Evidence That Peripheral Leptin Resistance in Omental Adipose Tissue and Liver Correlates with MASLD in Humans
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
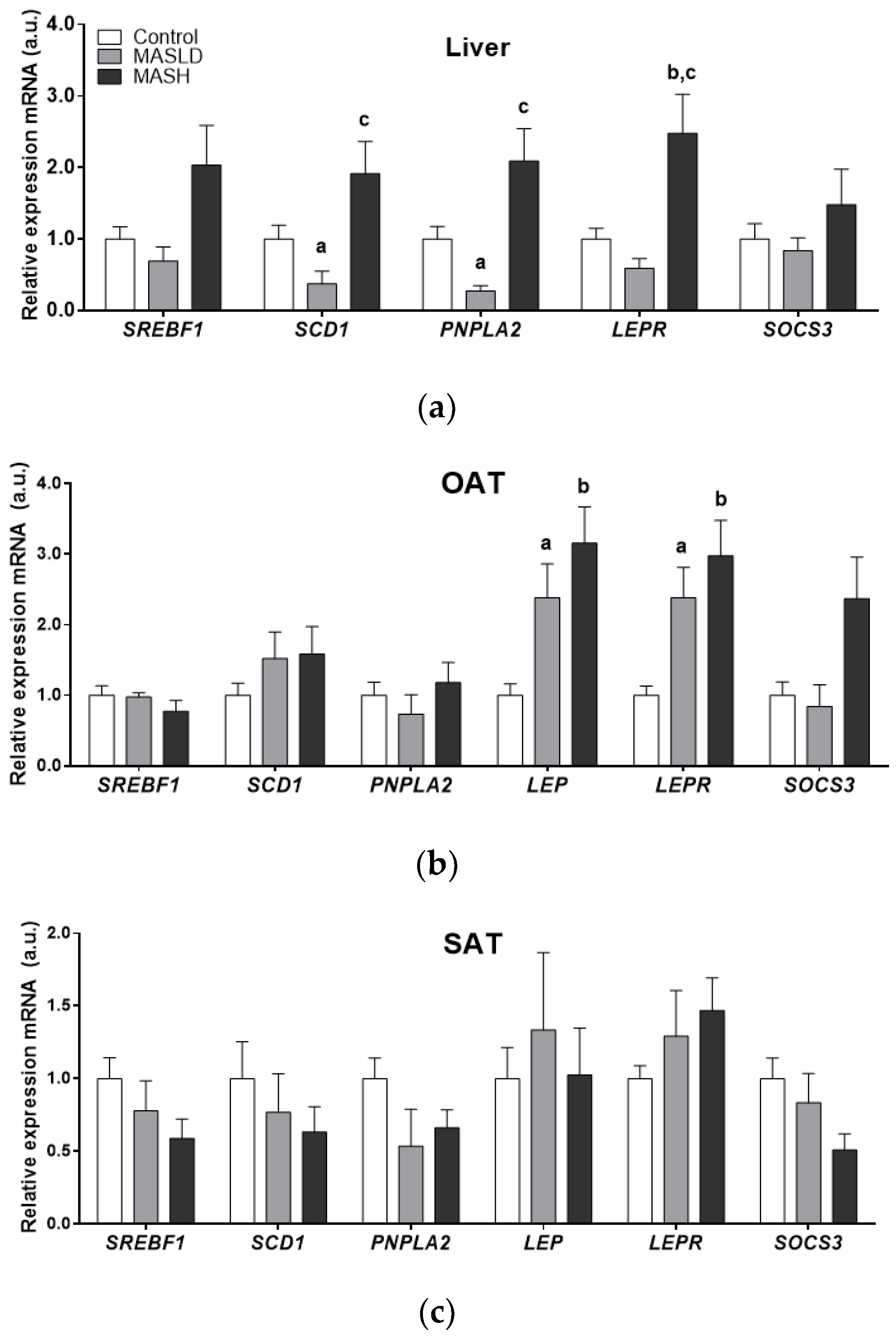
2.2. Analysis of mRNA Expression in the Study Tissues
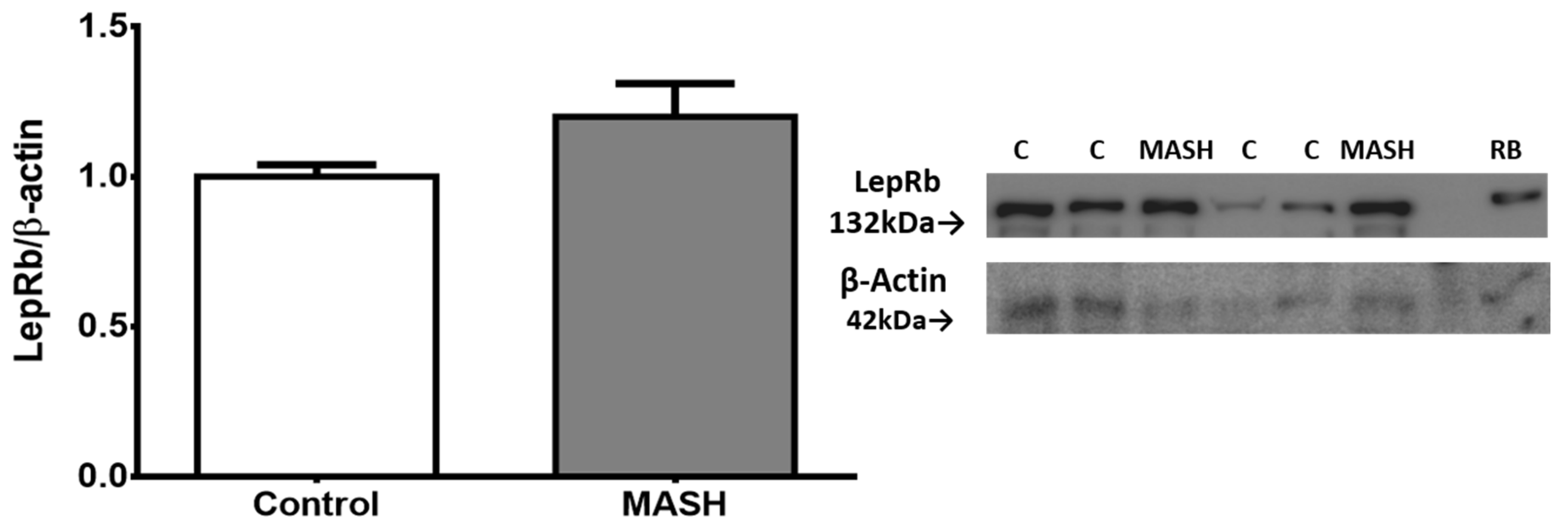
2.3. Protein Expression of the LEPR Long Isoform in Liver Tissue
2.4. Correlation between Clinical Data and mRNA Expression of Genes
3. Discussion
3.1. Cardiometabolic Criteria
3.2. The Relationship of Leptin with MASLD and MASH
3.3. Leptin Resistance
3.4. Leptin Receptor and the SOCS3 Inhibitor
4. Materials and Methods
4.1. Subjects
4.2. Analysis of mRNA Expression by Quantitative PCR
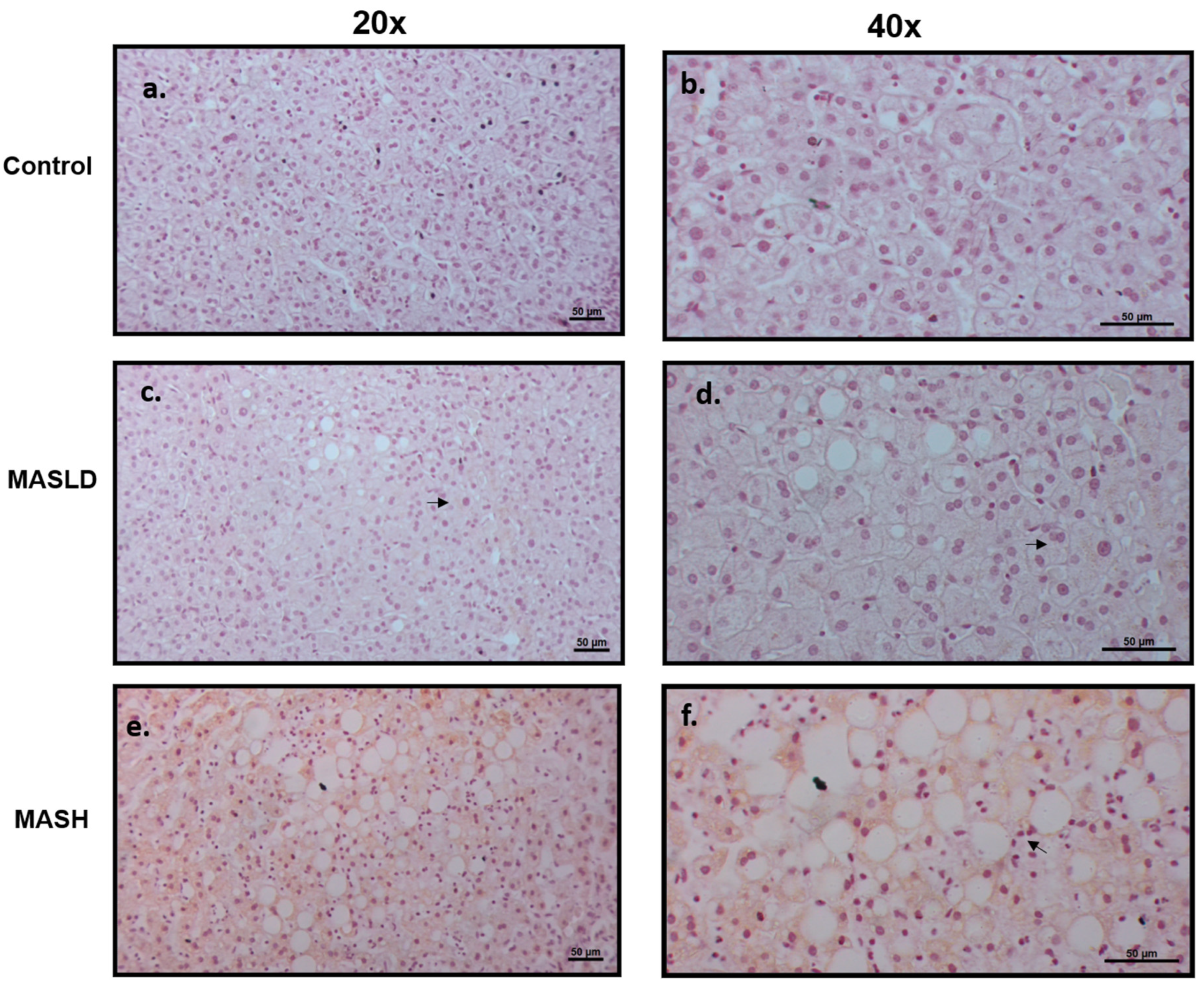
4.3. Liver Histology Evaluation and Human LEPR Immunohistochemistry
4.4. Protein Extraction and Western Blot Analysis
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Obesity Federation. World Obesity Atlas. 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 27 March 2024).
- García del Real, S. Influencia de la Enfermera Escolar en la Obesidad Infantil. NPunto 2019, 2, 25–43. [Google Scholar]
- Gobierno de México. Available online: https://www.gob.mx/issste/articulos/obesidad-infantil#:~:text=M%C3%A9xico (accessed on 24 March 2024).
- Parlati, L.; Régnier, M.; Guillou, H.; Postic, C. New Targets for NAFLD. JHEP Rep. 2021, 3, 100346. [Google Scholar] [CrossRef] [PubMed]
- Hackl, M.T.; Fürnsinn, C.; Schuh, C.M.; Krssak, M.; Carli, F.; Guerra, S.; Freudenthaler, A.; Baumgartner-Parzer, S.; Helbich, T.H.; Luger, A.; et al. Brain Leptin Reduces Liver Lipids by Increasing Hepatic Triglyceride Secretion and Lowering Lipogenesis. Nat. Commun. 2019, 10, 2717. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cortegana, C.; García-Galey, A.; Tami, M.; del Pino, P.; Carmona, I.; López, S.; Alba, G.; Sánchez-Margalet, V. Role of Leptin in Non-Alcoholic Fatty Liver Disease. Biomedicines 2021, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Derbala, M.; Al Naamani, K.; Ghazinian, H.; Fan, J.G.; Eslam, M. MAFLD criteria are better than MASLD criteria at predicting the risk of chronic kidney disease. Ann. Hepatol. 2024, 29, 101512. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Tan, J.S.; Pang, X.Z.; Lee, G.H. Metabolic dysfunction associated fatty liver disease: The new nomenclature and its impact. World J. Gastroenterol. 2023, 29, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Jeong, S.; Jang, H.; Koo, B.K.; Kim, W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: A nationwide cohort study. eClinicalMedicine 2023, 65, 102292. [Google Scholar] [CrossRef]
- De la Cruz-Color, L.; Hernández-Nazará, Z.H.; Maldonado-González, M.; Navarro-Muñíz, E.; Domínguez-Rosales, J.A.; Torres-Baranda, J.R.; del Carmen Ruelas-Cinco, E.; Ramírez-Meza, S.M.; Ruíz-Madrigal, B. Association of the PNPLA2, SCD1 and Leptin Expression with Fat Distribution in Liver and Adipose Tissue From Obese Subjects. Exp. Clin. Endocrinol. Diabetes 2019, 128, 715–722. [Google Scholar] [CrossRef]
- Perakakis, N.; Farr, O.M.; Mantzoros, C.S. Leptin in Leanness and Obesity. J. Am. Coll. Cardiol. 2021, 77, 745–760. [Google Scholar] [CrossRef]
- Cernea, S.; Roiban, A.L.; Both, E.; Huţanu, A. Serum Leptin and Leptin Resistance Correlations with NAFLD in Patients with Type 2 Diabetes. Diabetes Metab. Res. 2018, 34, e3050. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, N. There and Back Again: Leptin Actions in White Adipose Tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Yu, Y.; Jiang, L.; Li, W.; Yu, X.; Gonzalez, L.; Yang, G.; Ke, Z.; Li, W.; Li, C.; et al. Signaling through Tyr985 of Leptin Receptor as an Age/Diet-Dependent Switch in the Regulation of Energy Balance. Mol. Cell. Biol. 2010, 30, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Becerril, S.; Rodríguez, A.; Catalán, V.; Ramírez, B.; Unamuno, X.; Gómez-Ambrosi, J.; Frühbeck, G. iNOS Gene Ablation Prevents Liver Fibrosis in Leptin-Deficient Ob/Ob Mice. Genes 2019, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Wauman, J.; Zabeau, L.; Tavernier, J. The Leptin Receptor Complex: Heavier Than Expected? Front. Endocrinol. 2017, 8, 30. [Google Scholar] [CrossRef]
- Nason, S.R.; Kim, T.; Antipenko, J.P.; Finan, B.; DiMarchi, R.; Hunter, C.S.; Habegger, K.M. Glucagon-Receptor Signaling Reverses Hepatic Steatosis Independent of Leptin Receptor Expression. Endocrinology 2020, 161, bqz013. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Uña, M.; López-Mancheño, Y.; Diéguez, C.; Fernández-Rojo, M.A.; Novelle, M.G. Unraveling the Role of Leptin in Liver Function and Its Relationship with Liver Diseases. Int. J. Mol. Sci. 2020, 21, 9368. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Cho, Y.-K.; Kang, M.-S.; Yoo, T.-W.; Park, J.-H.; Kim, H.-J.; Park, D.-I.; Sohn, C.-I.; Jeon, W.-K.; Kim, B.-I.; et al. The Association between Increased Alanine Aminotransferase Activity and Metabolic Factors in Nonalcoholic Fatty Liver Disease. Metabolism 2006, 55, 1604–1609. [Google Scholar] [CrossRef]
- Salazar, M.R.; Carbajal, H.A.; Curciarello, J.O.; Aizpurua, M.; Adrover, R.E.; Riondet, B. Alanine-aminotransferase: An early marker for insulin resistance? Medicina 2007, 67, 125–130. [Google Scholar]
- Manzano-Nunez, R.; Santana-Dominguez, M.; Rivera-Esteban, J.; Sabiote, C.; Sena, E.; Bañares, J.; Tacke, F.; Pericàs, J.M. Non-Alcoholic Fatty Liver Disease in Patients with Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Clin. Med. 2023, 12, 856. [Google Scholar] [CrossRef]
- Mojiminiyi, O.A.; Safar, F.H.; Al Rumaih, H.; Diejomaoh, M. Variations in Alanine Aminotransferase Levels within the Normal Range Predict Metabolic and Androgenic Phenotypes in Women of Reproductive Age. Scand. J. Clin. Lab. Investig. 2010, 70, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-D.; Fan, Y.; Zhang, H.; Wang, P.; Yuan, J.-P.; Li, M.-J.; Zhan, X.-Y. Serum Leptin and Soluble Leptin Receptor in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2008, 14, 2888. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Aronis, K.N.; Kountouras, J.; Raptis, D.D.; Vasiloglou, M.F.; Mantzoros, C.S. Circulating Leptin in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Diabetologia 2015, 59, 30–43. [Google Scholar] [CrossRef]
- Casado, M.E.; Collado-Pérez, R.; Frago, L.M.; Barrios, V. Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies. Int. J. Mol. Sci. 2023, 24, 1422. [Google Scholar] [CrossRef] [PubMed]
- Sahin-Efe, A.; Upadhyay, J.; Ko, B.-J.; Dincer, F.; Park, K.H.; Migdal, A.; Vokonas, P.; Mantzoros, C. Irisin and Leptin Concentrations in Relation to Obesity, and Developing Type 2 Diabetes: A Cross Sectional and a Prospective Case-Control Study Nested in the Normative Aging Study. Metabolism 2018, 79, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.R.; Diab, D.L.; Baker, A.R.; Yerian, L.; Bajaj, H.; Gray-McGuire, C.; Schauer, P.R.; Gupta, M.; Feldstein, A.E.; Hazen, S.L.; et al. Triglyceride Levels and Not Adipokine Concentrations Are Closely Related to Severity of Nonalcoholic Fatty Liver Disease in an Obesity Surgery Cohort. Obesity 2009, 17, 1696–1701. [Google Scholar] [CrossRef]
- Hossain, I.A.; Akter, S.; Rahman, M.K.; Ali, L. Gender Specific Association of Serum Leptin and Insulinemic Indices with Nonalcoholic Fatty Liver Disease in Prediabetic Subjects. PLoS ONE 2015, 10, e0142165. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Wang, C.; Huang, Y.-Z.; Zhang, L.-L. Nonalcoholic Fatty Liver Disease Shows Significant Sex Dimorphism. World J. Clin. Cases 2022, 10, 1457–1472. [Google Scholar] [CrossRef]
- Licinio, J.; Negrão, A.B.; Mantzoros, C.; Kaklamani, V.; Wong, M.-L.; Bongiorno, P.B.; Negro, P.P.; Mulla, A.; Veldhuis, J.D.; Cearnal, L.; et al. Sex Differences in Circulating Human Leptin Pulse Amplitude: Clinical Implications1. J. Clin. Endocrinol. Metab. 1998, 83, 4140–4147. [Google Scholar] [CrossRef]
- Guo, Z.; Du, H.; Guo, Y.; Jin, Q.; Liu, R.; Yun, Z.; Zhang, J.; Li, X.; Ye, Y. Association between Leptin and NAFLD: A Two-Sample Mendelian Randomization Study. Eur. J. Med. Res. 2023, 28, 215. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Leptin in Nonalcoholic Fatty Liver Disease: A Narrative Review. Metabolism 2015, 64, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Moreno, N.R.; Balaguer, I.; Méndez-Giménez, L.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Portincasa, P.; Calamita, G.; Soveral, G.; et al. Leptin Administration Restores the Altered Adipose and Hepatic Expression of Aquaglyceroporins Improving the Non-Alcoholic Fatty Liver of Ob/Ob Mice. Sci. Rep. 2015, 5, 12067. [Google Scholar] [CrossRef] [PubMed]
- Leijian, G.; Kaixuan, X.; Shuyang, X.; Ningning, L.; Xinru, W.; Yankai, X.; Di, W. Profiles of Metabolic Gene Expression in the White Adipose, Liver and Hypothalamus in Leptin Knockout (LepΔI14/ΔI14) Rats. J. Biomed. Res. 2017, 31, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Oral, E.A.; Dufour, S.; Befroy, D.; Ariyan, C.; Yu, C.; Cline, G.W.; DePaoli, A.M.; Taylor, S.I.; Gorden, P.; et al. Leptin Reverses Insulin Resistance and Hepatic Steatosis in Patients with Severe Lipodystrophy. J. Clin. Investig. 2002, 109, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Becerril, S.; Rodríguez, A.; Catalán, V.; Méndez-Giménez, L.; Ramírez, B.; Sáinz, N.; Llorente, M.; Unamuno, X.; Gómez-Ambrosi, J.; Frühbeck, G. Targeted Disruption of the iNOS Gene Improves Adipose Tissue Inflammation and Fibrosis in Leptin-Deficient Ob/Ob Mice: Role of Tenascin C. Int. J. Obes. 2018, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Wolfs, M.G.M.; Gruben, N.; Rensen, S.S.; Verdam, F.J.; Greve, J.W.; Driessen, A.; Wijmenga, C.; Buurman, W.A.; Franke, L.; Scheja, L.; et al. Determining the Association between Adipokine Expression in Multiple Tissues and Phenotypic Features of Non-Alcoholic Fatty Liver Disease in Obesity. Nutr. Diabetes 2015, 5, e146. [Google Scholar] [CrossRef] [PubMed]
- Martelli, D.; Brooks, V.L. Leptin Increases: Physiological Roles in the Control of Sympathetic Nerve Activity, Energy Balance, and the Hypothalamic–Pituitary–Thyroid Axis. Int. J. Mol. Sci. 2023, 24, 2684. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, M.; Vlaicu, S.I.; Ciumărnean, L.; Milaciu, M.V.; Mărginean, C.; Florea, M.; Vesa, Ș.C.; Popa, M. Chronic Inflammation—A Link between Nonalcoholic Fatty Liver Disease (NAFLD) and Dysfunctional Adipose Tissue. Medicina 2022, 58, 641. [Google Scholar] [CrossRef]
- Pontes-da-Silva, R.M.; de Souza Marinho, T.; de Macedo Cardoso, L.E.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Obese Mice Weight Loss Role on Nonalcoholic Fatty Liver Disease and Endoplasmic Reticulum Stress Treated by a GLP-1 Receptor Agonist. Int. J. Obes. 2022, 46, 21–29. [Google Scholar] [CrossRef]
- Lu, P.; Yang, G.; Jiang, L.; He, W.; Wu, W.; Qi, L.; Shen, S.; Rao, J.; Zhang, P.; Xue, Z.; et al. Characterizing Disease Progression of Nonalcoholic Steatohepatitis in Leptin-Deficient Rats by Integrated Transcriptome Analysis. Exp. Biol. Med. 2020, 246, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, S.; Farrell, G.; Frost, L.; Kriketos, A.; Lin, R.; Liddle, C.; Samarasinghe, D.; George, J. Serum Leptin in NASH Correlates with Hepatic Steatosis but Not Fibrosis: A Manifestation of Lipotoxicity? Hepatology 2002, 36, 403–409. [Google Scholar] [CrossRef]
- Angulo, P.; Alba, L.M.; Petrovic, L.M.; Adams, L.A.; Lindor, K.D.; Jensen, M.D. Leptin, Insulin Resistance, and Liver Fibrosis in Human Nonalcoholic Fatty Liver Disease. J. Hepatol. 2004, 41, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Ikejima, K.; Honda, H.; Yoshikawa, M.; Hirose, M.; Kitamura, T.; Takey, Y.; Sato, N. Leptin Augments Inflammatory and Profibrogenic Responses in the Murine Liver Induced by Hepatotoxic Chemicals. Hepatology 2001, 34, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human Leptin Stimulates Proliferation and Activation of Human Circulating Monocytes. Cell. Immunol. 1999, 194, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, K.; MacIver, N.J. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front. Immunol. 2021, 11, 622468. [Google Scholar] [CrossRef] [PubMed]
- Palhinha, L.; Liechocki, S.; Hottz, E.D.; da Silva Pereira, J.A.; de Almeida, C.J.; Moraes-Vieira, P.M.M.; Bozza, P.T.; Maya-Monteiro, C.M. Leptin Induces Proadipogenic and Proinflammatory Signaling in Adipocytes. Front. Endocrinol. 2019, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- de Brito Monteiro, L.; Prodonoff, J.S.; Favero de Aguiar, C.; Correa-da-Silva, F.; Castoldi, A.; van Teijlingen Bakker, N.; Davanzo, G.G.; Castelucci, B.; da Silva Pereira, J.A.; Curtis, J.; et al. Leptin Signaling Suppression in Macrophages Improves Immunometabolic Outcomes in Obesity. Diabetes 2022, 71, 1546–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Huang, F.; Yao, Y.; Xu, L. Leptin Induces NAFLD Progression through Infiltrated CD8+ T Lymphocytes Mediating Pyroptotic-like Cell Death of Hepatocytes and Macrophages. Dig. Liver Dis. 2021, 53, 598–605. [Google Scholar] [CrossRef]
- Rosso, C.; Kazankov, K.; Younes, R.; Esmaili, S.; Marietti, M.; Sacco, M.; Carli, F.; Gaggini, M.; Salomone, F.; Møller, H.J.; et al. Crosstalk between Adipose Tissue Insulin Resistance and Liver Macrophages in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2019, 71, 1012–1021. [Google Scholar] [CrossRef]
- Zhao, D.; Cui, H.; Shao, Z.; Cao, L. Abdominal Obesity, Chronic Inflammation and the Risk of Non-Alcoholic Fatty Liver Disease. Ann. Hepatol. 2023, 28, 100726. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Evers, N.; Awazawa, M.; Nicholls, H.T.; Brönneke, H.S.; Dietrich, A.; Mauer, J.; Blüher, M.; Brüning, J.C. Obesogenic Memory Can Confer Long-Term Increases in Adipose Tissue but Not Liver Inflammation and Insulin Resistance after Weight Loss. Mol. Metab. 2016, 5, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Cottam, M.A.; Caslin, H.L.; Winn, N.C.; Hasty, A.H. Multiomics Reveals Persistence of Obesity-Associated Immune Cell Phenotypes in Adipose Tissue during Weight Loss and Weight Regain in Mice. Nat. Commun. 2022, 13, 2950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, N.; Zhu, Y.; Straub, L.; Zhang, Z.; Wang, M.-Y.; Zhu, Q.; Kusminski, C.M.; Elmquist, J.K.; Scherer, P.E. Partial Leptin Deficiency Confers Resistance to Diet-Induced Obesity in Mice. Mol. Metab. 2020, 37, 100995. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, K.; Nichols, A.G.; Alwarawrah, Y.; MacIver, N.J. Effects of T Cell Leptin Signaling on Systemic Glucose Tolerance and T Cell Responses in Obesity. PLoS ONE 2023, 18, e0286470. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.E.; Lowe, C.; Pretz, D.; Steger, J.; Williams, L.M.; Tups, A. High-Fat Diet Induces Leptin Resistance in Leptin-Deficient Mice. J. Neuroendocrinol. 2014, 26, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G., Jr.; Heymsfield, S.B.; Haft, C.; Kahn, B.B.; Laughlin, M.; Leibel, R.L.; Tschöp, M.H.; Yanovski, J.A. Challenges and Opportunities of Defining Clinical Leptin Resistance. Cell Metab. 2012, 15, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.L. Selective Leptin Resistance Revisited. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R566–R581. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lai, F.; Hou, Y.; Zheng, R. Leptin Signaling and Leptin Resistance. Med. Rev. 2022, 2, 363–384. [Google Scholar] [CrossRef]
- Peng, J.; Yin, L.; Wang, X. Central and Peripheral Leptin Resistance in Obesity and Improvements of Exercise. Horm. Behav. 2021, 133, 105006. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, Y.; Schultz, R.D.; Li, N.; He, Z.; Zhang, Z.; Caron, A.; Zhu, Q.; Sun, K.; Xiong, W.; et al. Partial Leptin Reduction as an Insulin Sensitization and Weight Loss Strategy. Cell Metab. 2019, 30, 706–719.e6. [Google Scholar] [CrossRef] [PubMed]
- Simonds, S.E.; Cowley, M.A.; Enriori, P.J. Leptin Increasing Sympathetic Nerve Outflow in Obesity. Adipocyte 2012, 1, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Genchi, V.A.; D’Oria, R.; Palma, G.; Caccioppoli, C.; Cignarelli, A.; Natalicchio, A.; Laviola, L.; Giorgino, F.; Perrini, S. Impaired Leptin Signalling in Obesity: Is Leptin a New Thermolipokine? Int. J. Mol. Sci. 2021, 22, 6445. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kwak, S.; Lee, J.-H.; Kang, S.; Lee, S.-P. Nonalcoholic Fatty Liver Disease Is an Early Predictor of Metabolic Diseases in a Metabolically Healthy Population. PLoS ONE 2019, 14, e0224626. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Surabattula, R.; Wang, X.Y. Determinants of Fibrosis Progression and Regression in NASH. J. Hepatol. 2018, 68, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Seufert, J.; Kieffer, T.J.; Leech, C.A.; Holz, G.G.; Moritz, W.; Ricordi, C.; Habener, J.F. Leptin Suppression of Insulin Secretion and Gene Expression in Human Pancreatic Islets: Implications for the Development of Adipogenic Diabetes Mellitus1. J. Clin. Endocrinol. Metab. 1999, 84, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Covey, S.D.; Wideman, R.D.; McDonald, C.; Unniappan, S.; Huynh, F.; Asadi, A.; Speck, M.; Webber, T.; Chua, S.C.; Kieffer, T.J. The Pancreatic β Cell Is a Key Site for Mediating the Effects of Leptin on Glucose Homeostasis. Cell Metab. 2006, 4, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Liolios, A.D.; Tritos, N.A.; Kaklamani, V.G.; Doulgerakis, D.E.; Griveas, I.; Moses, A.C.; Flier, J.S. Circulating Insulin Concentrations, Smoking, and Alcohol Intake Are Important Independent Predictors of Leptin in Young Healthy Men. Obes. Res. 1998, 6, 179–186. [Google Scholar] [CrossRef]
- Chiriacò, M.; Nesti, L.; Flyvbjerg, A.; Golay, A.; Nazare, J.-A.; Anderwald, C.-H.; Mitrakou, A.; Bizzotto, R.; Mari, A.; Natali, A. At Any Level of Adiposity, Relatively Elevated Leptin Concentrations Are Associated With Decreased Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2024, 109, 461–470. [Google Scholar] [CrossRef]
- Coppari, R.; Bjørbæk, C. Leptin Revisited: Its Mechanism of Action and Potential for Treating Diabetes. Nat. Rev. Drug Discov. 2012, 11, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Pérez-Pérez, A.; Vilariño-García, T.; Jiménez-Cortegana, C.; Muriana, F.J.G.; Millán-Linares, M.C.; Sánchez-Margalet, V. Nutritional Modulation of Leptin Expression and Leptin Action in Obesity and Obesity-Associated Complications. J. Nutr. Biochem. 2021, 89, 108561. [Google Scholar] [CrossRef] [PubMed]
- Castela, I.; Morais, J.; Barreiros-Mota, I.; Silvestre, M.P.; Marques, C.; Rodrigues, C.; Ismael, S.; Araújo, J.R.; Ângelo-Dias, M.; Martins, C.; et al. Decreased Adiponectin/Leptin Ratio Relates to Insulin Resistance in Adults with Obesity. Am. J. Physiol.-Endocrinol. Metab. 2023, 324, E115–E119. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.L.G.; Haynes, W.G.; Rahmouni, K.; Morgan, D.A.; Sivitz, W.I.; Mark, A.L. The Concept of Selective Leptin Resistance. Diabetes 2002, 51, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Magenes, V.C.; Rossi, V.; Fabiano, V.; Mameli, C.; Zuccotti, G. Lipodystrophies in Non-Insulin-Dependent Children: Treatment Options and Results from Recombinant Human Leptin Therapy. Pharmacol. Res. 2023, 187, 106629. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Wilson, H.; Rohner-Jeanrenaud, F.; Tschöp, M.H. Central Nervous System Regulation of Adipocyte Metabolism. Regul. Pept. 2008, 149, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, M.; Bo Jensen, P.; Blum, W.F.; Christoffersen, C.T.; Englaro, P.; Heinze, E.; Rascher, W.; Teller, W.; Tornqvist, H.; Hauner, H. Insulin and Cortisol Promote Leptin Production in Cultured Human Fat Cells. Diabetes 1996, 45, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, R.; Fantuzzi, G.; Fuller, J.; Dinarello, C.A.; Feingold, K.R.; Grunfeld, C. IL-1β Mediates Leptin Induction during Inflammation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 274, R204–R208. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Chen, J.; He, Y.; Ma, W.; Liu, X.; Sun, X. Effects of Multi-Organ Crosstalk on the Physiology and Pathology of Adipose Tissue. Front. Endocrinol. 2023, 14, 1198984. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamada, T.; Hosaka, S.; Kaneko, K.; Asai, Y.; Munakata, Y.; Seike, J.; Horiuchi, T.; Kodama, S.; Izumi, T.; et al. Inter-Organ Insulin-Leptin Signal Crosstalk from the Liver Enhances Survival during Food Shortages. Cell Rep. 2023, 42, 112415. [Google Scholar] [CrossRef]
- Korner, J.; Conroy, R.; Febres, G.J.; McMahon, D.; Conwell, I.; Karmally, W.; Aronne, L.J. Randomized Double-blind Placebo-controlled Study of Leptin Administration after Gastric Bypass. Obesity 2013, 21, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia Is Required for the Development of Leptin Resistance. PLoS ONE 2010, 5, e11376. [Google Scholar] [CrossRef] [PubMed]
- Balland, E.; Chen, W.; Tiganis, T.; Cowley, M.A. Persistent Leptin Signaling in the Arcuate Nucleus Impairs Hypothalamic Insulin Signaling and Glucose Homeostasis in Obese Mice. Neuroendocrinology 2019, 109, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, K.; Hebebrand, J.; Mika, C.; Heer, M.; Heussen, N.; Herpertz-Dahlmann, B. High Serum Leptin Levels Subsequent to Weight Gain Predict Renewed Weight Loss in Patients with Anorexia Nervosa. Psychoneuroendocrinology 2004, 29, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.; Fatima, A.; Tauseef, A.; Yousufzai, M.I.; Liaqat, I.; Naqvi, Q. Comparative and Predictive Significance of Serum Leptin Levels in Non-alcoholic Fatty Liver Disease. Cureus 2024, 16, e57943. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Shimomura, Y.; Nakanishi, Y.; Futawatari, T.; Ohtani, K.; Sato, N.; Mori, M. Estrogen Increases in Vivo Leptin Production in Rats and Human Subjects. J. Endocrinol. 1997, 154, 285–292. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, C.A.; Skowronski, A.A.; Rosenbaum, M. The Role of Leptin in the Development of Energy Homeostatic Systems and the Maintenance of Body Weight. Front. Physiol. 2021, 12, 789519. [Google Scholar] [CrossRef]
- Diéguez-Campa, C.E.; Angel-Chávez, L.I.; Reyes-Ruvalcaba, D.; Talavera-Zermeño, M.J.; Armendáriz-Cabral, D.A.; Torres-Muro, D.; Pérez-Neri, I. Leptin Levels and Q223R Leptin Receptor Gene Polymorphism in Obese Mexican Young Adults. EJIFCC 2020, 29, 197–207. [Google Scholar]
- Morabito, M.V.; Ravussin, Y.; Mueller, B.R.; Skowronski, A.A.; Watanabe, K.; Foo, K.S.; Lee, S.X.; Lehmann, A.; Hjorth, S.; Zeltser, L.M.; et al. Weight Perturbation Alters Leptin Signal Transduction in a Region-Specific Manner throughout the Brain. PLoS ONE 2017, 12, e0168226. [Google Scholar] [CrossRef]
- Ceccarini, G.; Pelosini, C.; Ferrari, F.; Magno, S.; Vitti, J.; Salvetti, G.; Moretto, C.; Marioni, A.; Buccianti, P.; Piaggi, P.; et al. Serum IGF-Binding Protein 2 (IGFBP-2) Concentrations Change Early after Gastric Bypass Bariatric Surgery Revealing a Possible Marker of Leptin Sensitivity in Obese Subjects. Endocrine 2019, 65, 86–93. [Google Scholar] [CrossRef]
- Mendoza-Herrera, K.; Florio, A.A.; Moore, M.; Marrero, A.; Tamez, M.; Bhupathiraju, S.N.; Mattei, J. The Leptin System and Diet: A Mini Review of the Current Evidence. Front. Endocrinol. 2021, 12, 749050. [Google Scholar] [CrossRef] [PubMed]
- Neseliler, S.; Hu, W.; Larcher, K.; Zacchia, M.; Dadar, M.; Scala, S.G.; Lamarche, M.; Zeighami, Y.; Stotland, S.C.; Larocque, M.; et al. Neurocognitive and Hormonal Correlates of Voluntary Weight Loss in Humans. Cell Metab. 2019, 29, 39–49.e4. [Google Scholar] [CrossRef]
- Nozari, Y.; Park, C.; Brietzke, E.; Iacobucci, M.; Gill, H.; McIntyre, R.S. Correlation between Improved Leptin Signaling and Cognitive Function Post Bariatric Surgery. J. Affect. Disord. 2023, 326, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.; Muzumdar, R.H.; Atzmon, G.; Ma, X.; Yang, X.; Einstein, F.H.; Barzilai, N. Resistance to Leptin Action Is the Major Determinant of Hepatic Triglyceride Accumulation In Vivo. FASEB J. 2006, 21, 53–60. [Google Scholar] [CrossRef]
- López, V.; Bonzón-Kulichenko, E.; Moltó, E.; Fernández-Agulló, T.; Arribas, C.; Andrés, A.; Gallardo, N. Food Restriction Is Required to Preserve the Antisteatotic Effects of Central Leptin in the Liver of Middle-Aged Rats. Obesity 2018, 26, 877–884. [Google Scholar] [CrossRef]
- Lu, C.-W.; Yang, K.-C.; Chi, Y.-C.; Wu, T.-Y.; Chiang, C.-H.; Chang, H.-H.; Huang, K.-C.; Yang, W.-S. Adiponectin–Leptin Ratio for the Early Detection of Lean Non-Alcoholic Fatty Liver Disease Independent of Insulin Resistance. Ann. Med. 2023, 55, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-F.; Cui, H.; Zhang, Q.; Morgan, D.A.; Thedens, D.R.; Nishimura, D.; Grobe, J.L.; Sheffield, V.C.; Rahmouni, K. The BBSome Controls Energy Homeostasis by Mediating the Transport of the Leptin Receptor to the Plasma Membrane. PLoS Genet. 2016, 12, e1005890. [Google Scholar] [CrossRef]
- Ogier, V.; Ziegler, O.; Méjean, L.; Nicolas, J.; Stricker-Krongrad, A. Obesity Is Associated with Decreasing Levels of the Circulating Soluble Leptin Receptor in Humans. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 496–503. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y.; Zhang, C.; Liu, M.; Xiong, H. Leptin Induces IL-6 and IL-8 Expression through Leptin Receptor Ob-Rb in Human Dental Pulp Fibroblasts. Acta Odontol. Scand. 2019, 77, 205–212. [Google Scholar] [CrossRef]
- Séron, K.; Couturier, C.; Belouzard, S.; Bacart, J.; Monté, D.; Corset, L.; Bocquet, O.; Dam, J.; Vauthier, V.; Lecœur, C.; et al. Endospanins Regulate a Postinternalization Step of the Leptin Receptor Endocytic Pathway. J. Biol. Chem. 2011, 286, 17968–17981. [Google Scholar] [CrossRef]
- Tsiotra, P.C.; Pappa, V.; Raptis, S.A.; Tsigos, C. Expression of the Long and Short Leptin Receptor Isoforms in Peripheral Blood Mononuclear Cells: Implications for Leptin’s Actions. Metabolism 2000, 49, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Li, Y.; Zheng, Q.; Jiang, L.; Myers, M.G., Jr.; Wu, W.-S.; Rui, L. LepRb+ Cell–Specific Deletion of Slug Mitigates Obesity and Nonalcoholic Fatty Liver Disease in Mice. J. Clin. Investig. 2023, 133, e156722. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Pelletier, N.E.; Wong, J.; Li, B.; Sdrulla, A.D.; Madden, C.J.; Marks, D.L.; Brooks, V.L. Leptin Increases Sympathetic Nerve Activity via Induction of Its Own Receptor in the Paraventricular Nucleus. eLife 2020, 9, e55357. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, L.; Persaud, A.; Feurdean, M.; Ahlawat, S.; Kim, H. The Association of Leptin with Severity of Non-Alcoholic Fatty Liver Disease: A Population-Based Study. Clin. Mol. Hepatol. 2018, 24, 392–401. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Butler, A.E.; Majeed, M.; Banach, M.; Sahebkar, A. Investigation of the Effect of Curcumin on Protein Targets in NAFLD Using Bioinformatic Analysis. Nutrients 2022, 14, 1331. [Google Scholar] [CrossRef] [PubMed]
- Papathanassoglou, E.; El-Haschimi, K.; Li, X.C.; Matarese, G.; Strom, T.; Mantzoros, C. Leptin Receptor Expression and Signaling in Lymphocytes: Kinetics During Lymphocyte Activation, Role in Lymphocyte Survival, and Response to High Fat Diet in Mice. J. Immunol. 2006, 176, 7745–7752. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Toda, C.; Okamoto, S. Regulatory Role of Leptin in Glucose and Lipid Metabolism in Skeletal Muscle. Indian. J. Endocr. Metab. 2012, 16, 562. [Google Scholar] [CrossRef]
- Singh, P.; Peterson, T.E.; Sert-Kuniyoshi, F.H.; Glenn, J.A.; Davison, D.E.; Romero-Corral, A.; Pusalavidyasagar, S.; Jensen, M.D.; Somers, V.K. Leptin signaling in adipose tissue: Role in lipid accumulation and weight gain. Circ. Res. 2012, 111, 599–603. [Google Scholar] [CrossRef]
- Hall, M.E.; Maready, M.W.; Hall, J.E.; Stec, D.E. Rescue of Cardiac Leptin Receptors inDb/DbMice Prevents Myocardial Triglyceride Accumulation. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E316–E325. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Ardid-Ruiz, A.; Ibars, M.; Mena, P.; Del Rio, D.; Muguerza, B.; Arola, L.; Aragonès, G.; Suárez, M. Resveratrol Treatment Enhances the Cellular Response to Leptin by Increasing OBRb Content in Palmitate-Induced Steatotic HepG2 Cells. Int. J. Mol. Sci. 2019, 20, 6282. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, J.; Lu, L.; Gao, L.; Zhu, S.; Sui, Y.; Cao, T.; Yang, T. Metformin Induces Pyroptosis in Leptin Receptor-Defective Hepatocytes via Overactivation of the AMPK Axis. Cell Death Dis. 2023, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Klöting, N. Leptin Receptor Compound Heterozygosity in Humans and Animal Models. Int. J. Mol. Sci. 2021, 22, 4475. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver Sympathetic Denervation Reverses Obesity-induced Hepatic Steatosis. J. Physiol. 2019, 597, 4565–4580. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Beghini, M.; Wolf, P.; Pfleger, L.; Hackl, M.; Bastian, M.; Freudenthaler, A.; Harreiter, J.; Zeyda, M.; Baumgartner-Parzer, S.; et al. Leptin Increases Hepatic Triglyceride Export via a Vagal Mechanism in Humans. Cell Metab. 2022, 34, 1719–1731.e5. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; O’Dwyer, S.M.; Webber, T.D.; Baker, R.K.; So, V.; Ellis, C.E.; Yoon, J.S.; Mojibian, M.; Glavas, M.M.; Karunakaran, S.; et al. Metabolic Effects of Leptin Receptor Knockdown or Reconstitution in Adipose Tissues. Sci. Rep. 2019, 9, 3307. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, J.M.; Fadel, J.R.; Reagan, L.P. Peripheral versus Central Insulin and Leptin Resistance: Role in Metabolic Disorders, Cognition, and Neuropsychiatric Diseases. Neuropharmacology 2022, 203, 108877. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and Metaflammation: The Yin and Yang of Type 2 Diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, R.; Hu, C. Leptin/obR Signaling Exacerbates Obesity-Related Neutrophilic Airway Inflammation through Inflammatory M1 Macrophages. Mol. Med. 2023, 29, 100. [Google Scholar] [CrossRef]
- Barnes, T.M.; Shah, K.; Allison, M.B.; Steinl, G.K.; Gordian, D.; Sabatini, P.V.; Tomlinson, A.J.; Cheng, W.; Jones, J.C.; Zhu, Q.; et al. Identification of the Leptin Receptor Sequences Crucial for the STAT3-Independent Control of Metabolism. Mol. Metab. 2020, 32, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zabeau, L.; Wauman, J.; Dam, J.; Van Lint, S.; Burg, E.; De Geest, J.; Rogge, E.; Silva, A.; Jockers, R.; Tavernier, J. A Novel Leptin Receptor Antagonist Uncouples Leptin’s Metabolic and Immune Functions. Cell. Mol. Life Sci. 2019, 76, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 Phosphorylation in Central Leptin Resistance. Nutr. Metab. 2021, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kusminski, C.M.; Elmquist, J.K.; Scherer, P.E. Leptin: Less Is More. Diabetes 2020, 69, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.B.; Buonfiglio, D.C.; Cardinali, L.I.; Furigo, I.C.; Ramos-Lobo, A.M.; Tirapegui, J.; Elias, C.F.; Donato, J., Jr. Inactivation of SOCS3 in Leptin Receptor-Expressing Cells Protects Mice from Diet-Induced Insulin Resistance but Does Not Prevent Obesity. Mol. Metab. 2014, 3, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Di Spiezio, A.; Sandin, E.S.; Dore, R.; Müller-Fielitz, H.; Storck, S.E.; Bernau, M.; Mier, W.; Oster, H.; Jöhren, O.; Pietrzik, C.U.; et al. The LepR-Mediated Leptin Transport across Brain Barriers Controls Food Reward. Mol. Metab. 2018, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Zhang, S.; Yuan, X.; Mo, W.; Wei, F.; Zhao, S.; Yang, W.; Liu, H.; Rong, X. Liraglutide Lowers Body Weight Set Point in DIO Rats and Its Relationship with Hypothalamic Microglia Activation. Obesity 2019, 28, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Schwertheim, S.; Alhardan, M.; Manka, P.P.; Sowa, J.-P.; Canbay, A.; Schmidt, H.H.-J.; Baba, H.A.; Kälsch, J. Higher pNRF2, SOCS3, IRF3, and RIG1 Tissue Protein Expression in NASH Patients versus NAFL Patients: pNRF2 Expression Is Concomitantly Associated with Elevated Fasting Glucose Levels. J. Pers. Med. 2023, 13, 1152. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-L.; Wang, Y.-C.; Hsu, Y.-A.; Chen, C.-S.; Weng, R.-C.; Lu, Y.-P.; Chuang, C.-Y.; Wan, L. Galectin-12 Modulates Kupffer Cell Polarization to Alter the Progression of Nonalcoholic Fatty Liver Disease. Glycobiology 2023, 33, 673–682. [Google Scholar] [CrossRef]
- Lule, K.O.; Akarsu, E.; Sayiner, Z.A.; Lule, N.O.; Balci, S.O.; Demirel, C.; Bozdag, Z.; Korkmaz, M.; Yilmaz, I. The Effects of Metformin, Pioglitazone, Exenatide and Exercise on Fatty Liver in Obese Diabetic Rats: The Role of IRS-1 and SOCS-3 Molecules. Inflammopharmacol 2022, 30, 243–250. [Google Scholar] [CrossRef]
- Tawfik, M.K.; Badran, D.I.; Keshawy, M.M.; Makary, S.; Abdo, M. Alternate-Day Fat Diet and Exenatide Modulate the Brain Leptin JAK2/STAT3/SOCS3 Pathway in a Fat Diet-Induced Obesity and Insulin Resistance Mouse Model. Arch. Med. Sci. 2023, 19, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Sun, K.; Wu, H.; Chen, X.; Tang, H.; Mao, J. PPARγ Alleviated Hepatocyte Steatosis through Reducing SOCS3 by Inhibiting JAK2/STAT3 Pathway. Biochem. Biophys. Res. Commun. 2018, 498, 1037–1044. [Google Scholar] [CrossRef]
- Lopez-Yus, M.; Lorente-Cebrian, S.; del Moral-Bergos, R.; Hörndler, C.; Garcia-Sobreviela, M.P.; Casamayor, C.; Sanz-Paris, A.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Identification of Novel Targets in Adipose Tissue Involved in Non-alcoholic Fatty Liver Disease Progression. FASEB J. 2022, 36, e22429. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Gu, P.; Wang, W.; Cao, L.; Zhang, L.; Li, J.; Mu, W.; Wang, H. Benzo[a]Pyrene Stimulates miR-650 Expression to Promote the Pathogenesis of Fatty Liver Disease and Hepatocellular Carcinoma via SOCS3/JAK/STAT3 Cascades. J. Mol. Cell Biol. 2021, 13, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Kabir, N.N.; Flores-Morales, A.; Rönnstrand, L. SOCS Proteins in Regulation of Receptor Tyrosine Kinase Signaling. Cell. Mol. Life Sci. 2014, 71, 3297–3310. [Google Scholar] [CrossRef]
- Darci-Maher, N.; Alvarez, M.; Arasu, U.T.; Selvarajan, I.; Lee, S.H.T.; Pan, D.Z.; Miao, Z.; Das, S.S.; Kaminska, D.; Örd, T.; et al. Cross-tissue omics analysis discovers ten adipose genes encoding secreted proteins in obesity-related non-alcoholic fatty liver disease. eBioMedicine 2023, 92, 104620. [Google Scholar] [CrossRef]
- Wang, W.; Kong, Y.; Wang, X.; Wang, Z.; Tang, C.; Li, J.; Yang, Q.; Chen, Y.Q.; Zhu, S. Identification of novel SCD1 inhibitor alleviates nonalcoholic fatty liver disease: Critical role of liver-adipose axis. Cell Commun. Signal. 2023, 21, 268. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Carr, R.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef]
- Filali-Mouncef, Y.; Hunter, C.; Roccio, F.; Zagkou, S.; Dupont, N.; Primard, C.; Proikas-Cezanne, T.; Reggiori, F. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy 2022, 18, 50–72. [Google Scholar] [CrossRef]
- Su, B.C.; Xiao, K.M.; Wang, K.L.; Yang, S.F.; Huang, Z.X.; Luo, J.W. ATGL promotes colorectal cancer growth by regulating autophagy process and SIRT1 expression. Med. Oncol. 2023, 40, 350. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and ?-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, P.C. Endocrine Diseases: Overview, 2nd ed.; Kristian, H., Ed.; International Encyclopedia of Public Health; Academic Press: Cambridge, MA, USA, 2017; pp. 332–341. [Google Scholar]

| Variable | Control n = 24 | MASLD n = 8 | MASH n = 11 |
|---|---|---|---|
| Sex (Male/Female) | 24 (3/21) | 8 (0/8) | 11 (3/8) |
| Age (years) | 36.0 ± 1.7 | 34.3 ± 3.8 | 39.7 ± 1.7 |
| Weight (kg) | 69.3 ± 2.6 | 69.9 ± 4.1 | 92.2 ± 9.7 |
| Height (cm) | 161.0 ± 1.3 | 159.9 ± 2.4 | 160.6 ± 2.9 |
| BMI (kg/m2) | 26.7 ± 0.9 | 27.3 ± 1.4 | 35.3 ± 2.8 a,b |
| Waist circumference (cm) | 84.3 ± 3.0 | 86.2 ± 3.4 | 102.2 ± 6.1 a |
| Abdominal Adiposity n (%) | 14 (58) | 6 (75) | 11 (100) |
| Hip circumference (cm) | 102.4 ± 2.5 | 103.8 ± 3.1 | 118.4 ± 6.3 a |
| Waist:hip ratio | 0.82 ± 0.02 | 0.83 ± 0.03 | 0.86 ± 0.03 |
| Fat (%) | 33.8 ± 2.2 | 37.7 ± 1.8 | 39.4 ± 3.2 |
| Fat mass (kg) | 24.6 ± 2.2 | 27.4 ± 3.3 | 33.9 ± 4.7 |
| Glucose (mg/dL) | 85.2 ± 3.3 | 90.4 ± 1.8 | 85.6 ± 3.9 |
| Insulin (mUI/mL) | 5.2 ± 0.6 | 8.4 ± 2.2 | 12.0 ± 3.0 a |
| HOMA-IR | 1.06 ± 0.14 | 1.90 ± 0.55 | 2.57 ± 0.66 a |
| Cholesterol (mg/dL) | 155.8 ± 10.0 | 170.3 ± 20.8 | 157.6 ± 17.2 |
| Triglycerides (mg/dL) | 117.0 ± 14.5 | 143.7 ± 20.7 | 118.1 ± 12.1 |
| LDL-c (mg/dL) | 89.2 ± 9.6 | 117.7 ± 26.4 | 97.3 ± 13.8 |
| VLDL-c (mg/dL) | 23.3 ± 2.9 | 31.3 ± 3.9 | 23.6 ± 2.4 |
| HDL-c (mg/dL) | 43.1 ± 3.1 | 38.9 ± 2.9 | 35.2 ± 3.4 |
| Low HDL-c n (%) | 18 (75) | 8 (100) | 9 (82) |
| ALT (U/L) | 22.6 ± 2.3 | 21.7 ± 2.7 | 34.5 ± 5.3 a |
| AST (U/L) | 21.2 ± 1.9 | 21.0 ± 2.1 | 29.3 ± 5.3 |
| GGT (U/L) | 28.0 ± 5.0 | 37.3 ± 9.0 | 47.5 ± 14.6 |
| Leptin (ng/mL) | 20.4 ± 2.5 | 14.9 ± 3.6 | 37.0 ± 9.0 a,b |
| a.u mRNA | PNPLA2 LIVER | SREBF1 LIVER | SREBF1 OAT | SCD1 LIVER | SCD1 SAT | LEP OAT | LEPR LIVER | LEPR OAT | SOCS3 OAT |
|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 0.376 * | ns | ns | ns | ns | 0.331 * | ns | ns | ns |
| Waist circumference (cm) | 0.337 * | 0.348 * | −0.357 * | ns | ns | ns | ns | ns | ns |
| Waist: hip ratio | ns | 0.371 * | ns | ns | ns | ns | ns | ns | 0.363 * |
| Fat (%) | 0.420 * | ns | ns | ns | ns | ns | ns | ns | ns |
| Fat mass (kg) | 0.455 ** | 0.421 * | ns | 0.371 * | ns | ns | ns | 0.431 ** | ns |
| Insulin (mUI/mL) | ns | ns | ns | ns | ns | 0.516 ** | ns | ns | 0.464 ** |
| HOMA-IR | ns | ns | ns | ns | ns | 0.530 * | ns | ns | 0.409 * |
| AST (U/L) | ns | ns | ns | ns | −0.383 * | ns | ns | ns | 0.481 ** |
| ALT (U/L) | ns | ns | ns | ns | −0.332 * | ns | ns | ns | 0.513 ** |
| Leptin (ng/mL) | 0.667 * | ns | ns | 0.477 ** | ns | 0.350 * | 0.430* | ns | ns |
| OAT | LEP OAT | LEPR OAT | LEPR LIVER | SCD1 LIVER |
|---|---|---|---|---|
| LEPR | 0.692 * | ns | ns | ns |
| PNPLA2 | 0.351 * | ns | ns | ns |
| SCD1 | 0.488 ** | 0.417 ** | ns | ns |
| SOCS3 | 0.388 * | ns | ns | ns |
| LIVER | ||||
| PNPLA2 | ns | ns | 0.419 ** | 0.446 ** |
| SCD1 | ns | ns | 0.577 ** | ns |
| SREBF1 | ns | ns | 0.349 * | 0.654 * |
| SOCS3 | ns | ns | ns | 0.352 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Cruz-Color, L.; Dominguez-Rosales, J.A.; Maldonado-González, M.; Ruíz-Madrigal, B.; Sánchez Muñoz, M.P.; Zaragoza-Guerra, V.A.; Espinoza-Padilla, V.H.; Ruelas-Cinco, E.d.C.; Ramírez-Meza, S.M.; Torres Baranda, J.R.; et al. Evidence That Peripheral Leptin Resistance in Omental Adipose Tissue and Liver Correlates with MASLD in Humans. Int. J. Mol. Sci. 2024, 25, 6420. https://doi.org/10.3390/ijms25126420
De la Cruz-Color L, Dominguez-Rosales JA, Maldonado-González M, Ruíz-Madrigal B, Sánchez Muñoz MP, Zaragoza-Guerra VA, Espinoza-Padilla VH, Ruelas-Cinco EdC, Ramírez-Meza SM, Torres Baranda JR, et al. Evidence That Peripheral Leptin Resistance in Omental Adipose Tissue and Liver Correlates with MASLD in Humans. International Journal of Molecular Sciences. 2024; 25(12):6420. https://doi.org/10.3390/ijms25126420
Chicago/Turabian StyleDe la Cruz-Color, Lucia, Jose Alfredo Dominguez-Rosales, Montserrat Maldonado-González, Bertha Ruíz-Madrigal, Martha P. Sánchez Muñoz, Vianney Alejandrina Zaragoza-Guerra, Victor H. Espinoza-Padilla, Elizabeth del C. Ruelas-Cinco, Sandra M. Ramírez-Meza, José R. Torres Baranda, and et al. 2024. "Evidence That Peripheral Leptin Resistance in Omental Adipose Tissue and Liver Correlates with MASLD in Humans" International Journal of Molecular Sciences 25, no. 12: 6420. https://doi.org/10.3390/ijms25126420
APA StyleDe la Cruz-Color, L., Dominguez-Rosales, J. A., Maldonado-González, M., Ruíz-Madrigal, B., Sánchez Muñoz, M. P., Zaragoza-Guerra, V. A., Espinoza-Padilla, V. H., Ruelas-Cinco, E. d. C., Ramírez-Meza, S. M., Torres Baranda, J. R., González-Gutiérrez, M. d. R., & Hernandez Nazara, Z. H. (2024). Evidence That Peripheral Leptin Resistance in Omental Adipose Tissue and Liver Correlates with MASLD in Humans. International Journal of Molecular Sciences, 25(12), 6420. https://doi.org/10.3390/ijms25126420






