.; Pace, N.P. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int. J. Environ. Res. Public. Health 2021, 18, 716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ben, Y.; Han, Y.; Zhang, Y.; Li, Y.; Chen, X. Phthalate exposure and risk of diabetes mellitus: Implications from a systematic review and meta-analysis. Environ. Res. 2022, 204, 112109. [Google Scholar] [CrossRef]
- Rancière, F.; Botton, J.; Slama, R.; Lacroix, M.Z.; Debrauwer, L.; Charles, M.A.; Roussel, R.; Balkau, B.; Magliano, D.J. Exposure to Bisphenol A and Bisphenol S and Incident Type 2 Diabetes: A Case-Cohort Study in the French Cohort D.E.S.I.R. Environ. Health Perspect. 2019, 127, 107013. [Google Scholar] [CrossRef]
- Yao, J.; Wang, F.; Zhang, Y.; Zhang, Z.; Bi, J.; He, J.; Li, P.; Han, X.; Wei, Y.; Zhang, X.; et al. Association of serum BPA levels with changes in lipid levels and dyslipidemia risk in middle-aged and elderly Chinese. Ecotoxicol. Environ. Saf. 2022, 241, 113819. [Google Scholar] [CrossRef]
- Gao, D.; Zou, Z.; Li, Y.; Chen, M.; Ma, Y.; Chen, L.; Wang, X.; Yang, Z.; Dong, Y.; Ma, J. Association between urinary phthalate metabolites and dyslipidemia in children: Results from a Chinese cohort study. Environ. Pollut. 2022, 295, 118632. [Google Scholar] [CrossRef]
- Han, C.; Hong, Y.C. Bisphenol A, Hypertension, and Cardiovascular Diseases: Epidemiological, Laboratory, and Clinical Trial Evidence. Curr. Hypertens. Rep. 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Teppala, S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J. Environ. Public Health 2012, 2012, 481641. [Google Scholar] [CrossRef]
- Liguori, F.; Moreno-Marrodan, C.; Barbaro, P. Biomass-derived chemical substitutes for bisphenol A: Recent advancements in catalytic synthesis. Chem. Soc. Rev. 2020, 49, 6329–6363. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gómez-Toledano, R. Relationship between emergent BPA-substitutes and renal and cardiovascular diseases in adult population. Environ. Pollut. 2022, 313, 120106. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, S.; Jin, H.; Tang, L.; Xia, M. Associations of bisphenol F and S, as substitutes for bisphenol A, with cardiovascular disease in American adults. J. Appl. Toxicol. 2023, 43, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fei, Q.; Liu, S.; Weng, X.; Liang, H.; Wu, Y.; Wen, L.; Hao, G.; Cao, G.; Jing, C. The bisphenol F and bisphenol S and cardiovascular disease: Results from NHANES 2013–2016. Environ. Sci. Eur. 2022, 34, 4. [Google Scholar] [CrossRef]
- Langsch, A.; David, R.M.; Schneider, S.; Sperber, S.; Haake, V.; Kamp, H.; Leibold, E.; Ravenzwaay, B.V.; Otter, R. Hexamoll® DINCH: Lack of in vivo evidence for obesogenic properties. Toxicol. Lett. 2018, 288, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piouka, A.; Livadas, S.; Piperi, C.; Katsikis, I.; Papavassiliou, A.G.; Panidis, D. Anti-mullerian hormone is associated with advanced glycosylated end products in lean women with polycystic ovary syndrome. Eur. J. Endocrinol. 2009, 160, 847–853. [Google Scholar] [CrossRef]
- Saleh, A.C.; Sabry, R.; Mastromonaco, G.F.; Favetta, L.A. BPA and BPS affect the expression of anti-Mullerian hormone (AMH) and its receptor during bovine oocyte maturation and early embryo development. Reprod. Biol. Endocrinol. 2021, 19, 119. [Google Scholar] [CrossRef]
- Talia, C.; Connolly, L.; Fowler, P.A. The insulin-like growth factor system: A target for endocrine disruptors? Environ. Int. 2021, 147, 106311. [Google Scholar] [CrossRef] [PubMed]
- Papatsoris, A.G.; Karamouzis, M.V.; Papavassiliou, A.G. Novel insights into the implication of the IGF-1 network in prostate cancer. Trends Mol. Med. 2005, 11, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Schlüsener, M.P.; Ternes, T.A.; Oehlmann, J. Identification of putative steroid receptor antagonists in bottled water: Combining bioassays and high-resolution mass spectrometry. PLoS ONE 2013, 8, e72472. [Google Scholar] [CrossRef]
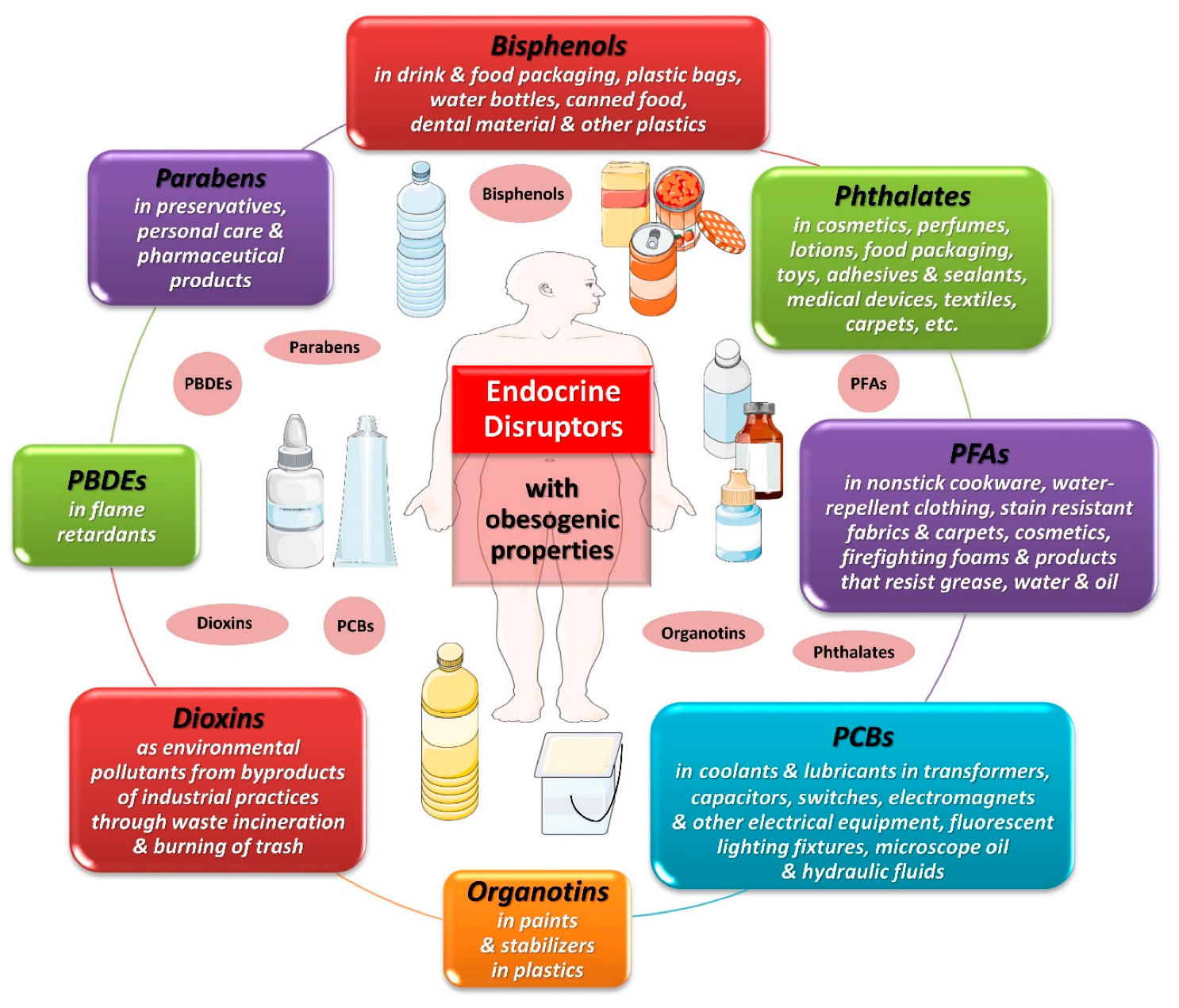
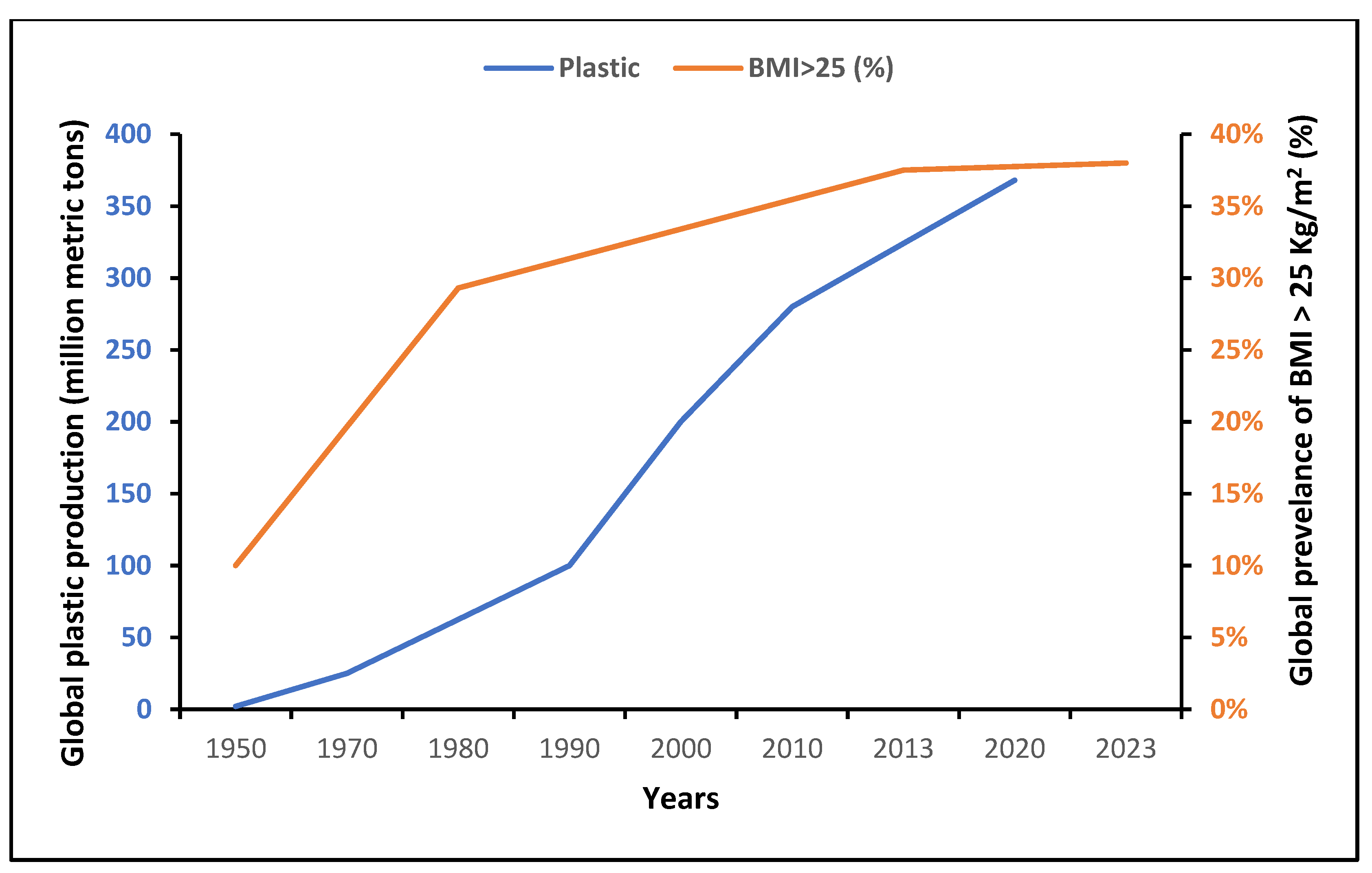
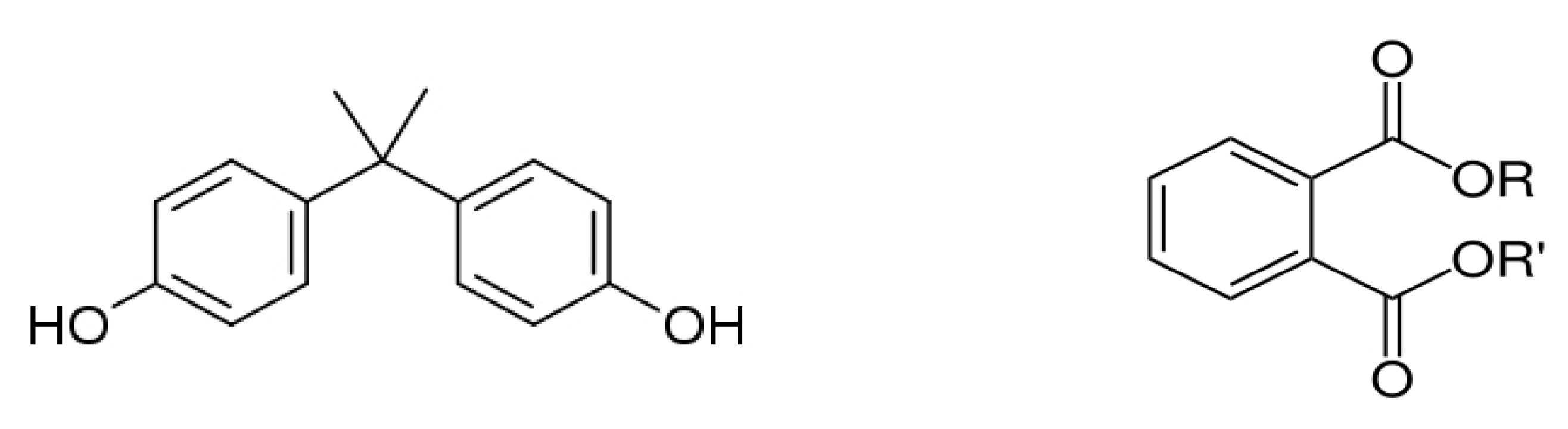
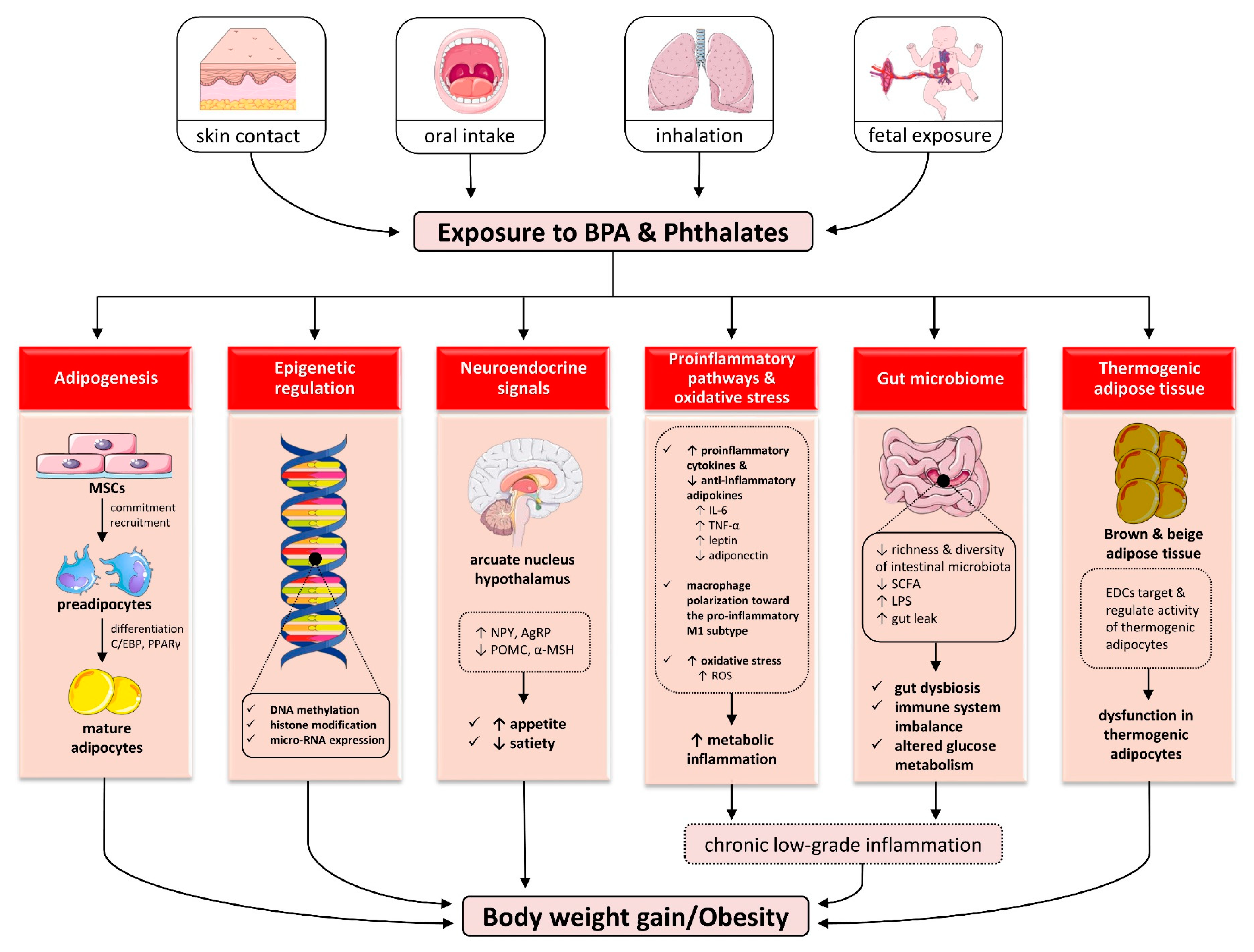
| Author, Year | Study Design/Population | Main Findings | Comments |
|---|---|---|---|
| Bisphenol A and obesity | |||
| Lin et al. (2023) [178] | Umbrella review of systematic reviews with meta-analyses on the association of BPA exposure with multiple outcomes, including obesity | - Higher BPA exposure significantly associated with obesity risk in both sexes (females: OR 1.51; males: OR 1.88) - Significant associations with generalized and abdominal obesity (OR 1.22 and 1.41, respectively) as well as overweight in adults (OR 1.25) - Significantly increased risk for type 2 DM (OR 1.28) | Higher BPA exposure associated with obesity in children and adults, with less heterogeneity among studies in females |
| Deodati et al. (2023) [179] | Case–control study among n = 122 children (n = 66 and n = 56 with and without obesity, respectively) matched for age and gender | - Significantly higher creatinine-adjusted urinary BPA concentrations in obesity than normal weight (10.77 vs. 5.50 µg/g, respectively) among girls, but not boys. - Significantly higher risk of obesity in children with BPA levels above the median eating packaged food (OR = 11.09) | Potential gender-specific relationship between BPA exposure and higher odds of childhood obesity in girls |
| Chen M et al. (2023) [180] | Cross-sectional study among n = 426 children aged 7 years old | - Urinary concentrations of BPA substitutes BPS, BPAF exhibit a significant positive association with BMI, WC, overweight/obesity only among boys - No associations between adiposity measures and BPA or other substitute compounds | Associations between BPS, BPAF, but not BPA exposure and obesity in boys |
| Bi J et al. (2022) [181] | Prospective observational study including n = 796 individuals with normal weight, among whom 133 developed overweight or obesity during follow-up | - Presence of a statistically significant inverted U-shaped relationship between serum BPA and incident overweight/obesity - Significant positive correlation between log10-BPA and increase in waist-to-hip ratio - Serum adiponectin mediates 46% of association between BPA and incident overweight/obesity | Non-monotonic relationship between baseline BPA and incident overweight/obesity among individuals with normal weight, potentially indirectly mediated by adiponectin |
| Choi et al. (2022) [182] | Cross-sectional study including 1046 adult participants in NHANES (2013–2016) and 3268 adult participants of the Korean National Environmental Health Survey (2015–2017) | Those in the higher urinary BPA tertiles had significantly higher odds for obesity (OR = 1.58 and 1.41 for 3rd and 2nd vs. 1st tertile, respectively) Similar associations for urinary BPF and BPS | Exposure not only to BPA but also to substitutes BPF and BPS is associated with adult obesity |
| Gajjar et al. (2021) [183] | Prospective observational cohort of n = 212 children with urinary BPA and BPS measurements at 8 years and body composition assessments at 8 years (bioimpedance) and 12 years (DXA) | No evidence for a synchronous or prospective association of urinary BPA or BPS with increased adiposity | |
| Wu et al. (2020) [184] | Cross-sectional study including n = 2372 children and adolescents (aged 6–19) participating in NHANES | BPA levels significantly associated with higher weight in a statistical approach implementing weighted quantile sum statistical model but not in other approaches | Evidence for an association between BPA exposure and childhood/adolescent obesity |
| Ribeiro et al. (2020) [185] | Meta-analysis of studies investigating BPA exposure and multiple adverse health outcomes, including obesity | - BPA is significantly associated with overweight (OR 1.254), obesity (OR 1.503) and increased WC (OR 1.503) in adults - OR 1.8 for childhood obesity | Positive association between BPA exposure and generalized as well as abdominal obesity |
| Wu et al. (2020) [186] | Meta-analysis of 10 observational studies | - Statistically significant dose–response positive relationship between BPA and overweight/obesity risk in both sexes - 11% increase in obesity risk for every 1 ng/mL of BPA | Continuous positive relationship between BPA and obesity irrespective of sex |
| Jacobson et al. (2019) [187] | Cross-sectional study including n = 1831 children and adolescents (aged 6–19) participating in NHANES | Urinary BPS and BPF but not total bisphenols or BPA were significantly associated with, particularly abdominal obesity | Substitute bisphenol exposure may predispose individuals to childhood/adolescent obesity |
| Liu et al. (2019) [188] | Cross-sectional study including n = 745 children and adolescents (aged 6–17 years) participating in NHANES | Urinary BPA (OR 1.74) and BPF (OR 1.54) are significantly associated with obesity, with stronger associations between boys Similar findings for abdominal obesity | Urinary BPA and its substitute BPF associated with obesity, particularly in boys |
| Zhang et al. (2019) [189] | Cross-sectional study including n = 1269 adults participating in NHANES | Among other chemicals, increased urinary BPA and BPS are significantly associated with higher obesity prevalence | Exposure to BPA, and BPS may predispose individuals to adulthood obesity, although the authors recommend considering the joint effects of different chemical exposures |
| Hao et al. (2018) [190] | Prospective study (mean follow-up: 4 years) among 888 Chinese adults without abdominal obesity at baseline | OR = 2.30 for incident abdominal obesity each unit increase in log [BPA] urinary concentration after adjustment for confounding factors Individuals in the lowest tertile of BPA concentrations had the lowest risk for incident central obesity (ORs 1.73 and 1.81 for those in the 2nd and 3rd tertiles, respectively) | Prospective association of BPA exposure with incident central obesity in Chinese adults |
| Do et al. (2017) [191] | Cross-sectional analysis of data from n = 4733 adults aged (18 to 79 years) | For each natural-log unit increase in urinary BPA concentration, significant increase of 0.33 kg/m2 in BMI and 1.00 cm in waist circumference | Dose–response relationship between BPA exposure and generalized as well as abdominal obesity |
| Song et al. (2014) [192] | Prospective (10 years) cohort study of 977 women with baseline measurements of urinary BPA and 9 phthalate biomarkers | After adjustment for dietary and lifestyle variables, those in the highest BPA quartile gained on average an additional 0.23 kg/year (0.07–0.38) of body weight during follow-up | BPA exposure is associated with greater longitudinal weight gain in women |
| Bhandari et al. (2013) [193] | Cross-sectional analysis of data from n = 2200 children and adolescents (aged 6 to 18 years) from NHANES (2003–2008) | - OR for obesity = 2.55 for children in the highest vs. lowest quartile of urinary BPA - Associations more robust among males and non-Hispanic Whites | BPA is associated with childhood/adolescent obesity, with potential gender- and race-specific effects |
| Shankar et al. (2012) [194] | Cross-sectional analysis of data from n = 3967 adult participants in NHANES (2003–2008) | ORs = 1.69 and 1.59 for generalized and abdominal obesity for the 4th vs. 1st quartile of urinary BPA concentrations, persistent after adjustment for several confounders and consistent among gender and race–ethnic groups | BPA exposure is associated with central and abdominal obesity in both genders and all race groups, irrespectively of traditional risk factors |
| Trasande et al. (2012) [195] | Cross-sectional analysis of data from n = of 2838 children and adolescents (aged 6–19 years) participating in NHANES | - Lowest prevalence of obesity in the 1st vs. 2nd-4th quartiles of ascending urinary BPA concentrations (10.3% vs. 20.1%, 19.0% and 22.3%, respectively) - Association of BPA and obesity significant in Whites but not Blacks or Hispanics | Association between BPA exposure and obesity likely exhibits race-specific effects |
| Wang et al. (2012) [196] | Cross-sectional study of n = 3390, aged >40 years | - Those in the highest quartile of urinary BPA concentrations showed significantly higher prevalence of generalized (OR = 1.50) and abdominal obesity (OR = 1.28) - Among participants without overweight or obesity, higher BPA was significantly associated with IR (OR 1.94) | Evidence for a positive association between BPA exposure and obesity, as well as IR among lean individuals |
| Carwile et al. (2011) [197] | Cross-sectional study including n = 2747 adults (aged 18–74) participating in NHANES (2003–2006) | Higher risk of general (OR 1.74) and abdominal (OR 1.58) obesity among individuals in the highest vs. lowest quartile of urinary BPA concentration | BPA exposure is associated with general and abdominal obesity in US adults |
| Phthalates and obesity | |||
| Deodati et al. (2023) [179] | Case–control study among n = 122 children (n = 66 and n = 56 with and without obesity, respectively) matched for age and gender | - Early downstream metabolites of Di(2-ethylhexyl) phthalate in urine significantly higher in girls with obesity than normal weight - Significant positive correlation of Di(2-ethylhexyl) phthalate metabolites with serum leptin levels | Significant correlation of certain phthalate metabolites with increased adiposity in girls |
| Stevens DR et al. (2023) [198] | Prospective study among n = 438 infants from the Healthy Start prospective pregnancy cohort. | - Significant inverse association between maternal urinary mono-benzyl and di- n-butyl phthalate at 28th gestational week and percentage fat mass at birth in male infants | Maternal phthalate exposure in pregnancy is inversely associated with fat mass in male, but not female, infants at birth |
| Li et al. (2023) [199] | Case–control study among n = 240 children with overweight/obesity (OBE) and n = 240 age- and gender-matched controls | Among 9 phthalates, monomethyl phthalate and monobutyl phthalate were significantly higher in controls than children with overweight/obesity but not after adjustment for physical activity and caloric intake. | No significant differences in phthalate concentrations between OBE and controls |
| Wu et al. (2022) [200] | Meta-analysis of observational studies for the association between phthalate compounds and obesity in adult and pediatric populations | - Mono-n-butyl-, monobutyl-, monoisobutyl-, monoethyl- and mono(2-ethyl-5-carboxypentyl) phthalate significantly associated with obesity, specific compounds more strongly correlate with general or abdominal obesity - Stronger associations in women and in studies from the United States and Europe | Compound-specific effects on general and abdominal obesity, with potential gender- and study-site-specific effects |
| Boyer et al. (2023) [201] | Measurement of the concentrations of 9 phthalates in n = 379 pregnant women, in relation to gestational weight gain (difference between pre-pregnancy and median 35.1 weeks weight) | Significant direct association between mono-(3-carboxypropyl) phthalate and mono-n-butyl phthalate was positively associated with gestational weight gain (1.81 kg and 0.77 kg at 35 weeks) interquartile range increase among women with obesity | Phthalate exposure is associated with greater weight gain in pregnancy, particularly among women with obesity at baseline |
| Vieyra et al. (2023) [202] | Prospective observational study n = 1125 participants of the Woman Health Initiative (WHI) with available urine phthalate measurements and DXA-based estimations of VAT and SAT | Significant positive associations of baseline di-isobutyl phthalate biomarkers, monocarboxy-isononly phthalate, and di(2-ethylhexyl) phthalate with VAT three years later, which persisted after adjustment for SAT | Higher levels of certain urinary phthalate compounds are longitudinally associated with higher VAT over time in postmenopausal women |
| Milankov et al. (2023) [203] | Cross-sectional study among n = 60 women with PCOS | Total urinary phthalate concentrations significantly positively correlate with BMI, waist circumference, waist-to-height ratio, VAI, FPG and HOMA-R | Increased phthalate exposure associated with obesity, insulin resistance and hyperglycemia in women with PCOS |
| Wang et al. (2023) [204] | Cross-sectional study among n = 798 students (7–10 years) | Significantly increased risk of abdominal obesity for the fourth vs. first quartile (OR = 5.29 and 3.73) and 273% (OR = 3.73; 95% CI: 1.57, 8.86) of urinary concentrations of monoethyl phthalate and monoisobutyl phthalate | Monoethyl-phthalate and monoisobutyl-phthalate exposure are associated with abdominal obesity in children |
| Peng et al. (2023) [205] | Prospective observational analysis of n = 1369 women in the Study of Women’s Health Across the Nation Multi-Pollutant Study | Significantly higher levels of spot urinary phthalates (except mono-carboxy-isononyl phthalate) were associated with faster increases in body fat percentage and fat mass, but not total body weight change over time | Urinary phthalate concentrations positively correlated with fat gain in middle-aged women |
| Kupsko et al. (2022) [206] | Prospective observational study of 514 mother–child pairs in pregnancy until twelve years post-term | Higher maternal urine di (2-ethylhexyl) phthalate metabolites significantly associated with greater odds of high and increasing weight in infants Higher di-isononyl phthalate metabolites significantly associated with greater odds increasing weight in infants | Exposure to certain phthalates during pregnancy exerts a significant impact in infant weight trajectories during childhood |
| Ribeiro et al. (2019) [207] | Meta-analysis of 29 studies for the association between phthalate compounds and obesity in adult and pediatric populations | - The low number of studies for many phthalate compounds precludes meta-analysis - Statistically significant association solely between mono(2-ethyl-5-carboxypentyl) phthalate and obesity in adults (OR = 1.67) | Positive association between many phthalate compounds and adiposity measures, most formally non-significant; possible publication-bias-related effects |
| Díaz Santana et al. (2019) [208] | Cross-sectional (n = 997) and prospective (n = 660) observational study among participants of the Woman Health Initiative (WHI) | - Significant positive associations between urinary phthalate biomarker concentrations and obesity in cross-sectional analysis - Baseline urinary mono-(2-ethyl-5-oxohexyl)-, monoethyl-, mono-hydroxybutyl- and mono-hydroxyisobutyl phthalate significantly correlate with weight gain after 3 years - No associations with weight changes at 6 years | Exposure to certain phthalates may predispose individuals to obesity and short-term weight gain |
| Rodriguez-Carmona, et al. (2019) [209] | Prospective cohort study among n = 178 pregnant women | Higher urinary mono-3-carboxypropyl pthalate is significantly associated with moderately increased weight gain over the next 5.2–10.7 years Higher mono-benzyl phthalate significantly associated with lower weight gain in the same timeframe | Prospective association between phthalate exposure in pregnancy and prospective weight changes in women |
| Buckley et al. (2016) [210] | Prospective cohort study assessing the fat mass of n = 180 children (4–9 years) in relation to maternal third-trimester urinary phthalate concentrations in pregnancy | - No continuous associations between maternal urinary phthalate concentrations and fat mass in offspring, without apparent gender-specific effects - 3.06% lower fat mass in children in the highest vs. lowest quartile of summed di(2-ethylhexyl) phthalate metabolites | No evidence for an impact of maternal phthalate exposure and increased infantile fat mass |
| Valvi et al. (2015) [211] | Prospective cohort study of n = 391 mothers with creatinine-adjusted measurements of urinary phthalates in the 1st and 3rd trimesters of pregnancy | High-molecular-weight phthalate metabolites in maternal urine significantly associated with lower BMI z-scores in boys and higher in girls 4–7 years of age | Potential gender-specific effects of maternal phthalate exposure and infantile BMI trajectories |
| Yaghjyan et al. (2015) [212] | Cross-sectional analysis including n = 6005 women without diabetes participating in NHANES (1999–2004) | - Significant positive associations between monobutylphthalate, mono-2-ethylhexyl- to mono(2-ethyl-5-hydroxyhexyl) phthalate ratio and BMI, WC | Among the observed associations, the higher mono-2-ethylhexyl- to mono(2-ethyl-5-hydroxyhexyl) phthalate ratio may be reflective of slower oxidative metabolism of mono-2-ethylhexyl-pthalate |
| Song et al. (2014) [192] | Prospective cohort study of n = 977 women with baseline measurements of urinary BPA and 9 phthalate biomarkers | After adjustment for dietary and lifestyle variables, significant albeit moderate positive dose–response relationship between phthalic acid, monobenzyl- and monobutyl-phthalate and weight gain over 10 years | Exposure to certain phthalates is associated with accelerated weight gain in women |
| Lind et al. (2012) [213] | Prospective cohort study among n = 1016 individuals of 70 years of age | Baseline serum concentrations of mono-isobutyl phthalate and mono-methyl phthalate significantly positively associated with DXA- and abdominal MRI-derived indices of adiposity in women two years later, but not in men | Circulating concentrations of certain phthalates are associated with increased adiposity only in women, suggesting possible sex-specific associations of phthalates with obesity |
| Hatch et al. (2008) [214] | Cross-sectional analysis of data of n = 4369 NHANES participants (1999–2002) | - Positive trends of BMI and WC across quartiles of concentrations of mono-benzyl, mono-2-ethyl-5-oxohexyl, mono-ethyl, mono-n-butyl, mono-2-ethyl-5-hydroxyhexyl, particularly among males 20–59 years old - Similar trends across mono-ethyl phthalate quartiles in adolescent girls, less strong in adult women - Inverse trend for mono-2-ethylhexyl phthalate in adolescent girls and several inverse associations in adults 60–80 years - No associations in children | Exposure to several phthalates may be associated with increased adiposity, but age-group- and gender-specific effects likely exist |
| Stahlhut et al. (2007) [78] | Cross-sectional analysis of data of n = 1443 men participating in NHANES (1999–2002) | - Urinary concentrations of monobenzylphthalate, mono(2-ethyl-5-hydroxyhexyl) phthalate, mono(2-ethyl-5-oxohexyl) phthalate and monoethylphthalate are significantly associated with increased waist circumference, after adjustment for confounders - Monobutylphthalate, monobenzylphthalate and monoethylphthalate concentrations exhibit significant positive correlations with HOMA-R | Exposure to certain phthalates is associated with higher abdominal obesity and insulin resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalamaga, M.; Kounatidis, D.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Psallida, S.; Papavassiliou, A.G. The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies. Int. J. Mol. Sci. 2024, 25, 675. https://doi.org/10.3390/ijms25010675
Dalamaga M, Kounatidis D, Tsilingiris D, Vallianou NG, Karampela I, Psallida S, Papavassiliou AG. The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies. International Journal of Molecular Sciences. 2024; 25(1):675. https://doi.org/10.3390/ijms25010675
Chicago/Turabian StyleDalamaga, Maria, Dimitrios Kounatidis, Dimitrios Tsilingiris, Natalia G. Vallianou, Irene Karampela, Sotiria Psallida, and Athanasios G. Papavassiliou. 2024. "The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies" International Journal of Molecular Sciences 25, no. 1: 675. https://doi.org/10.3390/ijms25010675
APA StyleDalamaga, M., Kounatidis, D., Tsilingiris, D., Vallianou, N. G., Karampela, I., Psallida, S., & Papavassiliou, A. G. (2024). The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies. International Journal of Molecular Sciences, 25(1), 675. https://doi.org/10.3390/ijms25010675









