MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction
Abstract
:1. Introduction
2. Results
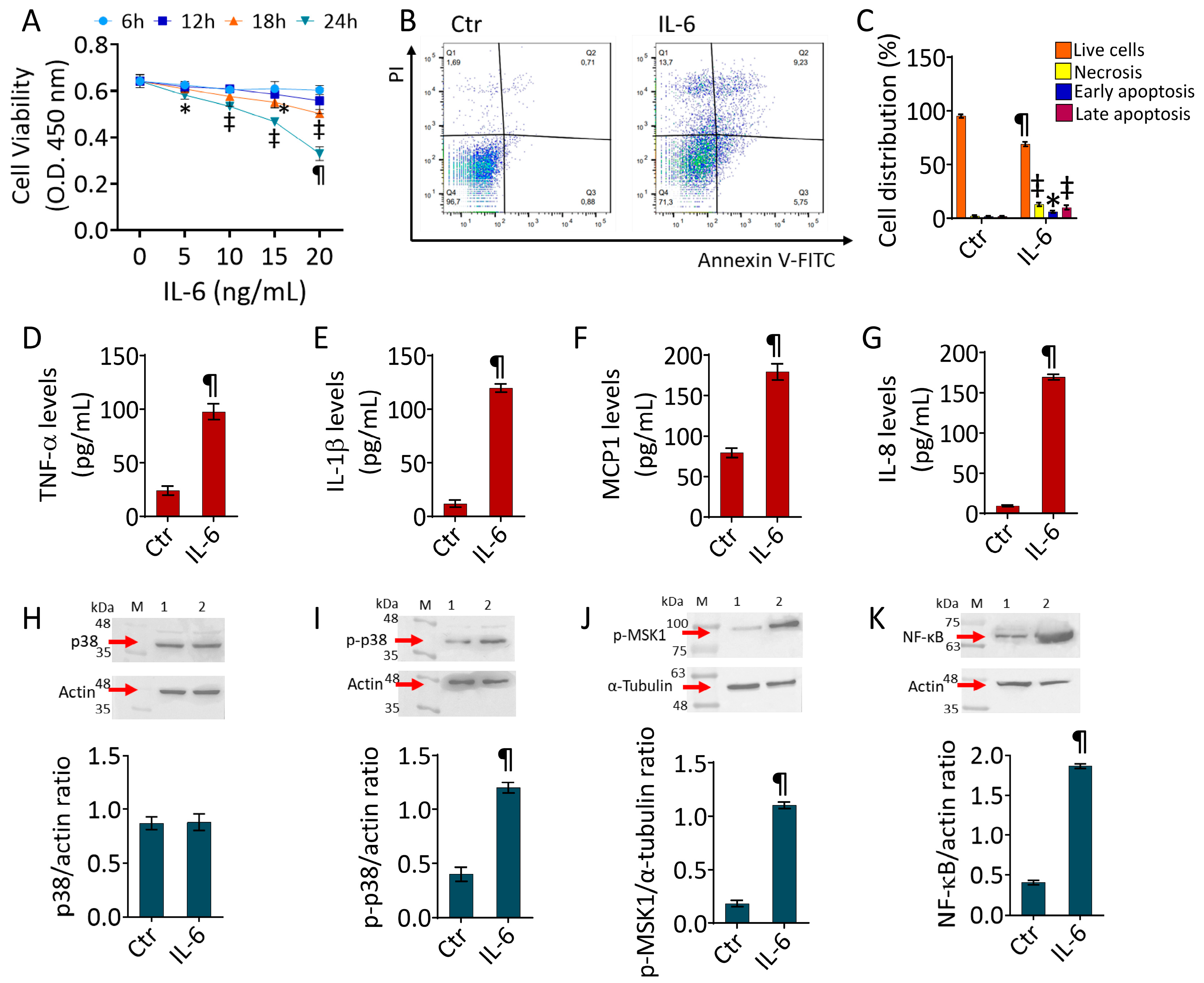
2.1. IL-6-Induced Inflammatory State
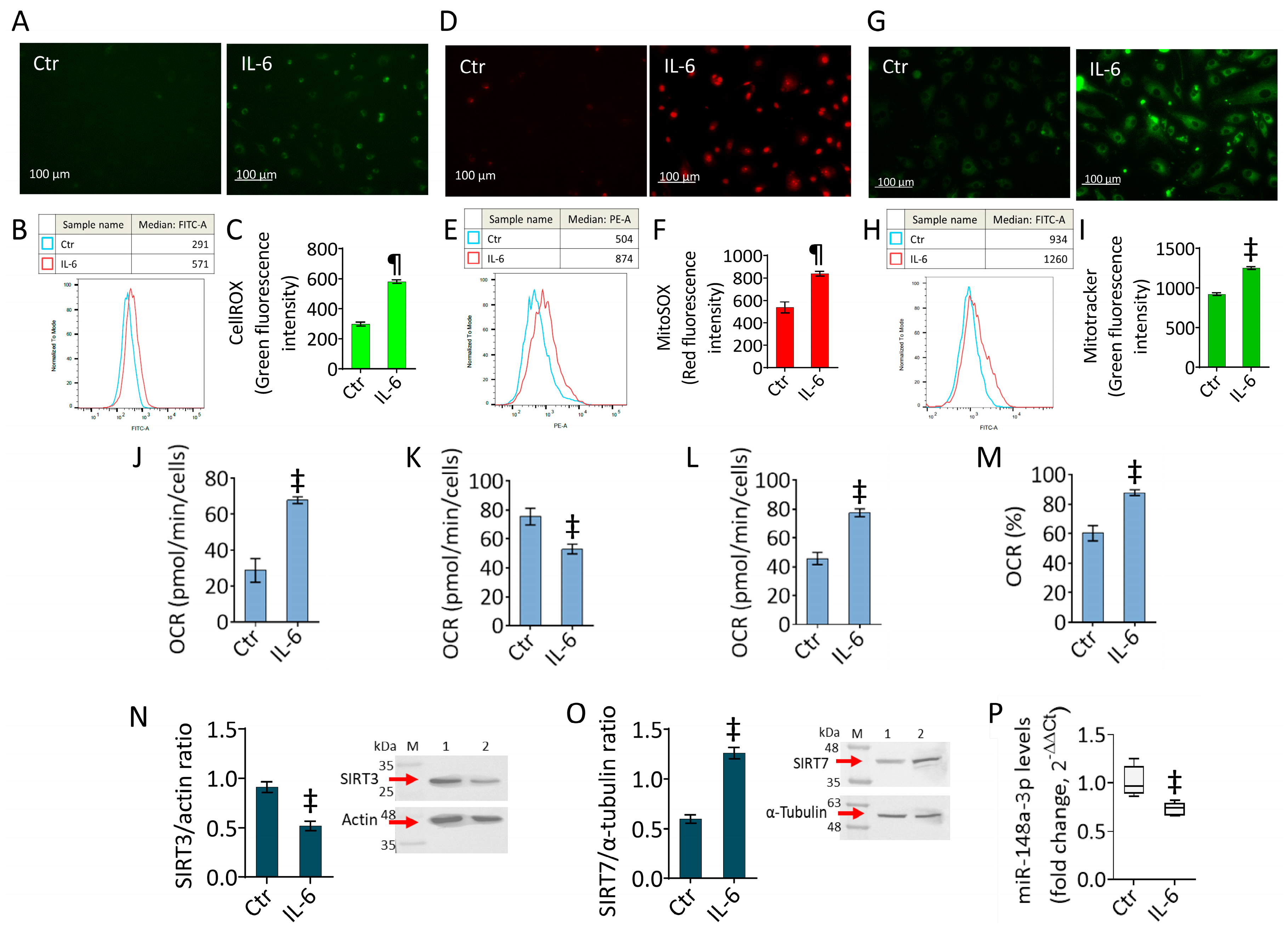
2.2. IL-6 Triggers Oxidative Stress and Mitochondrial Alteration
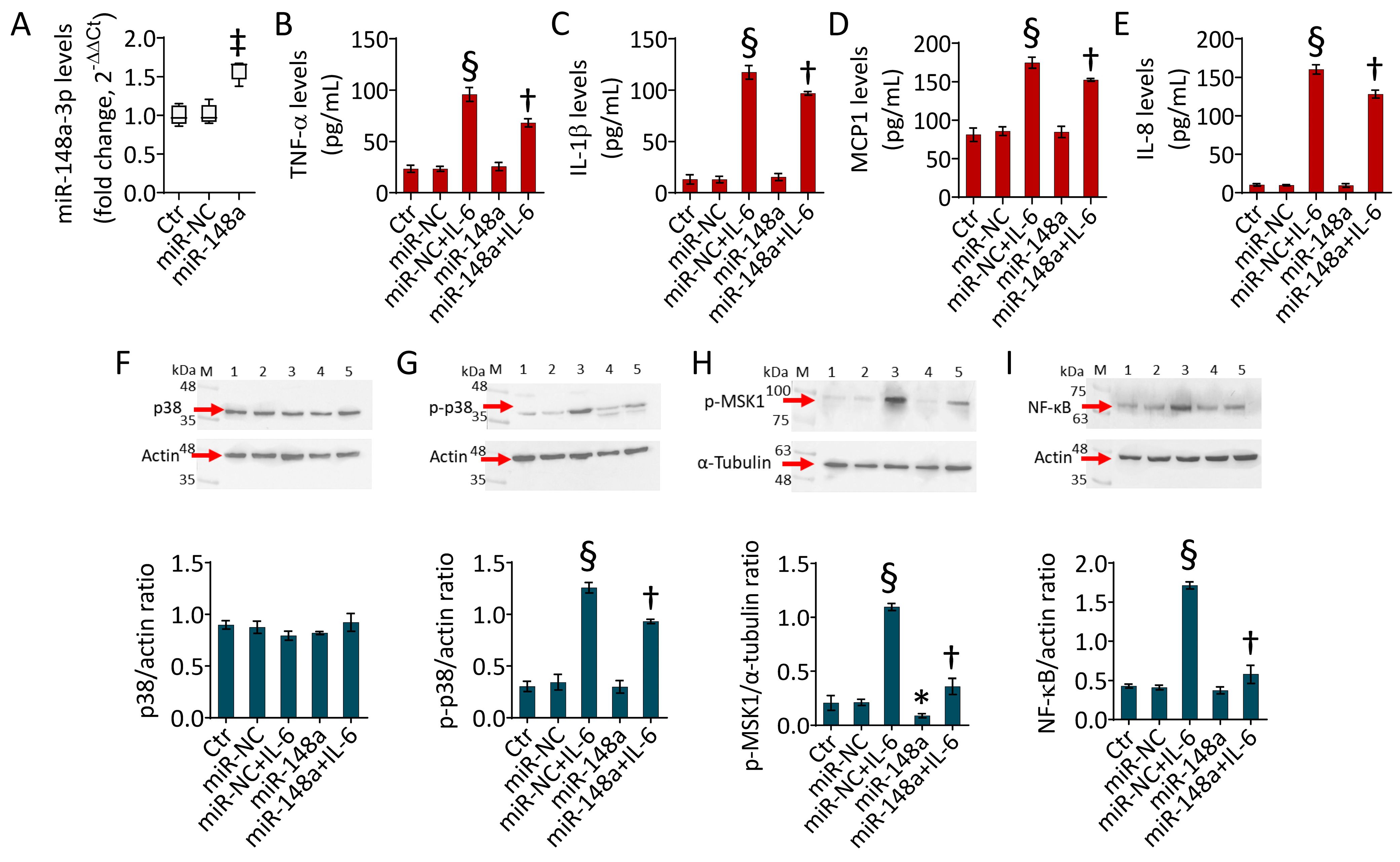
2.3. MiR-148a Opposes the IL-6-Induced Inflammatory State
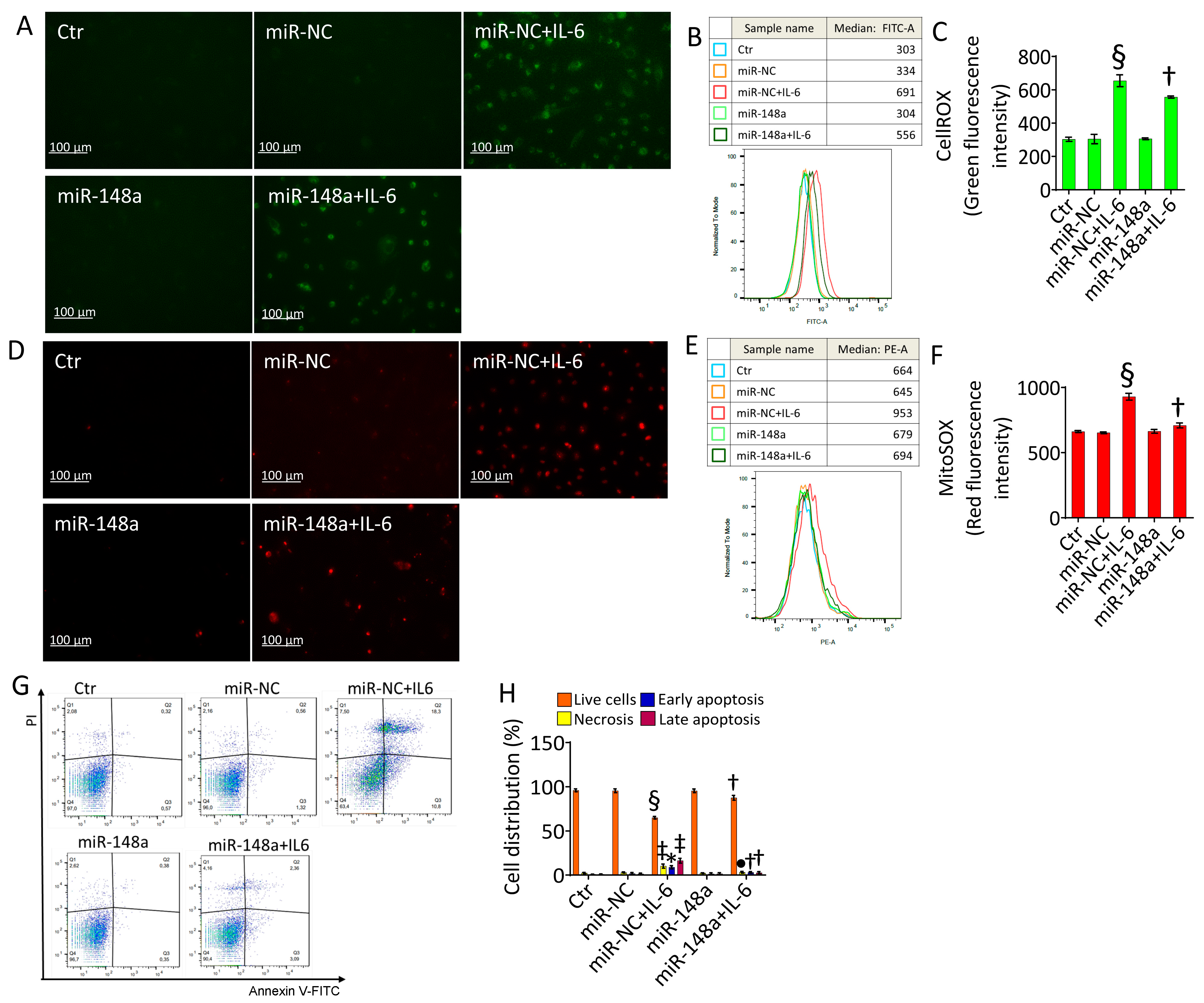
2.4. MiR-148a Ameliorates Oxidative Stress and Apoptosis Caused by IL-6
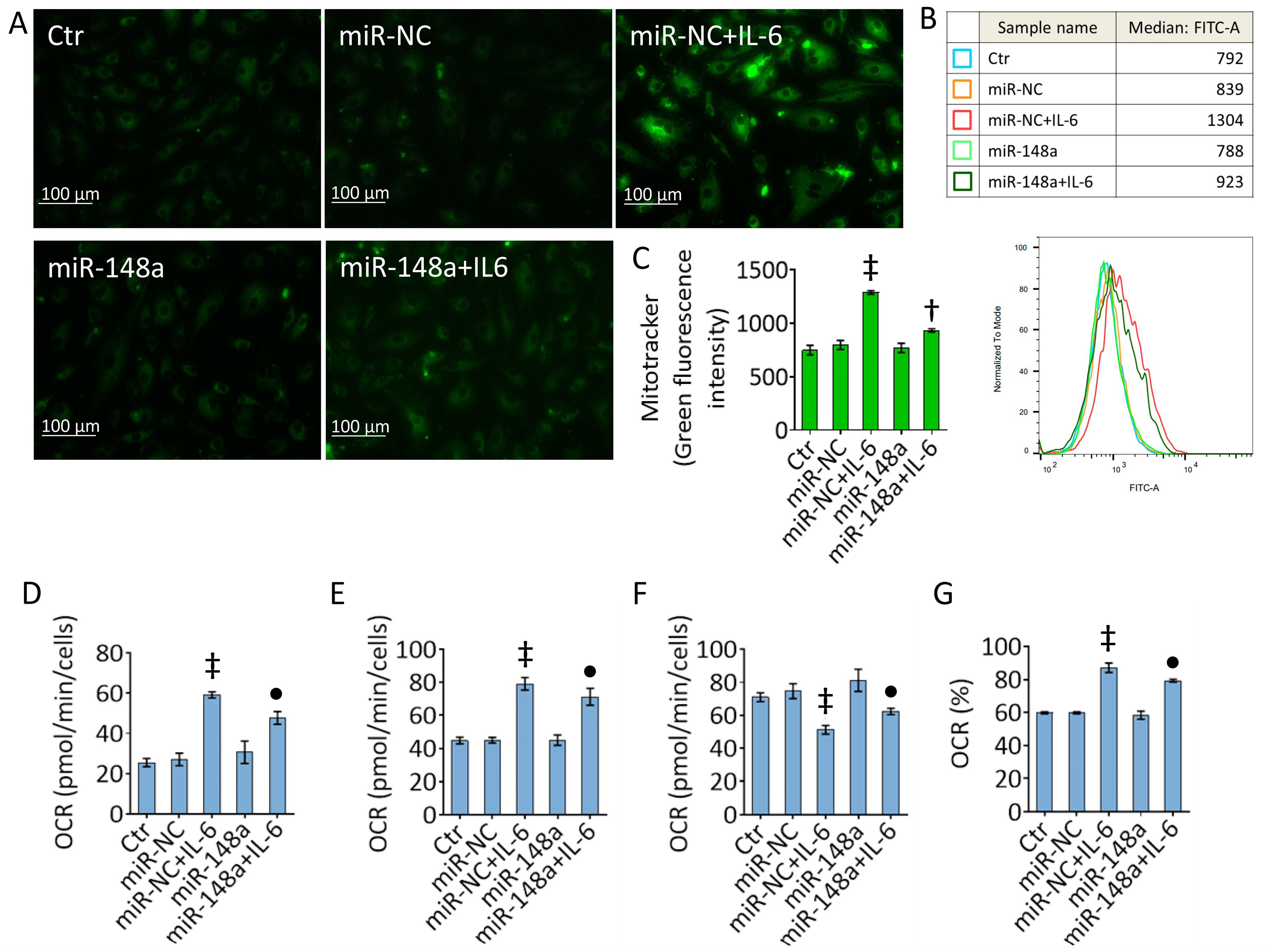
2.5. MiR-148a Decreases the IL-6 Effects on Mitochondrial Damage
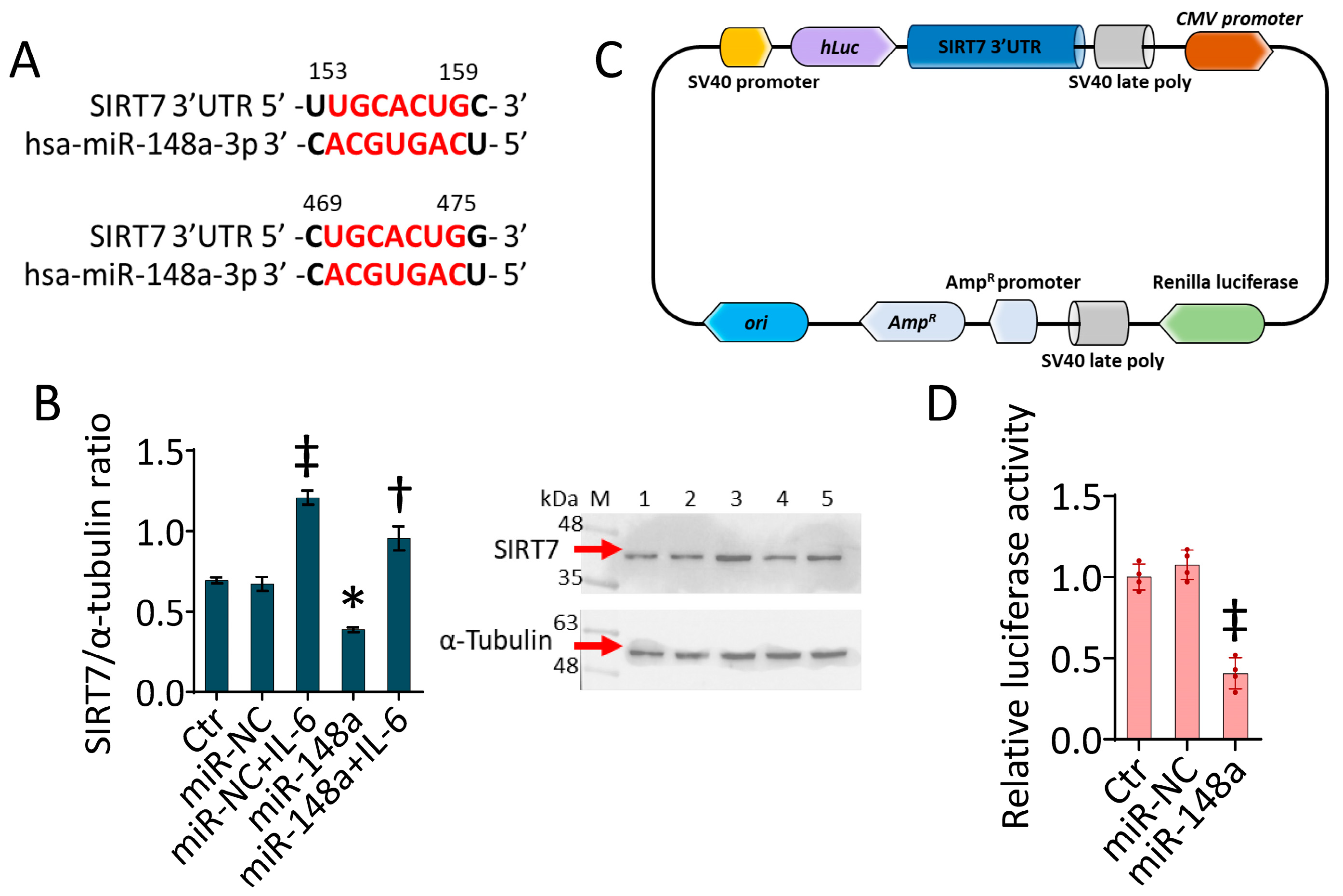
2.6. SIRT7 as a Target of miR-148a-3p
3. Discussion
4. Materials and Methods
4.1. Cell Growth, Treatment and Mimic Transfection
4.2. Cell Viability
4.3. Quantitative Real-Time PCR
4.4. Cytokine Levels
4.5. Oxidative Stress Detection
4.6. Mitochondrial Analysis
4.7. Apoptotic Cell Death
4.8. Protein Analysis by Immunoblotting
4.9. Bioinformatics Analysis
4.10. Luciferase Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, V.; Oikonomou, E.; Anastasiou, A.; Lampsas, S.; Zakynthinos, G.E.; Kalogeras, K.; Katsioupa, M.; Kapsali, M.; Kourampi, I.; Pesiridis, T.; et al. Molecular Mechanisms and Therapeutic Implications of Endothelial Dysfunction in Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 4321. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Ma, L.; Xu, Q.; Zhang, X.; Jin, C. Noncoding RNAs, Versatile regulators of endothelial dysfunction. Life Sci. 2023, 334, 122246. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Huang, X.Y.; Yu, H.Z.; Xue, Y.; Zhu, P.L. Circular RNA circ-RELL1 regulates inflammatory response by miR-6873-3p/MyD88/NF-κB axis in endothelial cells. Biochem. Biophys. Res. Commun. 2020, 525, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, J.; Zhang, Q.; Luo, Q.; Liu, B. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol. Res. 2021, 54, 11. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hu, J.; Zhang, A.; Li, F.; Li, X. miR-155 induces endothelial cell apoptosis and inflammatory response in atherosclerosis by regulating Bmal1. Exp. Ther. Med. 2020, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Yang, Y.; Ji, S.; Wang, S.; Peng, X.; Tian, C.; Sun, R.C.; Yu, T.; Chu, X.M. MicroRNA-302c-3p inhibits endothelial cell pyroptosis via directly targeting NOD-, LRR- and pyrin domain-containing protein 3 in atherosclerosis. J. Cell Mol. Med. 2021, 25, 4373–4386. [Google Scholar] [CrossRef]
- Li, P.; Zhong, X.; Li, J.; Liu, H.; Ma, X.; He, R.; Zhao, Y. MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated endothelial cell pyroptosis through FOXO3 down-regulation in atherosclerosis. Biochem. Biophys. Res. Commun. 2018, 503, 2833–2840. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, W.; Yin, W.; Wang, X.; Wang, J.; Zhu, X.; Xu, S. Circular RNA circ_0090231 promotes atherosclerosis in vitro by enhancing NLR family pyrin domain containing 3-mediated pyroptosis of endothelial cells. Bioengineered 2021, 12, 10837–10848. [Google Scholar] [CrossRef]
- Song, Y.; Yang, L.; Guo, R.; Lu, N.; Shi, Y.; Wang, X. Long noncoding RNA MALAT1 promotes high glucose-induced human endothelial cells pyroptosis by affecting NLRP3 expression through competitively binding miR-22. Biochem. Biophys. Res. Commun. 2019, 509, 359–366. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, Y.; Yin, Y.; Li, Q.; He, J.; Jing, Y.; Qi, Y.T.; Xu, Q.; Li, W.; Lu, B.; et al. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J. Mol. Cell Biol. 2013, 5, 3–13. [Google Scholar] [CrossRef]
- Goedeke, L.; Rotllan, N.; Canfrán-Duque, A.; Aranda, J.F.; Ramírez, C.M.; Araldi, E.; Lin, C.S.; Anderson, N.N.; Wagschal, A.; de Cabo, R.; et al. MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat. Med. 2015, 21, 1280–1289. [Google Scholar] [CrossRef]
- Shang, L.; Quan, A.; Sun, H.; Xu, Y.; Sun, G.; Cao, P. MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from Apoe deficient mice through restricting circular RNA 0003575. Gene 2019, 711, 143948. [Google Scholar] [CrossRef]
- Wang, F.; Ge, J.; Huang, S.; Zhou, C.; Sun, Z.; Song, Y.; Xu, Y.; Ji, Y. KLF5/LINC00346/miR-148a-3p axis regulates inflammation and endothelial cell injury in atherosclerosis. Int. J. Mol. Med. 2021, 48, 152. [Google Scholar] [CrossRef]
- Wang, K.; Huang, X.T.; Miao, Y.P.; Bai, X.L.; Jin, F. MiR-148a-3p attenuates apoptosis and inflammation by targeting CNTN4 in atherosclerosis. Ann. Transl. Med. 2022, 10, 1201. [Google Scholar] [CrossRef]
- Yin, M.; Lu, J.; Guo, Z.; Zhang, Y.; Liu, J.; Wu, T.; Guo, K.; Luo, T.; Guo, Z. Reduced SULT2B1b expression alleviates ox-LDL-induced inflammation by upregulating miR-148-3P via inhibiting the IKKβ/NF-κB pathway in macrophages. Aging 2021, 13, 3428–3442. [Google Scholar] [CrossRef]
- Bai, X.; Li, J.; Li, L.; Liu, M.; Liu, Y.; Cao, M.; Tao, K.; Xie, S.; Hu, D. Extracellular Vesicles From Adipose Tissue-Derived Stem Cells Affect Notch-miR148a-3p Axis to Regulate Polarization of Macrophages and Alleviate Sepsis in Mice. Front. Immunol. 2020, 11, 1391. [Google Scholar] [CrossRef]
- Martino, E.; D’Onofrio, N.; Balestrieri, A.; Mele, L.; Sardu, C.; Marfella, R.; Campanile, G.; Balestrieri, M.L. MiR-15b-5p and PCSK9 inhibition reduces lipopolysaccharide-induced endothelial dysfunction by targeting SIRT4. Cell Mol. Biol. Lett. 2023, 28, 66. [Google Scholar] [CrossRef]
- Tang, P.; Wang, Y.; Yang, X.; Wu, Z.; Chen, W.; Ye, Y.; Jiang, Y.; Lin, L.; Lin, B.; Lin, B. Protective Role of Endothelial SIRT1 in Deep Vein Thrombosis and Hypoxia-induced Endothelial Dysfunction Mediated by NF-κB Deacetylation. Inflammation 2023, 46, 1887–1900. [Google Scholar] [CrossRef]
- Cao, X.; Wu, V.W.Y.; Han, Y.; Hong, H.; Wu, Y.; Kong, A.P.S.; Lui, K.O.; Tian, X.Y. Role of Argininosuccinate Synthase 1 -Dependent L-Arginine Biosynthesis in the Protective Effect of Endothelial Sirtuin 3 Against Atherosclerosis. Adv. Sci. 2024, 11, e2307256. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Kim, N.; Herman, A.B.; Gorospe, M.; Lee, J.S. Factors and Pathways Modulating Endothelial Cell Senescence in Vascular Aging. Int. J. Mol. Sci. 2022, 23, 10135. [Google Scholar] [CrossRef] [PubMed]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front. physiol 2021, 12, 733696. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; He, J.; Wang, S.; Wang, J.; Liu, J.; Wang, Y. Molecular mechanisms of endothelial dysfunction in coronary microcirculation dysfunction. J. Thromb. Thrombolysis 2023, 56, 388–397. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Prattichizzo, F.; Marfella, R.; Sardu, C.; Martino, E.; Scisciola, L.; Marfella, L.; Grotta, R.; Frigé, C.; Paolisso, G.; et al. SIRT3 mediates the effects of PCSK9 inhibitors on inflammation, autophagy, and oxidative stress in endothelial cells. Theranostics 2023, 13, 531–542. [Google Scholar] [CrossRef]
- Martino, E.; Balestrieri, A.; Anastasio, C.; Maione, M.; Mele, L.; Cautela, D.; Campanile, G.; Balestrieri, M.L.; D’Onofrio, N. SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance. Antioxidants 2022, 11, 1611. [Google Scholar] [CrossRef]
- He, X.; Zeng, H.; Chen, J.X. Emerging role of SIRT3 in endothelial metabolism, angiogenesis, and cardiovascular disease. J. Cell. Physiol. 2019, 234, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Huang, C.; Huang, Y.; Hong, L.; Wang, H.; Zhou, Z.; Qiu, Y. SIRT4 suppresses inflammatory responses in human umbilical vein endothelial cells. Cardiovasc. Toxicol. 2015, 15, 217–223. [Google Scholar] [CrossRef]
- Liberale, L.; Akhmedov, A.; Vlachogiannis, N.I.; Bonetti, N.R.; Nageswaran, V.; Miranda, M.X.; Puspitasari, Y.M.; Schwarz, L.; Costantino, S.; Paneni, F.; et al. Sirtuin 5 promotes arterial thrombosis by blunting the fibrinolytic system. Cardiovasc. Res. 2021, 117, 2275–2288. [Google Scholar] [CrossRef]
- Diaz-Cañestro, C.; Merlini, M.; Bonetti, N.R.; Liberale, L.; Wüst, P.; Briand-Schumacher, S.; Klohs, J.; Costantino, S.; Miranda, M.; Schoedon-Geiser, G.; et al. Sirtuin 5 as a novel target to blunt blood-brain barrier damage induced by cerebral ischemia/reperfusion injury. Int. J. Cardiol. 2018, 260, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, C.; Tang, X.; Sun, S.; Liu, S.; Yang, L.; Chen, Y.; Yang, Q.; Wei, T.W.; Wu, X.; et al. Endothelium-specific SIRT7 targeting ameliorates pulmonary hypertension through KLF4 deacetylation. Cardiovasc. Res. 2024, 120, cvae011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Yang, X.; Lai, P.; Mou, Y.; Deng, J.; Li, X.; Wang, H.; Liu, X.; Zhou, L.; et al. H2O2 down-regulates SIRT7’s protective role of endothelial premature dysfunction via microRNA-335-5p. Biosci. Rep. 2022, 42, BSR20211775. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, X.; Zhang, S.; Deng, Z.; Wang, B.; Shi, W.; Xie, H.; Liu, B.; Li, J. Aging-Conferred SIRT7 Decline Inhibits Rosacea-Like Skin Inflammation by Modulating Toll-Like Receptor 2-NF-κB Signaling. J. Invest. Dermatol. 2022, 142, 2580–2590.e6. [Google Scholar] [CrossRef] [PubMed]
- Wyman, A.E.; Nguyen, T.T.T.; Karki, P.; Tulapurkar, M.E.; Zhang, C.O.; Kim, J.; Feng, T.G.; Dabo, A.J.; Todd, N.W.; Luzina, I.G.; et al. SIRT7 deficiency suppresses inflammation, induces EndoMT, and increases vascular permeability in primary pulmonary endothelial cells. Sci. Rep. 2020, 10, 12497. [Google Scholar] [CrossRef]
- Vigili de Kreutzenberg, S.; Giannella, A.; Ceolotto, G.; Faggin, E.; Cappellari, R.; Mazzucato, M.; Fraccaro, C.; Tarantini, G.; Avogaro, A.; Fadini, G.P. A miR-125/Sirtuin-7 pathway drives the pro-calcific potential of myeloid cells in diabetic vascular disease. Diabetologia 2022, 65, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Li, M.; Jiang, X.; Huang, X.; Gu, S.; Ye, J.; Zhu, L.; Hou, M.; Zan, T. Young fibroblast-derived exosomal microRNA-125b transfers beneficial effects on aged cutaneous wound healing. J. Nanobiotechnology 2022, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Zhang, S.; Wu, S.; Zhu, Q.; Li, W. MiR-770 promotes oral squamous cell carcinoma migration and invasion by regulating the Sirt7/Smad4 pathway. Iubmb Life 2021, 73, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, H.; Yuan, J.; Qiao, L.; Dong, L.; Wang, Y. Downregulation of circular RNA circPVT1 restricts cell growth of hepatocellular carcinoma through downregulation of Sirtuin 7 via microRNA-3666. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1291–1300. [Google Scholar] [CrossRef]
- Gupta, L.; Thomas, J.; Ravichandran, R.; Singh, M.; Nag, A.; Panjiyar, B.K. Inflammation in Cardiovascular Disease: A Comprehensive Review of Biomarkers and Therapeutic Targets. Cureus 2023, 15, e45483. [Google Scholar] [CrossRef]
- Abubakar, M.; Rasool, H.F.; Javed, I.; Raza, S.; Abang, L.; Hashim, M.M.A.; Saleem, Z.; Abdullah, R.M.; Faraz, M.A.; Hassan, K.M.; et al. Comparative Roles of IL-1, IL-6, IL-10, IL-17, IL-18, 1L-22, IL-33, and IL-37 in Various Cardiovascular Diseases With Potential Insights for Targeted Immunotherapy. Cureus 2023, 15, e42494. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, M.M.; Lindkvist, M.; Fälker, K.; Kumawat, A.K.; Paramel, G.; Grenegård, M.; Sirsjö, A.; Ljungberg, L.U. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ye, D.; Wang, Z.; Pan, H.; Lu, X.; Wang, M.; Xu, Y.; Yu, J.; Zhang, J.; Zhao, M.; et al. The Role of Interleukin-6 Family Members in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 818890. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Khan, M.A.; Klip, I.T.; Meyer, S.; de Boer, R.A.; Jaarsma, T.; Hillege, H.; van Veldhuisen, D.J.; van der Meer, P.; Voors, A.A. Biomarker Profiles in Heart Failure Patients with Preserved and Reduced Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e003989. [Google Scholar] [CrossRef] [PubMed]
- Razaghizad, A.; Aziz, H.; Zhang, G.K.; Ferreira, J.P.; White, W.B.; Mehta, C.R.; Bakris, G.L.; Zannad, F.; Sharma, A. Pathophysiological Sex Differences in Heart Failure Progression After Acute Coronary Syndrome, Insights From the EXAMINE Trial. J. Card. Fail. 2023, 7:S1071-9164(23)00862-X. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, S.; Codina-Fauteux, V.A.; de Bellefon, S.M.; Leblanc, F.; Beaudoin, M.; Simon, M.M.; Dali, R.; Kwan, T.; Lo, K.S.; Pastinen, T.; et al. Integrative analysis of vascular endothelial cell genomic features identifies AIDA as a coronary artery disease candidate gene. Genome Biol. 2019, 20, 133. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Prattichizzo, F.; Martino, E.; Anastasio, C.; Mele, L.; La Grotta, R.; Sardu, C.; Ceriello, A.; Marfella, R.; Paolisso, G.; et al. MiR-27b attenuates mitochondrial oxidative stress and inflammation in endothelial cells. Redox Biol. 2023, 62, 102681. [Google Scholar] [CrossRef]
- Syed, N.H.; Mussa, A.; Elmi, A.H.; Jamal Al-Khreisat, M.; Ahmad Mohd Zain, M.R.; Nurul, A.A. Role of MicroRNAs in Inflammatory Joint Diseases, A Review. Immunol. Invest. 2023, 53, 185–209. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Hong, L.; Shao, L.; Lai, H.; Zhu, F.; Lan, J. Downregulation of MicroRNA-29-3p Following Percutaneous Coronary Intervention, An Implication of YY1/IRAK1 Pathway in the Post-Vascular Injury Inflammation. Acta Cardiol. Sin. 2023, 39, 742–754. [Google Scholar]
- Zhu, H.; Liang, H.; Gao, Z.; Zhang, X.; He, Q.; He, C.; Cai, C.; Chen, J. MiR-483-5p downregulation alleviates ox-LDL induced endothelial cell injury in atherosclerosis. BMC Cardiovasc. Disord. 2023, 23, 521. [Google Scholar] [CrossRef]
- Ohta, M.; Kihara, T.; Toriuchi, K.; Aoki, H.; Iwaki, S.; Kakita, H.; Yamada, Y.; Aoyama, M. IL-6 promotes cell adhesion in human endothelial cells via microRNA-126-3p suppression. Exp. Cell Res. 2020, 393, 112094. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.; Vermeulen, L.; Haegeman, G.; Frossard, N. Ser276 phosphorylation of NF-kB p65 by MSK1 controls SCF expression in inflammation. PLoS ONE. 2009, 4, e4393. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Forteath, C.; Hussain, M.S.; Reyskens, K.; Belch, J.J.F.; Lang, C.C.; Mordi, I.R.; Bhalraam, U.; Arthur, J.S.C.; Khan, F. Mitogen and Stress-Activated Kinases 1 and 2 Mediate Endothelial Dysfunction. Int. J. Mol. Sci. 2021, 22, 8655. [Google Scholar] [CrossRef] [PubMed]
- Matuszcak, C.; Lindner, K.M.; Eichelmann, A.K.; Hussey, D.J.; Haier, J.; Hummel, R. microRNAs, Key regulators of chemotherapy response and metastatic potential via complex control of target pathways in esophageal adenocarcinoma. Surg. Oncol. 2018, 27, 392–401. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Q.; Sun, X.; Wang, Q.; Zou, G.; Wang, D.; Zhuang, B.; Juan, Z.; Zhang, R.; Zhang, D. Therapeutic Effects of Salvianolic Acid B on Angiotensin II-Induced Atrial Fibrosis by Regulating Atrium Metabolism via Targeting AMPK/FoxO1/miR-148a-3p Axis. J. Cardiovasc. Transl. Res. 2023, 16, 341–357. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Jiang, L.; Mo, C.; Luo, M.; Hu, K. circFTO upregulates transforming growth factor-alpha through sponging miR-148a-3p to regulate high glucose-induced ARPE-19 cells injury. Bioengineered 2022, 13, 11489–11502. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, L.; Liu, J.; Zhu, T.; Xie, Z.; Sun, X.; Zhang, H. Metformin Protects against Oxidative Stress Injury Induced by Ischemia/Reperfusion via Regulation of the lncRNA-H19/miR-148a-3p/Rock2 Axis. Oxid. Med. Cell Longev. 2019, 2019, 8768327. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Benavides, G.A.; Mitchell, T.; Dranka, B.P.; Ferrick, D.; Singal, A.K.; Ballinger, S.W.; Bailey, S.M.; et al. The Bioenergetic Health Index: A new concept in mitochondrial translational research. Clin. Sci. 2014, 127, 367–373. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Li, Z.; Chen, Z.; Zhi, X.; Xu, J.; Li, Q.; Wang, L.; Huang, X.; Wang, L.; et al. MicroRNA-148a-3p enhances cisplatin cytotoxicity in gastric cancer through mitochondrial fission induction and cyto-protective autophagy suppression. Cancer Lett. 2017, 410, 212–227. [Google Scholar] [CrossRef]
- Martino, E.; Balestrieri, A.; Aragona, F.; Bifulco, G.; Mele, L.; Campanile, G.; Balestrieri, M.L.; D’Onofrio, N. MiR-148a-3p Promotes Colorectal Cancer Cell Ferroptosis by Targeting SLC7A11. Cancers 2023, 15, 4342. [Google Scholar] [CrossRef]
- Miyasato, Y.; Yoshizawa, T.; Sato, Y.; Nakagawa, T.; Miyasato, Y.; Kakizoe, Y.; Kuwabara, T.; Adachi, M.; Ianni, A.; Braun, T.; et al. Sirtuin 7 Deficiency Ameliorates Cisplatin-induced Acute Kidney Injury Through Regulation of the Inflammatory Response. Sci. Rep. 2018, 8, 5927. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhu, Y.; Han, H.; Zhang, Q.; Cui, K.; Shen, H.; Zhang, J.; Yan, J.; Prochownik, E.; Li, Y. MicroRNA-148a deficiency promotes hepatic lipid metabolism and hepatocarcinogenesis in mice. Cell Death Dis. 2017, 8, e2916. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Balestrieri, A.; Mele, L.; Sardu, C.; Marfella, R.; D’Onofrio, N.; Campanile, G.; Balestrieri, M.L. Milk Exosomal miR-27b Worsen Endoplasmic Reticulum Stress Mediated Colorectal Cancer Cell Death. Nutrients 2022, 14, 5081. [Google Scholar] [CrossRef]
- Martino, E.; Luce, A.; Balestrieri, A.; Mele, L.; Anastasio, C.; D’Onofrio, N.; Balestrieri, M.L.; Campanile, G. Whey Improves In Vitro Endothelial Mitochondrial Function and Metabolic Redox Status in Diabetic State. Antioxidants 2023, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasio, C.; Donisi, I.; Colloca, A.; D’Onofrio, N.; Balestrieri, M.L. MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction. Int. J. Mol. Sci. 2024, 25, 5087. https://doi.org/10.3390/ijms25105087
Anastasio C, Donisi I, Colloca A, D’Onofrio N, Balestrieri ML. MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction. International Journal of Molecular Sciences. 2024; 25(10):5087. https://doi.org/10.3390/ijms25105087
Chicago/Turabian StyleAnastasio, Camilla, Isabella Donisi, Antonino Colloca, Nunzia D’Onofrio, and Maria Luisa Balestrieri. 2024. "MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction" International Journal of Molecular Sciences 25, no. 10: 5087. https://doi.org/10.3390/ijms25105087
APA StyleAnastasio, C., Donisi, I., Colloca, A., D’Onofrio, N., & Balestrieri, M. L. (2024). MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction. International Journal of Molecular Sciences, 25(10), 5087. https://doi.org/10.3390/ijms25105087







