Upregulation of Hepatic Glutathione S-Transferase Alpha 1 Ameliorates Metabolic Dysfunction-Associated Steatosis by Degrading Fatty Acid Binding Protein 1
Abstract
:1. Introduction
2. Results
2.1. GSTA1 Is a Potential Regulator of Lipid Accumulation
2.2. Expression of GSTA1 Is Negatively Related to the Accumulation of LD during MASLD
2.3. GSTA1 Suppresses the Accumulation of LD In Vitro
2.4. GSTA1 Reduces the Accumulation of LD in the Mouse Liver
2.5. Upregulation of GSTA1 Expression by Bicyclol Attenuates Steatosis
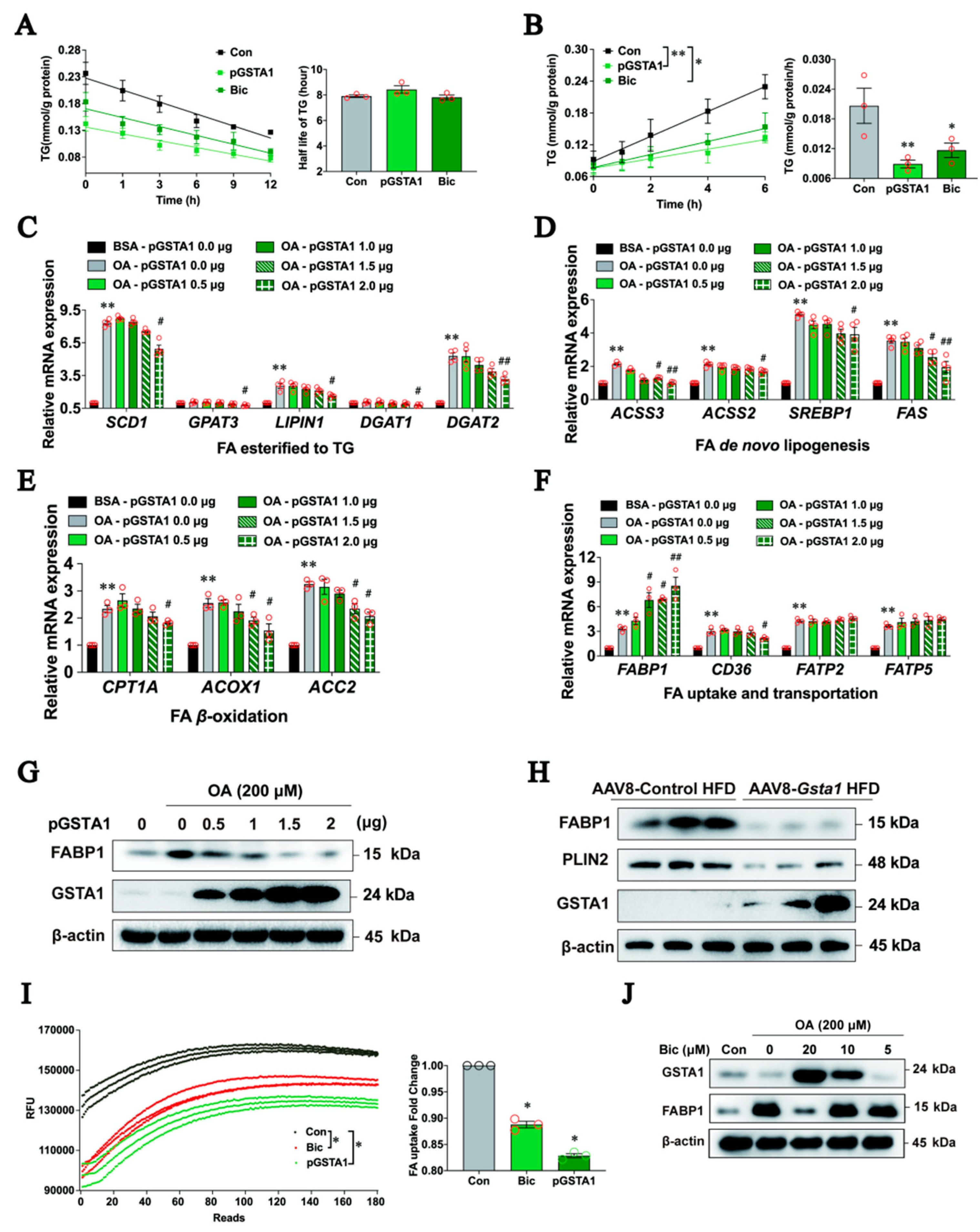
2.6. GSTA1 Inhibits the Uptake and Transportation of Free Fatty Acids in Hepatocytes
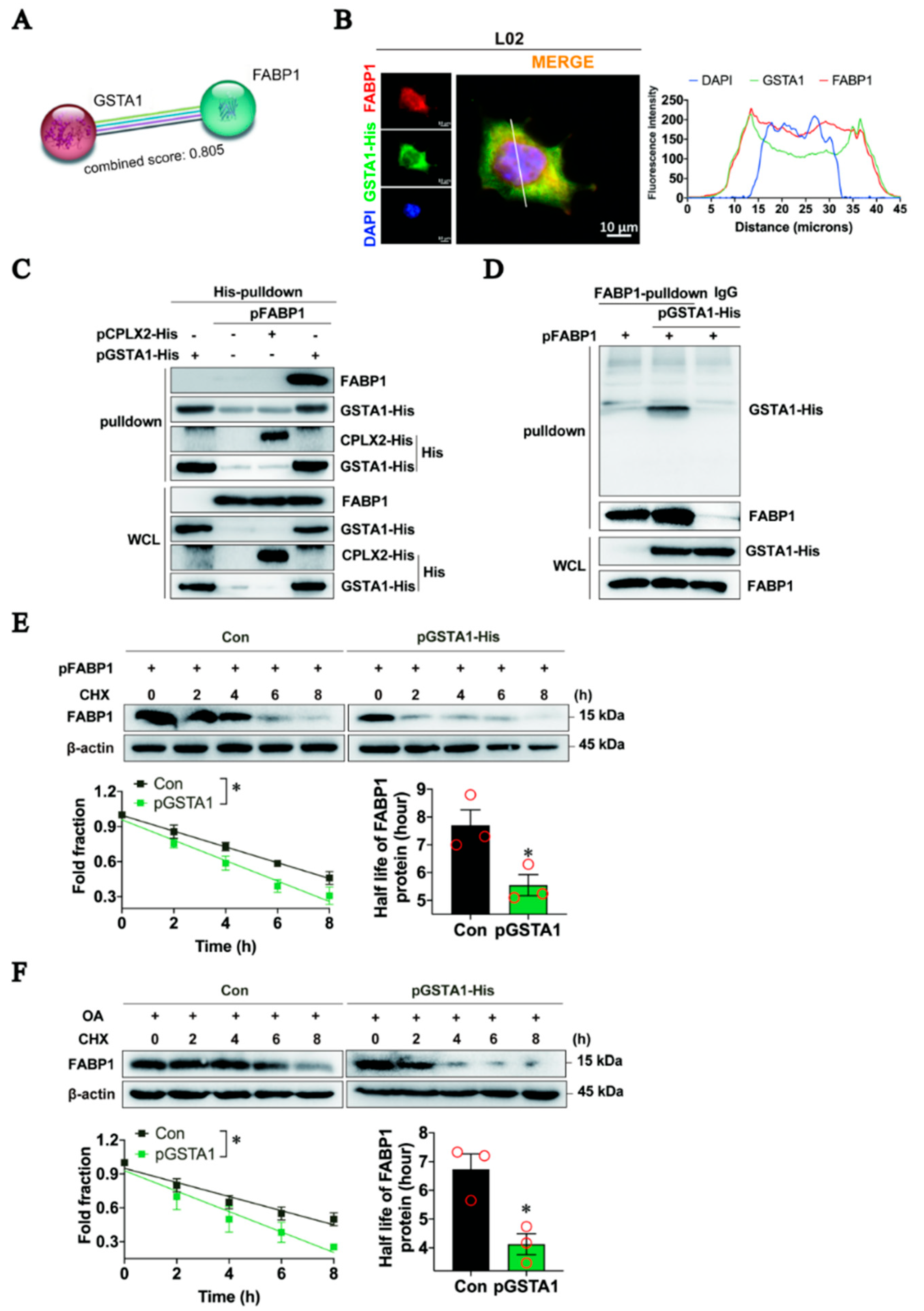
2.7. GSTA1 Interacts Directly with FABP1 and Promotes FABP1 Degradation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cytotoxicity Assay
4.3. Nile Red Staining
4.4. Proteomic Analysis
4.5. Real-Time Quantitative RT-PCR Analysis
4.6. Plasmid Construction and Transfection Assay
4.7. GSTA1 Expression in the Liver of MASLD Mice
4.8. Construction of the Adenovirus Vector and Infection
4.9. Biochemical Parameter Tests
4.10. Histological Examination
4.11. TG Degradation and Synthesis Assay
4.12. Free Fatty Acid Uptake and Transportation Assay
4.13. Histidine (His) Pull-Down Experiments
4.14. Co-Immunoprecipitation
4.15. Immunofluorescence Staining
4.16. Western Blot Analyses
4.17. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lonardo, A.; Mantovani, A.; Petta, S.; Carraro, A.; Byrne, C.D.; Targher, G. Metabolic mechanisms for and treatment of NAFLD or NASH occurring after liver transplantation. Nat. Rev. Endocrinol. 2022, 18, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Raponi, M. Pediatric non-alcoholic fatty liver disease: Preventive and therapeutic value of lifestyle intervention. World J. Gastroenterol. 2009, 15, 6017–6022. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.K.; Tang, M.; Lei, L.; Li, J.R.; Sun, H.; Jiang, J.; Dong, B.; Li, H.Y.; Jiang, J.D.; et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 2024, 16, 2304159. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Poulsen, K.L.; Wu, L.; Liu, S.; Miyata, T.; Song, Q.; Wei, Q.; Zhao, C.; Lin, C.; Yang, J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target. Ther. 2022, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef]
- D’Amico, F.; Baumann, C.; Rousseau, H.; Danese, S.; Peyrin-Biroulet, L. Phase I, II and III Trials in Inflammatory Bowel Diseases: A Practical Guide for the Non-specialist. J. Crohn’s Colitis 2020, 14, 710–718. [Google Scholar] [CrossRef]
- Ratziu, V.; Sanyal, A.; Harrison, S.A.; Wong, V.W.; Francque, S.; Goodman, Z.; Aithal, G.P.; Kowdley, K.V.; Seyedkazemi, S.; Fischer, L.; et al. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020, 72, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M.; Pares, A.; Kowdley, K.V.; Heneghan, M.A.; Caldwell, S.; Pratt, D.; Bonder, A.; Hirschfield, G.M.; Levy, C.; Vierling, J.; et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J. Hepatol. 2021, 74, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, S.A.; Loustaud-Ratti, V.; Bureau, C.; Lawitz, E.; Abdelmalek, M.; Alkhouri, N.; Francque, S.; Girma, H.; Darteil, R.; et al. Hepatic and renal improvements with FXR agonist vonafexor in individuals with suspected fibrotic NASH. J. Hepatol. 2023, 78, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, E.; Grace, Y.; Bolson, A.; DellaVecchia, M.J.; Ruble, M. Emerging therapies for the treatment of nonalcoholic steatohepatitis: A systematic review. Pharmacotherapy 2021, 41, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Allen, A.M.; Dubourg, J.; Noureddin, M.; Alkhouri, N. Challenges and opportunities in NASH drug development. Nat. Med. 2023, 29, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Kokkorakis, M.; Boutari, C.; Hill, M.A.; Kotsis, V.; Loomba, R.; Sanyal, A.J.; Mantzoros, C.S. Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: Trials, opportunities, and challenges. Metabolism 2024, 154, 155835. [Google Scholar] [CrossRef] [PubMed]
- Teslenko, I.; Trudeau, J.; Luo, S.; Watson, C.J.W.; Chen, G.; Truica, C.I.; Lazarus, P. Influence of Glutathione-S-Transferase A1*B Allele on the Metabolism of the Aromatase Inhibitor, Exemestane, in Human Liver Cytosols and in Patients Treated With Exemestane. J. Pharmacol. Exp. Ther. 2022, 382, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, S.; Schindeldecker, M.; Drebber, U.; Witzel, H.R.; Weinmann, A.; Dries, V.; Schirmacher, P.; Roth, W.; Straub, B.K. Lipid Droplet-Associated Proteins Perilipin 1 and 2: Molecular Markers of Steatosis and Microvesicular Steatotic Foci in Chronic Hepatitis C. Int. J. Mol. Sci. 2022, 23, 15456. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chen, E.; Li, L.; Saha, P.; Lee, H.J.; Huang, L.S.; Shelness, G.S.; Chan, L.; Chang, B.H. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 2017, 13, 1130–1144. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Guo, J.; Wang, Q.; Zhu, S.; Gao, S.; Yang, C.; Wei, M.; Pan, X.; Zhu, W.; et al. Cytotoxic and Antitumor Effects of Curzerene from Curcuma longa. Planta Med. 2017, 83, 23–29. [Google Scholar] [CrossRef]
- Sui, X.; Wang, K.; Song, K.; Xu, C.; Song, J.; Lee, C.W.; Liao, M.; Farese, R.V., Jr.; Walther, T.C. Mechanism of action for small-molecule inhibitors of triacylglycerol synthesis. Nat. Commun. 2023, 14, 3100. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jia, M.; Liu, Y.; Wang, S.; Tang, Y.; Li, X.; Jiang, X.; Wu, Z.; Lou, Y.; Fan, G. Identification of bicyclol metabolites in rat plasma, urine and feces by UPLC-Q-TOF-MS/MS and evaluation of the efficacy and safety of these metabolites based on network pharmacology and molecular docking combined with toxicity prediction. J. Pharm. Biomed. Anal. 2022, 220, 114947. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Hydes, T.; Hamid, A.; Cuthbertson, D.J. Emerging and Established Therapeutic Approaches for Nonalcoholic Fatty Liver Disease. Clin. Ther. 2021, 43, 1476–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yan, Y.; Xiao, Z.; Wang, M.; Xu, M.; Wang, Z.; Wang, Y.; Zhuang, Z.; Yang, D.; Chen, G.; et al. Bicyclol ameliorates nonalcoholic fatty liver disease in mice via inhibiting MAPKs and NF-κB signaling pathways. Biomed. Pharmacother. 2021, 141, 111874. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, N.N.; Li, J.R.; Dong, B.; Wang, M.X.; Tan, J.L.; Wang, X.K.; Jiang, J.; Lei, L.; Li, H.Y.; et al. Combined Use of Bicyclol and Berberine Alleviates Mouse Nonalcoholic Fatty Liver Disease. Front. Pharmacol. 2022, 13, 843872. [Google Scholar] [CrossRef]
- Li, H.; Liu, N.N.; Li, J.R.; Wang, M.X.; Tan, J.L.; Dong, B.; Lan, P.; Zhao, L.M.; Peng, Z.G.; Jiang, J.D. Bicyclol ameliorates advanced liver diseases in murine models via inhibiting the IL-6/STAT3 signaling pathway. Biomed. Pharmacother. 2022, 150, 113083. [Google Scholar] [CrossRef]
- Zhang, X.; Heckmann, B.L.; Xie, X.; Saarinen, A.M.; Liu, J. Regulation of FSP27 protein stability by AMPK and HSC70. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E1047–E1056. [Google Scholar] [CrossRef]
- Garreau de Loubresse, N.; Prokhorova, I.; Holtkamp, W.; Rodnina, M.V.; Yusupova, G.; Yusupov, M. Structural basis for the inhibition of the eukaryotic ribosome. Nature 2014, 513, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Gluchowski, N.L.; Gabriel, K.R.; Chitraju, C.; Bronson, R.T.; Mejhert, N.; Boland, S.; Wang, K.; Lai, Z.W.; Farese, R.V., Jr.; Walther, T.C. Hepatocyte Deletion of Triglyceride-Synthesis Enzyme Acyl CoA: Diacylglycerol Acyltransferase 2 Reduces Steatosis Without Increasing Inflammation or Fibrosis in Mice. Hepatology 2019, 70, 1972–1985. [Google Scholar] [CrossRef]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm. Sin. B 2022, 12, 558–580. [Google Scholar] [CrossRef]
- Tirinato, L.; Pagliari, F.; Limongi, T.; Marini, M.; Falqui, A.; Seco, J.; Candeloro, P.; Liberale, C.; Di Fabrizio, E. An Overview of Lipid Droplets in Cancer and Cancer Stem Cells. Stem Cells Int. 2017, 2017, 1656053. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shao, X.; Shen, J.; Lin, Q.; Zhu, X.; Li, S.; Li, J.; Zhou, W.; Qi, C.; Ni, Z. Downregulation of PPARα mediates FABP1 expression, contributing to IgA nephropathy by stimulating ferroptosis in human mesangial cells. Int. J. Biol. Sci. 2022, 18, 5438–5458. [Google Scholar] [CrossRef]
- Yan, T.; Luo, Y.; Yan, N.; Hamada, K.; Zhao, N.; Xia, Y.; Wang, P.; Zhao, C.; Qi, D.; Yang, S.; et al. Intestinal peroxisome proliferator-activated receptor α-fatty acid-binding protein 1 axis modulates nonalcoholic steatohepatitis. Hepatology 2023, 77, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Poetsch, T.; Ju, J.; Eyler, D.E.; Dang, Y.; Bhat, S.; Merrick, W.C.; Green, R.; Shen, B.; Liu, J.O. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat. Chem. Biol. 2010, 6, 209–217. [Google Scholar] [CrossRef]
- Ratziu, V.; Tacke, F. At the dawn of potent therapeutics for fatty liver disease—introducing the miniseries on promising pharmacological targets for NASH. J. Hepatol. 2023, 79, 261–262. [Google Scholar] [CrossRef]
- Hu, B.; Hu, S.; Huang, H.; Wei, Q.; Ren, M.; Huang, S.; Tian, X.; Su, J. Insecticides induce the co-expression of glutathione S-transferases through ROS/CncC pathway in Spodoptera exigua. Pestic. Biochem. Physiol. 2019, 155, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, H.; Wei, Q.; Ren, M.; Mburu, D.K.; Tian, X.; Su, J. Transcription factors CncC/Maf and AhR/ARNT coordinately regulate the expression of multiple GSTs conferring resistance to chlorpyrifos and cypermethrin in Spodoptera exigua. Pest. Manag. Sci. 2019, 75, 2009–2019. [Google Scholar] [CrossRef]
- Buratti, F.M.; Scardala, S.; Funari, E.; Testai, E. The conjugation of microcystin-RR by human recombinant GSTs and hepatic cytosol. Toxicol. Lett. 2013, 219, 231–238. [Google Scholar] [CrossRef]
- Wu, J.; Jia, S.; Xu, B.; Yao, X.; Shao, J.; Yao, J.; Cen, D.; Yao, X. Bicyclol attenuates high fat diet-induced non-alcoholic fatty liver disease/non-alcoholic steatohepatitis through modulating multiple pathways in mice. Front. Pharmacol. 2023, 14, 1157200. [Google Scholar] [CrossRef]
- Ma, X.; Chang, Y.; Zhang, Y.; Muhammad, I.; Shi, C.; Li, R.; Li, C.; Li, Z.; Lin, Y.; Han, Q.; et al. Effects of C2-Ceramide and Oltipraz on Hepatocyte Nuclear Factor-1 and Glutathione S-Transferase A1 in Acetaminophen-Mediated Acute Mice Liver Injury. Front. Pharmacol. 2018, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Kurzawski, M.; Dziedziejko, V.; Urasińska, E.; Post, M.; Wójcicki, M.; Miętkiewski, J.; Droździk, M. Nuclear factor erythroid 2-like 2 (Nrf2) expression in end-stage liver disease. Environ. Toxicol. Pharmacol. 2012, 34, 87–95. [Google Scholar] [CrossRef]
- Reljic, Z.; Zlatovic, M.; Savic-Radojevic, A.; Pekmezovic, T.; Djukanovic, L.; Matic, M.; Pljesa-Ercegovac, M.; Mimic-Oka, J.; Opsenica, D.; Simic, T. Is increased susceptibility to Balkan endemic nephropathy in carriers of common GSTA1 (*A/*B) polymorphism linked with the catalytic role of GSTA1 in ochratoxin a biotransformation? Serbian case control study and in silico analysis. Toxins 2014, 6, 2348–2362. [Google Scholar] [CrossRef]
- Wang, X.H.; Cui, X.X.; Sun, X.Q.; Wang, X.H.; Li, X.C.; Qi, Y.; Li, W.; Han, M.Y.; Muhammad, I.; Zhang, X.Y. High Fat Diet-Induced Hepatic 18-Carbon Fatty Acids Accumulation Up-Regulates CYP2A5/CYP2A6 via NF-E2-Related Factor 2. Front. Pharmacol. 2017, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Sieglaff, D.H.; Yuan, C.S.; Su, J.; Arumanayagam, A.S.; Firouzbakht, S.; Cantu Pompa, J.J.; Reynolds, F.D.; Zhou, X.B.; Cvoro, A.; et al. Gene specific actions of thyroid hormone receptor subtypes. PLoS ONE 2013, 8, e52407. [Google Scholar] [CrossRef] [PubMed]
- Dum, D.; Ocokoljic, A.; Lennartz, M.; Hube-Magg, C.; Reiswich, V.; Höflmayer, D.; Jacobsen, F.; Bernreuther, C.; Lebok, P.; Sauter, G.; et al. FABP1 expression in human tumors: A tissue microarray study on 17,071 tumors. Virchows Arch. 2022, 481, 945–961. [Google Scholar] [CrossRef]
- Schroeder, F.; McIntosh, A.L.; Martin, G.G.; Huang, H.; Landrock, D.; Chung, S.; Landrock, K.K.; Dangott, L.J.; Li, S.; Kaczocha, M.; et al. Fatty Acid Binding Protein-1 (FABP1) and the Human FABP1 T94A Variant: Roles in the Endocannabinoid System and Dyslipidemias. Lipids 2016, 51, 655–676. [Google Scholar] [CrossRef]
- You, H.; Wen, X.; Wang, X.; Zhu, C.; Chen, H.; Bu, L.; Zhang, J.; Qu, S. Derlin-1 ameliorates nonalcoholic hepatic steatosis by promoting ubiquitylation and degradation of FABP1. Free Radic. Biol. Med. 2023, 207, 260–271. [Google Scholar] [CrossRef]
- Atshaves, B.P.; Martin, G.G.; Hostetler, H.A.; McIntosh, A.L.; Kier, A.B.; Schroeder, F. Liver fatty acid-binding protein and obesity. J. Nutr. Biochem. 2010, 21, 1015–1032. [Google Scholar] [CrossRef]
- McIntosh, A.L.; Atshaves, B.P.; Martin, G.G.; Landrock, D.; Milligan, S.; Landrock, K.K.; Huang, H.; Storey, S.M.; Mackie, J.; Schroeder, F.; et al. Effect of liver fatty acid binding protein (L-FABP) gene ablation on lipid metabolism in high glucose diet (HGD) pair-fed mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 985–1004. [Google Scholar] [CrossRef]
- Prinetti, A.; Mitro, N. FABP1 in wonderland. J. Neurochem. 2016, 138, 371–373. [Google Scholar] [CrossRef]
- Dharmarajan, S.; Newberry, E.P.; Montenegro, G.; Nalbantoglu, I.; Davis, V.R.; Clanahan, M.J.; Blanc, V.; Xie, Y.; Luo, J.; Fleshman, J.W., Jr.; et al. Liver fatty acid-binding protein (L-Fabp) modifies intestinal fatty acid composition and adenoma formation in ApcMin/+ mice. Cancer Prev. Res. 2013, 6, 1026–1037. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Gu, W.; Cui, B.; Xu, M.; Yan, Q.; Wang, W.; Ning, G.; Hong, J. Serum liver fatty acid binding protein levels correlate positively with obesity and insulin resistance in Chinese young adults. PLoS ONE 2012, 7, e48777. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Gao, Y.; Chen, S.; Wang, Z.; Wang, H.; Tang, Y.; Su, H.; Lu, F.; Dong, H.; Fang, K. Diosgenin attenuates non-alcoholic fatty liver disease in type 2 diabetes through regulating SIRT6-related fatty acid uptake. Phytomedicine 2023, 111, 154661. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.T.; Wu, C.C.; Hung, W.C.; Lee, T.L.; Hsuan, C.F.; Wei, C.T.; Lu, Y.C.; Yu, T.H.; Chung, F.M.; Lee, Y.J.; et al. FABP1 and FABP2 as markers of diabetic nephropathy. Int. J. Med. Sci. 2020, 17, 2338–2345. [Google Scholar] [CrossRef]
- Wang, G.; Bonkovsky, H.L.; de Lemos, A.; Burczynski, F.J. Recent insights into the biological functions of liver fatty acid binding protein 1. J. Lipid Res. 2015, 56, 2238–2247. [Google Scholar] [CrossRef]
- Khalifa, O.; Al-Akl, N.S.; Errafii, K.; Arredouani, A. Exendin-4 alleviates steatosis in an in vitro cell model by lowering FABP1 and FOXA1 expression via the Wnt/-catenin signaling pathway. Sci. Rep. 2022, 12, 2226. [Google Scholar] [CrossRef]
- Wolfrum, C.; Buhlmann, C.; Rolf, B.; Börchers, T.; Spener, F. Variation of liver-type fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and antisense RNA affects the rate of fatty acid uptake. Biochim. Biophys. Acta 1999, 1437, 194–201. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, Y.; Zhou, Z.; Cai, Z.; Jiao, S.; Huang, W.; Wang, B.; Chen, S.; Wang, W.; Cao, Z.; et al. Discovery of the First-in-Class Intestinal Restricted FXR and FABP1 Dual Modulator ZLY28 for the Treatment of Nonalcoholic Fatty Liver Disease. J. Med. Chem. 2023, 66, 6082–6104. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Li, H.; Dong, B.; Jiang, J.; Liu, N.; Tan, J.; Wang, X.; Lei, L.; Li, H.; et al. Down-Regulating the High Level of 17-Beta-Hydroxysteroid Dehydrogenase 13 Plays a Therapeutic Role for Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 5544. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Li, H.; Tang, M.; Lei, L.; Li, H.-Y.; Dong, B.; Li, J.-R.; Wang, X.-K.; Sun, H.; Li, J.-Y.; et al. Upregulation of Hepatic Glutathione S-Transferase Alpha 1 Ameliorates Metabolic Dysfunction-Associated Steatosis by Degrading Fatty Acid Binding Protein 1. Int. J. Mol. Sci. 2024, 25, 5086. https://doi.org/10.3390/ijms25105086
Jiang J, Li H, Tang M, Lei L, Li H-Y, Dong B, Li J-R, Wang X-K, Sun H, Li J-Y, et al. Upregulation of Hepatic Glutathione S-Transferase Alpha 1 Ameliorates Metabolic Dysfunction-Associated Steatosis by Degrading Fatty Acid Binding Protein 1. International Journal of Molecular Sciences. 2024; 25(10):5086. https://doi.org/10.3390/ijms25105086
Chicago/Turabian StyleJiang, Jing, Hu Li, Mei Tang, Lei Lei, Hong-Ying Li, Biao Dong, Jian-Rui Li, Xue-Kai Wang, Han Sun, Jia-Yu Li, and et al. 2024. "Upregulation of Hepatic Glutathione S-Transferase Alpha 1 Ameliorates Metabolic Dysfunction-Associated Steatosis by Degrading Fatty Acid Binding Protein 1" International Journal of Molecular Sciences 25, no. 10: 5086. https://doi.org/10.3390/ijms25105086
APA StyleJiang, J., Li, H., Tang, M., Lei, L., Li, H. -Y., Dong, B., Li, J. -R., Wang, X. -K., Sun, H., Li, J. -Y., Xu, J. -C., Gong, Y., Jiang, J. -D., & Peng, Z. -G. (2024). Upregulation of Hepatic Glutathione S-Transferase Alpha 1 Ameliorates Metabolic Dysfunction-Associated Steatosis by Degrading Fatty Acid Binding Protein 1. International Journal of Molecular Sciences, 25(10), 5086. https://doi.org/10.3390/ijms25105086





