Insights into Plastic Degradation Processes in Marine Environment by X-ray Photoelectron Spectroscopy Study
Abstract
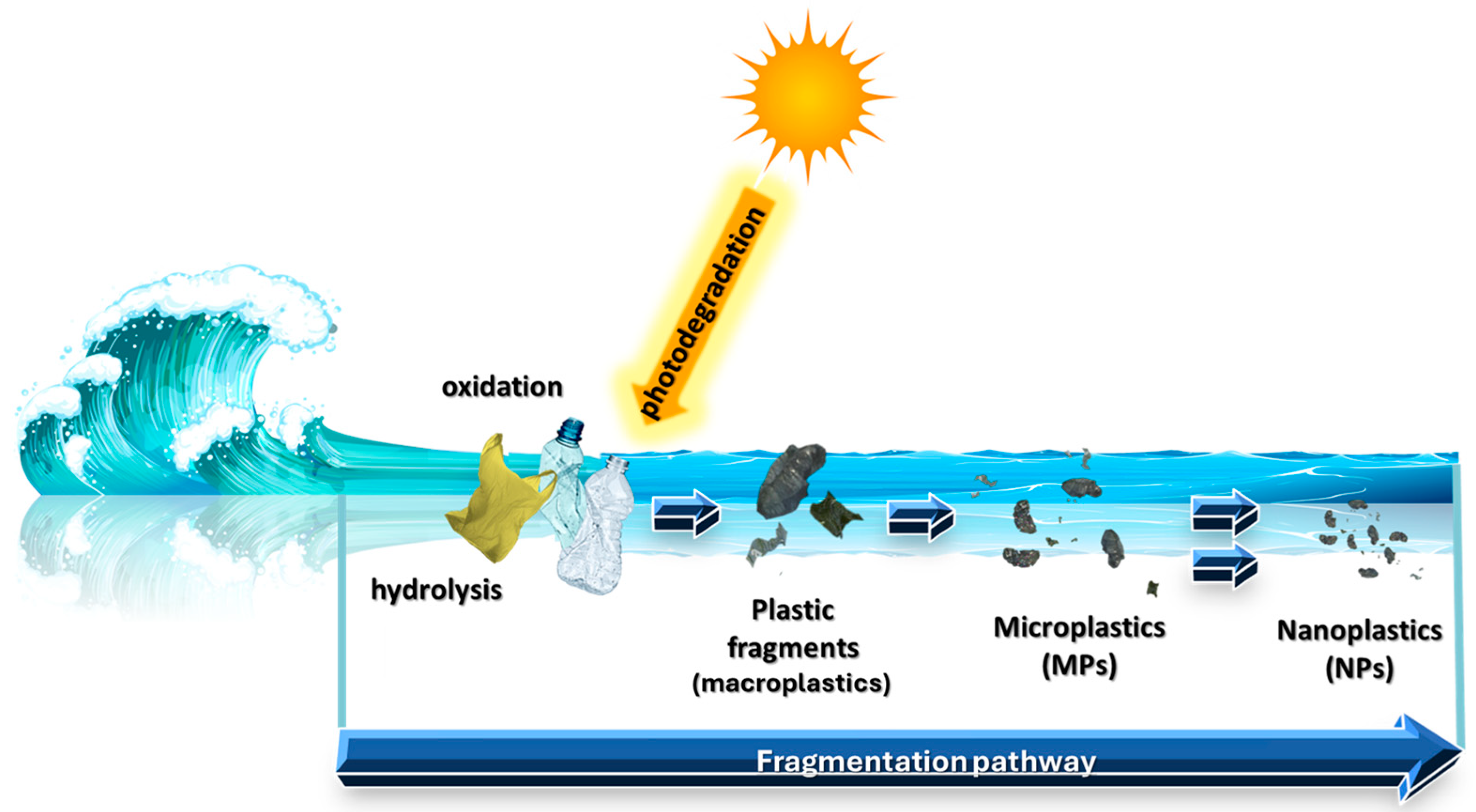
:1. Introduction
2. Results and Discussions
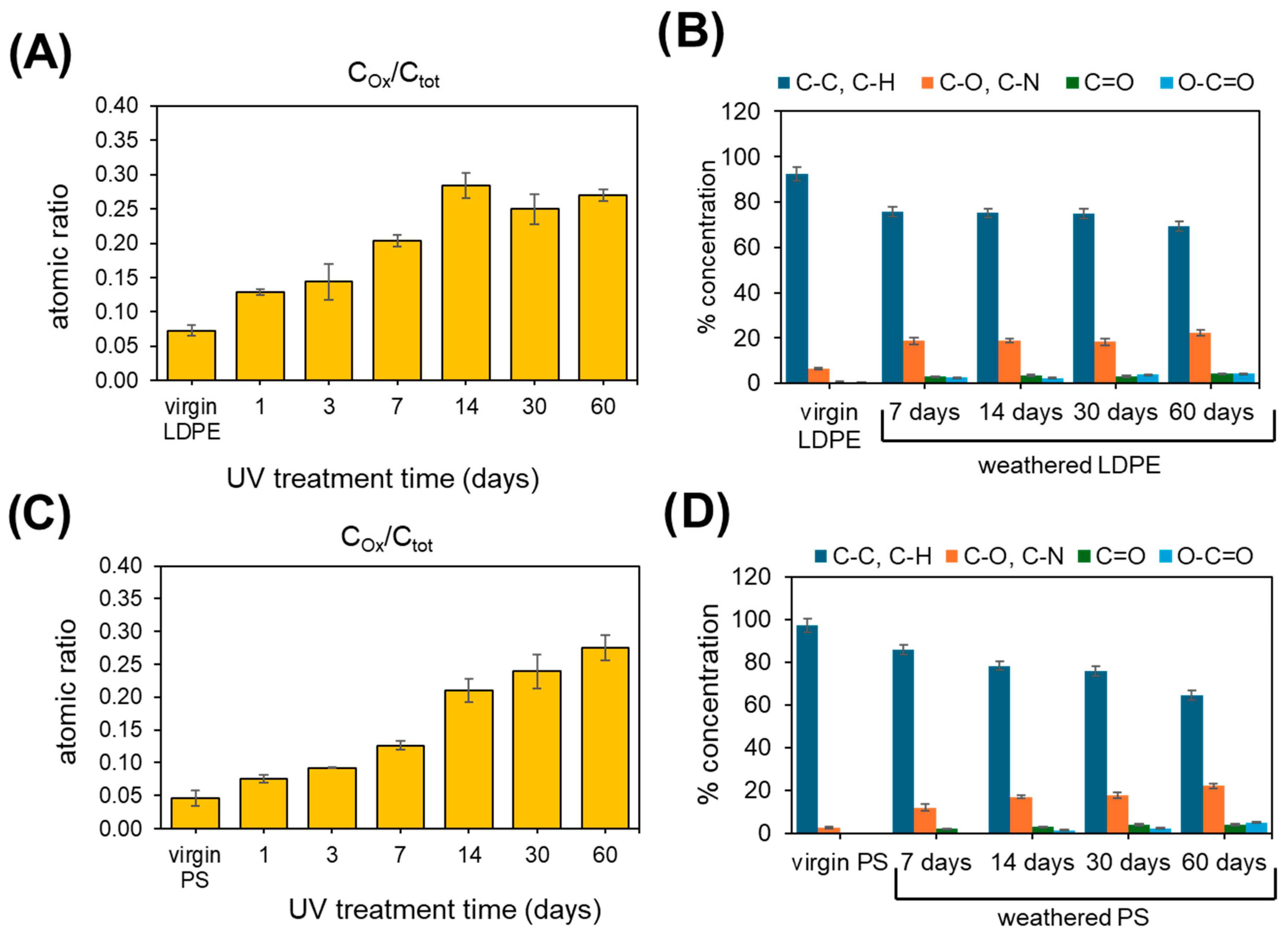
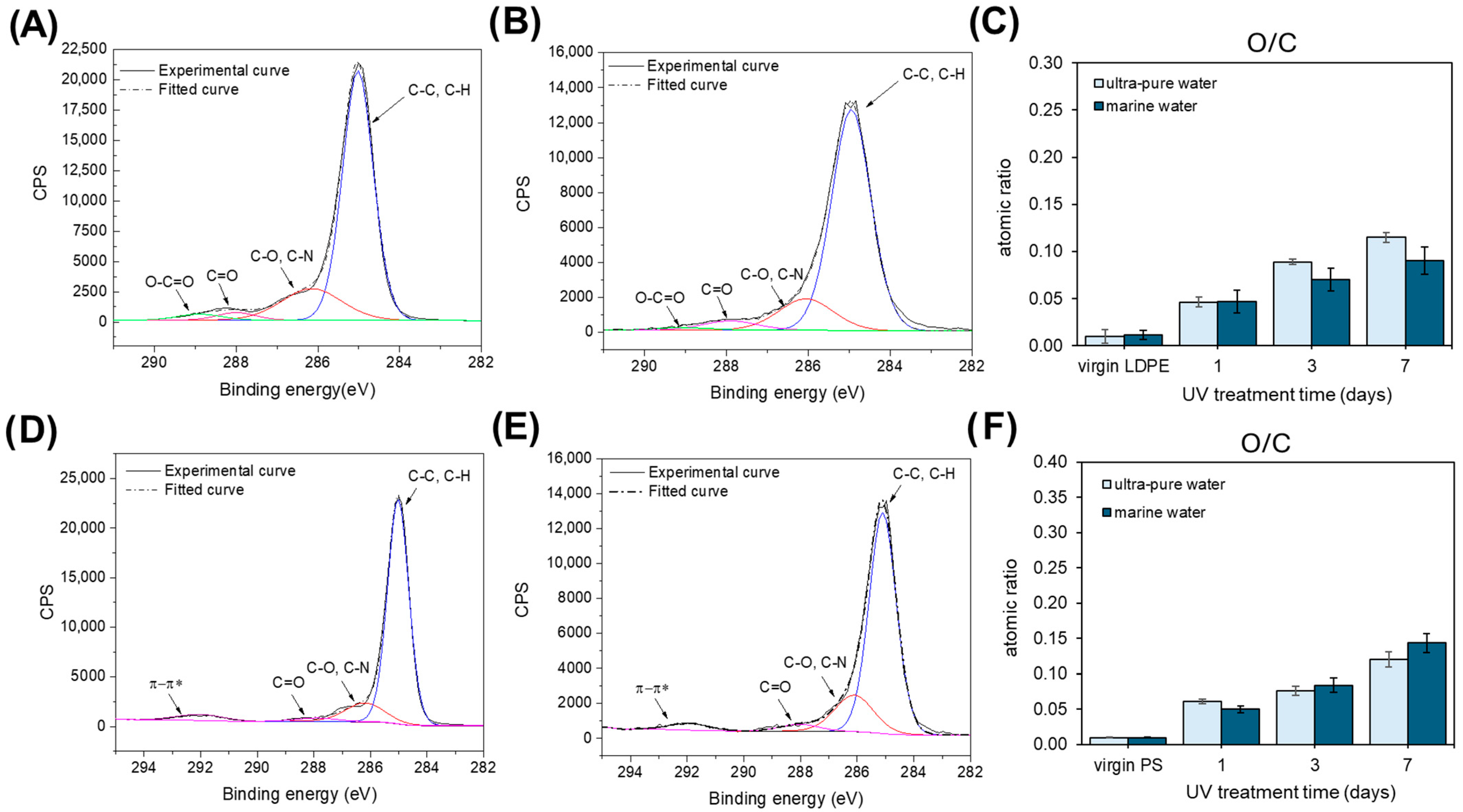
2.1. XPS Analysis of Pristine and Artificially Weathered LDPE and PS
2.2. Plastics Characterization by FTIR and Raman Spectroscopy
2.3. Contact Angle Measurements on Artificially Weathered LDPE and PS Samples
2.4. XPS Analysis of Naturally Weathered LDPE and PS Samples Collected from Real Marine Environment (“La Strea beach”, Porto Cesareo, Italy)
3. Materials and Methods
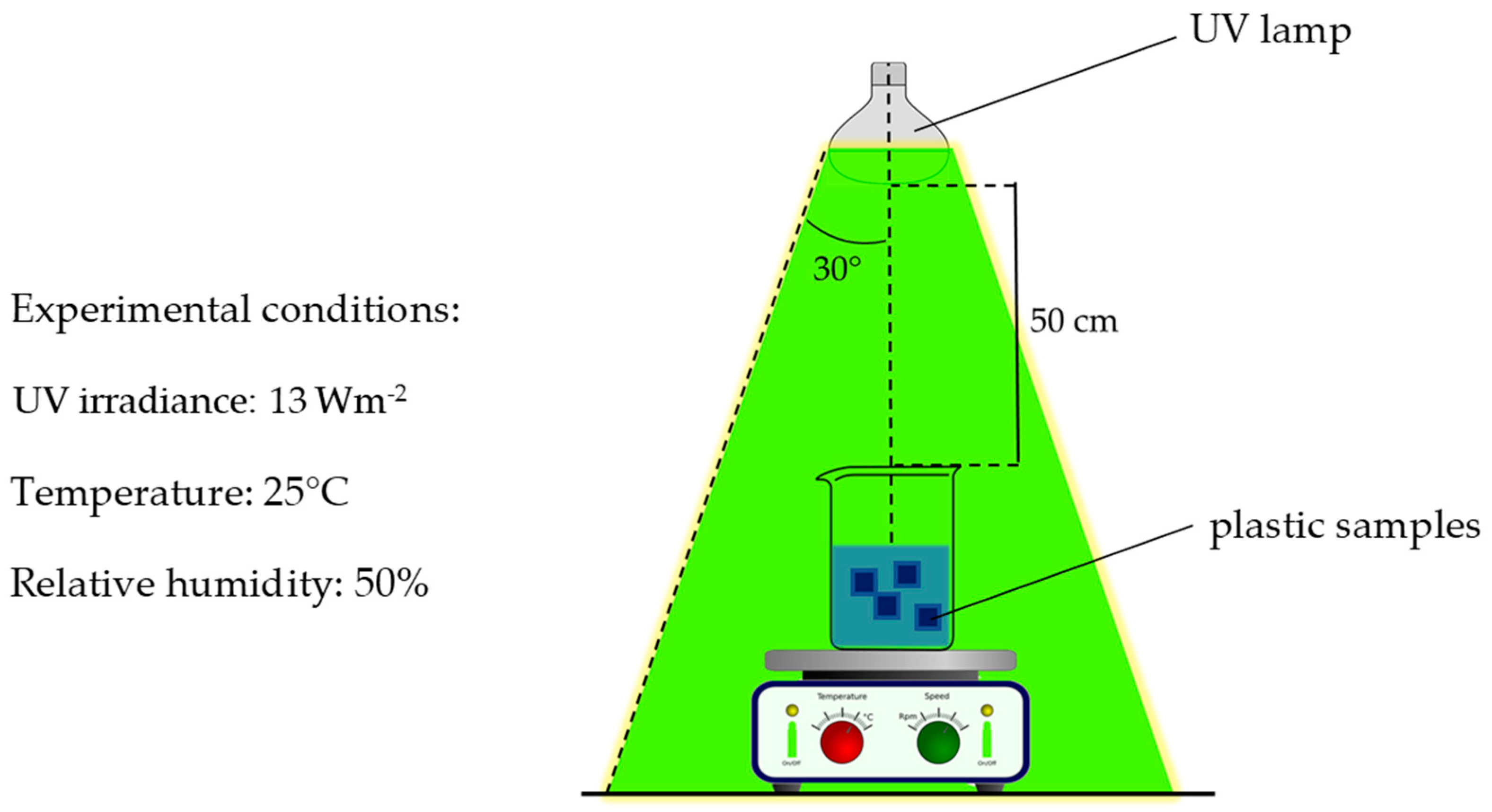
3.1. Artificial Weathering Treatment
3.2. Sampling of Naturally Weathered Plastics from Marine Environments
3.3. XPS Analysis of Plastic Samples
3.4. Plastics Characterization by Contact Angle Measurements
3.5. Plastics Characterization by Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yildizhan, F.S. A Technical and Industrial Analysis of Global Plastics Market, Trade, Financing, and Operations. Sci. Prepr. 2021. [Google Scholar] [CrossRef]
- Gabbott, S.; Key, S.; Russell, C.; Yonan, Y.; Zalasiewicz, J. The Geography and Geology of Plastics: Their Environmental Distribution and Fate. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 33–63. [Google Scholar]
- Di Giulio, T.; Mazzotta, E.; Malitesta, C. Molecularly Imprinted Polyscopoletin for the Electrochemical Detection of the Chronic Disease Marker Lysozyme. Biosensors 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef] [PubMed]
- Bank, M.S.; Hansson, S.V. The Microplastic Cycle: An Introduction to a Complex Issue. In Microplastic in the Environment: Pattern and Process; Environmental Contamination Remediation and Management; Springer International Publishing: Cham, Switzerland, 2022; pp. 115–118. [Google Scholar]
- Hale, R.C.; Seeley, M.E.; King, A.E.; Yu, L.H. Analytical Chemistry of Plastic Debris: Sampling, Methods, and Instrumentation. In Microplastic in the Environment: Pattern and Process; Bank, M.S., Ed.; Environmental Contamination Remediation and Management; Springer International Publishing: Cham, Switzerland, 2022; pp. 137–155. [Google Scholar]
- Mičušík, M.; Kleinová, A.; Oros, M.; Šimon, P.; Dubaj, T.; Procházka, M.; Omastová, M. Plastic ingestion by the Wels catfish (Silurus glanis L.): Detailed chemical analysis and degradation state evaluation. Toxicol. Rep. 2021, 8, 1869–1876. [Google Scholar] [CrossRef]
- Arp, H.P.H.; Kühnel, D.; Rummel, C.; MacLeod, M.; Potthoff, A.; Reichelt, S.; Rojo-Nieto, E.; Schmitt-Jansen, M.; Sonnenberg, J.; Toorman, E.; et al. Weathering Plastics as a Planetary Boundary Threat: Exposure, Fate, and Hazards. Environ. Sci. Technol. 2021, 55, 7246–7255. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.; Barnes, P.; Bornman, J.; Gouin, T.; Madronich, S.; White, C.; Zepp, R.; Jansen, M. Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation. Sci. Total. Environ. 2022, 851, 158022. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- Binda, G.; Spanu, D.; Monticelli, D.; Pozzi, A.; Bellasi, A.; Bettinetti, R.; Carnati, S.; Nizzetto, L. Unfolding the interaction between microplastics and (trace) elements in water: A critical review. Water Res. 2021, 204, 117637. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Recyclability of four types of plastics exposed to UV irradiation in a marine environment. Waste Manag. 2018, 79, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.C.W. On the Possible Initiation of Photooxidation by Charge-Transfer Excitation. J. Phys. Chem. 1965, 69, 4317–4325. [Google Scholar] [CrossRef]
- Gijsman, P. Reply to “comment on oxygen charge-transfer complexes as peroxidation initiators in polymers”. Polym. Degrad. Stab. 1998, 60, 217–219. [Google Scholar] [CrossRef]
- Grause, G.; Chien, M.-F.; Inoue, C. Changes during the weathering of polyolefins. Polym. Degrad. Stab. 2020, 181, 109364. [Google Scholar] [CrossRef]
- McGreer, M. Testing the Effects of UV-C Radiation on Materials. JOT-International Surf. Technol. 2021, 14, 46–47. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging effects on low- and high-density polyethylene, polypropylene and polystyrene under UV irradiation: An insight into decomposition mechanism by Py-GC/MS for microplastic analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Gijsman, P.; Meijers, G.; Vitarelli, G. Comparison of the UV-degradation chemistry of polypropylene, polyethylene, polyamide 6 and polybutylene terephthalate. Polym. Degrad. Stab. 1999, 65, 433–441. [Google Scholar] [CrossRef]
- Massey, S.; Roy, D.; Adnot, A. Study of natural ageing of polypropylene by X-ray photoelectron spectroscopy. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 2003, 208, 236–241. [Google Scholar] [CrossRef]
- Massey, S.; Adnot, A.; Rjeb, A.; Roy, D. Action of water in the degradation of low-density polyethylene studied by X-ray photoelectron spectroscopy. Express Polym. Lett. 2007, 1, 506–511. [Google Scholar] [CrossRef]
- Briggs, D. XPS Studies of Polymer Surface Modifications and Adhesion Mechanisms. J. Adhes. 1982, 13, 287–301. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. Adv. 2022, 12. [Google Scholar] [CrossRef]
- Baio, J.E.; Graham, D.J.; Castner, D.G. Surface analysis tools for characterizing biological materials. Chem. Soc. Rev. 2020, 49, 3278–3296. [Google Scholar] [CrossRef]
- Rella, S.; Mazzotta, E.; Caroli, A.; De Luca, M.; Bucci, C.; Malitesta, C. Investigation of polydopamine coatings by X-ray Photoelectron Spectroscopy as an effective tool for improving biomolecule conjugation. Appl. Surf. Sci. 2018, 447, 31–39. [Google Scholar] [CrossRef]
- Malitesta, C.; Losito, I.; Sabbatini, L.; Zambonin, P. New findings on polypyrrole chemical structure by XPS coupled to chemical derivatization labelling. J. Electron Spectrosc. Relat. Phenom. 1995, 76, 629–634. [Google Scholar] [CrossRef]
- Malitesta, C.; Guascito, M.R.; Mazzotta, E.; Picca, R.A. X-Ray Photoelectron Spectroscopy characterization of electrosynthesized poly(3-thiophene acetic acid) and its application in Molecularly Imprinted Polymers for atrazine. Thin Solid Films 2010, 518, 3705–3709. [Google Scholar] [CrossRef]
- Losito, I.; Malitesta, C.; De Bari, I.; Calvano, C.-D. X-ray photoelectron spectroscopy characterization of poly(2,3-diaminophenazine) films electrosynthesised on platinum. Thin Solid Films 2005, 473, 104–113. [Google Scholar] [CrossRef]
- Malitesta, C.; Losito, I.; Zambonin, P.G. Molecularly Imprinted Electrosynthesized Polymers: New Materials for Biomimetic Sensors. Anal. Chem. 1999, 71, 1366–1370. [Google Scholar] [CrossRef]
- Kong, J.; Lee, D.; Kim, H. Surface modification of low-density polyethylene (LDPE) film and improvement of adhesion between evaporated copper metal film and LDPE. J. Appl. Polym. Sci. 2001, 82, 1677–1690. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, K.Y.; Lee, Y.H.; Kim, H.C. An Application of Plasma Treatment to Improve Shear and Bending Behavior of HDPE/Steel Composites. J. Korean Phys. Soc. 2009, 54, 986–991. [Google Scholar] [CrossRef]
- Ba, O.M.; Marmey, P.; Anselme, K.; Duncan, A.C.; Ponche, A. Surface composition XPS analysis of a plasma treated polystyrene: Evolution over long storage periods. Colloids Surfaces B Biointerfaces 2016, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Clément, F.; Held, B.; Soulem, N.; Martinez, H. A study on the aging process of polystyrene thin films treated under DC pulsed discharges conditions in oxygen and argon-oxygen mixtures. Eur. Phys. J. Appl. Phys. 2002, 21, 59–66. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Wu, W.-M.; Luo, J.; Hou, D. Microplastic generation from field-collected plastic gauze: Unveiling the aging processes. J. Hazard. Mater. 2024, 467, 133615. [Google Scholar] [CrossRef] [PubMed]
- Dorey, S.; Gaston, F.; Marque, S.R.; Bortolotti, B.; Dupuy, N. XPS analysis of PE and EVA samples irradiated at different γ-doses. Appl. Surf. Sci. 2018, 427, 966–972. [Google Scholar] [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stabil. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Zhang, Y.; Pedersen, J.N.; Eser, B.E.; Guo, Z. Biodegradation of polyethylene and polystyrene: From microbial deterioration to enzyme discovery. Biotechnol. Adv. 2022, 60, 107991. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, X.; Shi, X.; Zhang, Y. A polystyrene-degrading Acinetobacter bacterium isolated from the larvae of Tribolium castaneum. Sci. Total. Environ. 2020, 726, 138564. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Wu, W.-M.; Zhao, J.; Jiang, L. Evidence of Polyethylene Biodegradation by Bacterial Strains from the Guts of Plastic-Eating Waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Lang, M.; Yu, X.; Wu, R.; Yang, X.; Guo, X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020, 393, 122515. [Google Scholar] [CrossRef]
- Stark, N.M.; Matuana, L.M. Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy. Polym. Degrad. Stab. 2004, 86, 1–9. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Zhao, S.; Xia, T.; Guo, X.; Wang, T.; Zhu, L. Formation of Environmentally Persistent Free Radicals on Microplastics under Light Irradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.; Adnot, A.; Rjeb, A.; Roy, D. Study of natural aging of industrial low density polyethylene by X-ray photoelectron spectroscopy. Plast. Rubber Compos. 2008, 37, 7–12. [Google Scholar] [CrossRef]
- de la Orden, M.; Montes, J.; Urreaga, J.M.; Bento, A.; Ribeiro, M.; Pérez, E.; Cerrada, M. Thermo and photo-oxidation of functionalized metallocene high density polyethylene: Effect of hydrophilic groups. Polym. Degrad. Stab. 2015, 111, 78–88. [Google Scholar] [CrossRef]
- Hamzah, M.; Khenfouch, M.; Rjeb, A.; Sayouri, S.; Houssaini, D.S.; Darhouri, M.; Srinivasu, V. Surface chemistry changes and microstructure evaluation of low density nanocluster polyethylene under natural weathering: A spectroscopic investigation. J. Physics: Conf. Ser. 2018, 984, 012010. [Google Scholar] [CrossRef]
- Mailhot, B.; Jarroux, N.; Gardette, J.-L. Comparative analysis of the photo-oxidation of polystyrene and poly(α-methylstyrene). Polym. Degrad. Stab. 2000, 68, 321–326. [Google Scholar] [CrossRef]
- Aktas, C.; Polat, O.; Beitollahpoor, M.; Farzam, M.; Pesika, N.S.; Sahiner, N. Force-Based Characterization of the Wetting Properties of LDPE Surfaces Treated with CF4 and H2 Plasmas. Polymers 2023, 15, 2132. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadou, I.; Paraskevopoulos, K.; Chrissafis, K.; Pavlidou, E.; Stamkopoulos, T.-G.; Bikiaris, D. Effect of different nanoparticles on HDPE UV stability. Polym. Degrad. Stab. 2011, 96, 151–163. [Google Scholar] [CrossRef]
- Menezes, D.B.; Reyer, A.; Marletta, A.; Musso, M. Glass transition of polystyrene (PS) studied by Raman spectroscopic investigation of its phenyl functional groups. Mater. Res. Express 2017, 4, 015303. [Google Scholar] [CrossRef]
- Presser, V.; Keuper, M.; Berthold, C.; Nickel, K.G. Experimental Determination of the Raman Sampling Depth in Zirconia Ceramics. Appl. Spectrosc. 2009, 63, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Maraschin, N. Polyethylene, Low Density. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2005; pp. 57–89. [Google Scholar]
- Malpass, D.B. Downstream Aspects of Polyethylene. In Introduction to Industrial Polyethylene: Properties, Catalysts, and Processes; Scrivener: Salem, MA, USA, 2010; pp. 14–99. [Google Scholar]
- Tanvir, A.; Faruq, M.; Nor Azah, Y. Applications of Polystyrene and Its Role as a Base in Industrial Chemistry. In Polystyrene: Synthesis, Characteristics and Applications; Lynwood, C., Ed.; Nova Science: Hauppauge, NY, USA, 2014; pp. 269–280. [Google Scholar]
- Gray, J.E. Polystyrene: Properties, Performance, and Applications; Nova Science: Hauppauge, NY, USA, 2011; pp. 1–55. [Google Scholar]
- Yeung, C.W.S.; Teo, J.Y.Q.; Loh, X.J.; Lim, J.Y.C. Polyolefins and Polystyrene as Chemical Resources for a Sustainable Future: Challenges, Advances, and Prospects. ACS Mater. Lett. 2021, 3, 1660–1676. [Google Scholar] [CrossRef]
- Ebrahimpour, Z.; Pliekhova, O.; Cabrera, H.; Abdelhamid, M.; Korte, D.; Gadedjisso-Tossou, K.S.; Niemela, J.; Stangar, U.L.; Franko, M. Photodegradation mechanisms of reactive blue 19 dye under UV and simulated solar light irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119481. [Google Scholar] [CrossRef]
- Halim Hamid, S. (Ed.) Jean-Luc Gardette Fundamental and Technical Aspects of the Photooxidation of Polymers. In Handbook of Polymer Degradation; CRC: Boca Raton, FL, USA, 2000; pp. 697–702. [Google Scholar]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; La Mantia, F.; Malatesta, V. Photo-oxidation behaviour of polyethylene/multi-wall carbon nanotube composite films. Polym. Degrad. Stab. 2009, 94, 162–170. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Andersson, S.O.; Karlsson, S. The mechanism of biodegradation of polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, Q.; Yang, X.; Wang, F.; Zhang, X. An evaluation model to predict microplastics generation from polystyrene foams and experimental verification. J. Hazard. Mater. 2023, 446, 130673. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Takada, H., Karapanagioti, H.K., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2019; Volume 78, pp. 146–147. [Google Scholar]
- Shi, X.; Chen, Z.; Liu, X.; Wei, W.; Ni, B.-J. The photochemical behaviors of microplastics through the lens of reactive oxygen species: Photolysis mechanisms and enhancing photo-transformation of pollutants. Sci. Total. Environ. 2022, 846, 157498. [Google Scholar] [CrossRef]
- Mailhot, B.; Gardette, J.L. Polystyrene photooxidation. 2. A pseudo wavelength effect. Macromolecules 1992, 25, 4127–4133. [Google Scholar] [CrossRef]
- Maglić, L.; Zec, D.; Frančić, V. Ballast water sediment elemental analysis. Mar. Pollut. Bull. 2016, 103, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, A.; Iemmo, L.; Urban, F.; Palomba, M.; Carotenuto, G.; Longo, A.; Sorrentino, A.; Giubileo, F.; Barucca, G.; Rovere, M.; et al. Graphite platelet films deposited by spray technique on low density polyethylene substrates. Mater. Today Proc. 2019, 20, 87–90. [Google Scholar] [CrossRef]
- Perraki, M.; Skliros, V.; Mecaj, P.; Vasileiou, E.; Salmas, C.; Papanikolaou, I.; Stamatis, G. Identification of Microplastics Using µ-Raman Spectroscopy in Surface and Groundwater Bodies of SE Attica, Greece. Water 2024, 16, 843. [Google Scholar] [CrossRef]
- Yabagi, J.A.; Kimpa, M.I.; Muhammad, M.N.; Bin Rashid, S.; Zaidi, E.; Agam, M.A. The effect of gamma irradiation on chemical, morphology and optical properties of polystyrene nanosphere at various exposure time. IOP Conf. Series: Mater. Sci. Eng. 2018, 298, 012004. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Kita, Y.; Matsukawa, K.; Yamaguchi, H.; Ochiai, S.; Siesler, H.W.; Ozaki, Y. Molecular Structure, Crystallinity and Morphology of Polyethylene/Polypropylene Blends Studied by Raman Mapping, Scanning Electron Microscopy, Wide Angle X-Ray Diffraction, and Differential Scanning Calorimetry. Polym. J. 2006, 38, 1127–1136. [Google Scholar] [CrossRef]
- Fan, Y.; Cornelius, C.J. Raman spectroscopic and gas transport study of a pentablock ionomer complexed with metal ions and its relationship to physical properties. J. Mater. Sci. 2012, 48, 1153–1161. [Google Scholar] [CrossRef]
- Mazilu, M.; De Luca, A.C.; Riches, A.; Herrington, C.S.; Dholakia, K. Optimal algorithm for fluorescence suppression of modulated Raman spectroscopy. Opt. Express 2010, 18, 11382–11395. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.; Shibahara, K.; Nakane, K. Melt-Electrospun Polyethylene Nanofiber Obtained from Polyethylene/Polyvinyl Butyral Blend Film. Polymers 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Zaki, M. The optical, wettability and hardness properties of polyethylene improved by alpha particle irradiations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.-I.; Sawa, T.; Kuzuya, M. Plasma-Assisted Immobilization of Bio-Molecules on LDPE Surface. J. Photopolym. Sci. Technol. 2003, 16, 71–74. [Google Scholar] [CrossRef]
- Curti, P.S.; de Moura, M.R.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C.; Moliterno, R.A. Surface modification of polystyrene and poly(ethylene terephtalate) by grafting poly(N-isopropylacrylamide). J. Mater. Sci. Mater. Med. 2002, 13, 1175–1180. [Google Scholar] [CrossRef]
- Anjum, M.R.; Mushtaq, S.; Abbas, M.A.; Mahmood, A.; Nasir, H.; Janjua, H.A.; Malik, Q.; Ahmad, N.M. Antialgal Synergistic Polystyrene Blended with Polyethylene Glycol and Silver Sulfadiazine for Healthcare Applications. Adv. Polym. Technol. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Julienne, F.; Delorme, N.; Lagarde, F. From macroplastics to microplastics: Role of water in the fragmentation of polyethylene. Chemosphere 2019, 236, 124409. [Google Scholar] [CrossRef] [PubMed]
- Khiabani, P.S.; Kashi, M.B.; Zhang, X.; Pardehkhorram, R.; Markhali, B.P.; Soeriyadi, A.H.; Micolich, A.P.; Gooding, J.J. A graphene-based sensor for real time monitoring of sun exposure. Carbon 2018, 138, 215–218. [Google Scholar] [CrossRef]
- Sahan, M. The measurements of the global solar radiation and solar ultraviolet radiation during 2018 year. AIP Conf. Proc. 2019, 2178, 030016. [Google Scholar] [CrossRef]
- Ineichen, P. Long Term Satellite Global, Beam and Diffuse Irradiance Validation. Energy Procedia 2014, 48, 1586–1596. [Google Scholar] [CrossRef]
- Han, W.; Shin, J.; Shin, J.H. Low-cost, open-source contact angle analyzer using a mobile phone, commercial tripods and 3D printed parts. HardwareX 2022, 12, e00327. [Google Scholar] [CrossRef]
- Fritz, M.; Lauschke, T.; Schlebrowski, T.; Beucher, L.; Schweyen, P.; Alenezi, B.; Hahn, B.; Dierkes, G.; Ternes, T.; Fischer, C.B. Photoaging phenomena of biodegradable polybutylene succinate and conventional low density polyethylene by artificial weathering—A comparative surface study. Appl. Surf. Sci. 2022, 590, 153058. [Google Scholar] [CrossRef]







| Raman Shift (cm−1) | Assignments (LDPE) | Assignments (PS) |
|---|---|---|
| 3061 | C-H aromatic ring stretching | |
| 2885 | C-H (methyl) stretching | |
| 2846 | C-H (methyl) stretching | |
| 1603 | C=C aromatic ring stretching | |
| 1582 | C=C aromatic ring stretching | |
| 1465 | CH2 bending | |
| 1441 | CH2 bending | |
| 1418 | CH2 bending | |
| 1295 | CH2 twisting | |
| 1201 | Aromatic ring vibration | |
| 1170 | CH2 rocking | |
| 1155 | C-C stretching | |
| 1129 | C-C symmetric stretching | |
| 1061 | C-C antisymmetric stretching | |
| 1033 | C-H bending | |
| 1001 | Aromatic breathing | |
| 798 | C-H bending | |
| 621 | Ring deformation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giulio, T.; De Benedetto, G.E.; Ditaranto, N.; Malitesta, C.; Mazzotta, E. Insights into Plastic Degradation Processes in Marine Environment by X-ray Photoelectron Spectroscopy Study. Int. J. Mol. Sci. 2024, 25, 5060. https://doi.org/10.3390/ijms25105060
Di Giulio T, De Benedetto GE, Ditaranto N, Malitesta C, Mazzotta E. Insights into Plastic Degradation Processes in Marine Environment by X-ray Photoelectron Spectroscopy Study. International Journal of Molecular Sciences. 2024; 25(10):5060. https://doi.org/10.3390/ijms25105060
Chicago/Turabian StyleDi Giulio, Tiziano, Giuseppe Egidio De Benedetto, Nicoletta Ditaranto, Cosimino Malitesta, and Elisabetta Mazzotta. 2024. "Insights into Plastic Degradation Processes in Marine Environment by X-ray Photoelectron Spectroscopy Study" International Journal of Molecular Sciences 25, no. 10: 5060. https://doi.org/10.3390/ijms25105060
APA StyleDi Giulio, T., De Benedetto, G. E., Ditaranto, N., Malitesta, C., & Mazzotta, E. (2024). Insights into Plastic Degradation Processes in Marine Environment by X-ray Photoelectron Spectroscopy Study. International Journal of Molecular Sciences, 25(10), 5060. https://doi.org/10.3390/ijms25105060







