Long Non-Coding RNAs in Sjögren’s Disease
Abstract
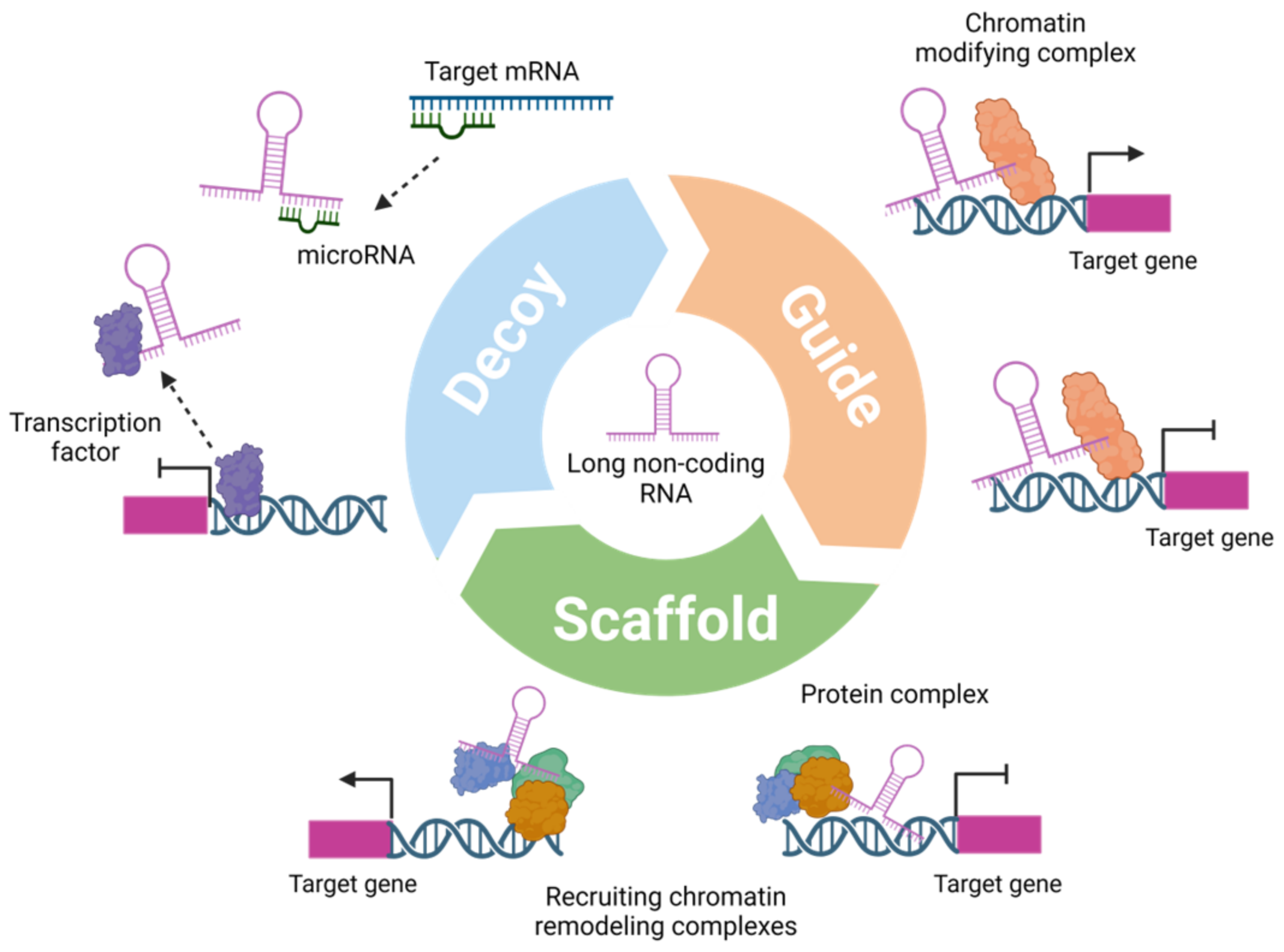
:1. Introduction
2. Skewed X Chromosomal Inactivation
3. Long, Non-Coding RNAs Link Genetic Risk to Cell-Type Specific Function
4. Long, Non-Coding RNAs as Biomarkers
5. Immunomodulatory Properties of Long, Non-Coding RNAs
6. Conclusions
| lcnRNA | NONCODE Gene ID | Origin | Method | No. of Patients (Discovery Cohort) | Validation Method | No. of Patients (Validation Cohort) | Up- or Downregulation (SjD versus Control) | Reference |
|---|---|---|---|---|---|---|---|---|
| lnc-DC | n.a. | blood plasma | qPCR | 149 pSjD, 50 SLE, 50 RA, 109 HC | n.v. | up | [60] | |
| LINC01871 | NONHSAG026917.2 | whole blood | RNA-seq | 50 SjD § (27 Ro+, 23 Ro−), 27 HC | qPCR | 22 SjD (14 Ro+, 8 Ro−), 24 HC | up in SjD (Ro+ and Ro−) | [61] |
| NRIR | NONHSAT068918.2 | up in SjD Ro+ | ||||||
| OAS123-AS1 | n.a. | |||||||
| MX1-AS1 | n.a. | |||||||
| GBP5-AS1 | n.a. | |||||||
| TPTEP1-202 | n.a. | PBMCs | RNA-seq | 5 pSjD, 5 HC | qPCR | 16 pSjD, 6 HC | down | [72] |
| LINC00426 | NONHSAG013150.2 | up | ||||||
| CYTOR | NONHSAG077527.2 | |||||||
| NRIR | NONHSAG026902.2 | |||||||
| BISPR | NONHSAG025088.3 | |||||||
| LINC00657 | NONHSAG031696.3 | PBMCs | microarray analysis | 8 pSjD, 8 HC | qPCR | n.a. | down | [70] |
| LINC00511 | NONHSAG022655.3 | up up | ||||||
| CTD-2020K17.1 | n.a. | |||||||
| GABPB1-AS1 | NONHSAG016861.3 | PBMCs | RNA-seq | 4 pSjD, 4 HC | qPCR | 30 pSjD, 15 HC | up | [71] |
| PSMA3-AS1 | NONHSAG015097.3 | |||||||
| TMEVPG1 (NeST, IFN-γ-AS1) | NONHSAG011599.2 | CD4+ T cells | qPCR | 20 pSjD, 10 HC | n.v. | up | [76] | |
| NEAT1 | NONHSAG008670.3 | CD4+ T cells | qPCR | 20 pSjD, 10 HC | n.v. | up | [74] | |
| PVT1 | n.a. | CD4+ T cells | qPCR | 25 SjD, 25 HC | n.v. | up | [73] | |
| LINC00487 | NONHSAG026900.2 | B-cells | microarray analysis | 6 pSjD, 6 HC | qPCR | 14 pSjD, 12 HC | up | [78] |
| CTA-250D10.23 | n.a. | parotid gland | microarray analysis | 19 pSjD, 20 HC, 20 nSS | microarray analysis (labial SG) * | up | [68] | |
| KIAA0125 | n.a. | |||||||
| LOC100505812 | n.a. | |||||||
| BZRAP1-AS1 | n.a. | |||||||
| LINC01215 | NONHSAT194282.1 | |||||||
| PSMB8-AS1 | NONHSAT108940.2 | |||||||
| ITGB2-AS1 | NONHSAT082896.2 | |||||||
| LOC100505549 | n.a. | |||||||
| LOC101929272 | n.a. | |||||||
| ENST00000420219.1 | n.a. | labial SG | microarray analysis | 4 pSjD, 4 C (mucocele excision) | qPCR | 30 pSjD, 16 C (mucocele excision) | up | [67] |
| ENST00000455309.1 | NONHSAG028948.3 | |||||||
| NR_002712 | n.a. | |||||||
| ENST00000546086.1 | NONHSAG011610.3 | |||||||
| n340599 | NONHSAG041352.2 | |||||||
| TCONS_l2_00014794 | n.a. | |||||||
| n336161 | n.a. | |||||||
| lnc-UTS2D-1:1 | n.a. |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zou, Y.; Xu, H. Involvement of long noncoding RNAs in the pathogenesis of autoimmune diseases. J. Transl. Autoimmun. 2020, 3, 100044. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2023, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Uszczynska-Ratajczak, B.; Lagarde, J.; Frankish, A.; Guigó, R.; Johnson, R. Towards a complete map of the human long non-coding RNA transcriptome. Nat. Rev. Genet. 2018, 19, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, L.; Chen, L.L. The Diversity of Long Noncoding RNAs and Their Generation. Trends Genet. 2017, 33, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Ramilowski, J.A.; Yip, C.W.; Agrawal, S.; Chang, J.C.; Ciani, Y.; Kulakovskiy, I.V.; Mendez, M.; Ooi, J.L.C.; Ouyang, J.F.; Parkinson, N.; et al. Functional annotation of human long noncoding RNAs via molecular phenotyping. Genome Res. 2020, 30, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.; Li, Y.; Song, T.; Wu, Y.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Pei, D.; et al. NONCODEV6: An updated database dedicated to long non-coding RNA annotation in both animals and plants. Nucleic Acids Res. 2021, 49, D165–D171. [Google Scholar] [CrossRef]
- RNAcentral Consortium; A Sweeney, B.; I Petrov, A.; E Ribas, C.; Finn, R.D.; Bateman, A.; Szymanski, M.; Karlowski, W.M.; E Seemann, S.; Gorodkin, J.; et al. Rnacentral 2021: Secondary Structure Integration, Improved Sequence Search and New Member Databases. Nucleic Acids Res. 2021, 49, D212–D220. [Google Scholar]
- Li, Z.; Liu, L.; Feng, C.; Qin, Y.; Xiao, J.; Zhang, Z.; Ma, L. Lncbook 2.0: Integrating Human Long Non-Coding Rnas with Multi-Omics Annotations. Nucleic Acids Res. 2023, 51, D186–D191. [Google Scholar] [CrossRef]
- Geisler, S.; Coller, J. Rna in Unexpected Places: Long Non-Coding Rna Functions in Diverse Cellular Contexts. Nat. Rev. Mol. Cell Biol. 2013, 14, 699–712. [Google Scholar] [CrossRef]
- Romero-Barrios, N.; Legascue, M.F.; Benhamed, M.; Ariel, F.; Crespi, M. Splicing Regulation by Long Noncoding Rnas. Nucleic Acids Res. 2018, 46, 2169–2184. [Google Scholar] [CrossRef]
- Yoon, J.H.; Abdelmohsen, K.; Kim, J.; Yang, X.; Martindale, J.L.; Tominaga-Yamanaka, K.; White, E.J.; Orjalo, A.V.; Rinn, J.L.; Kreft, S.G.; et al. Scaffold Function of Long Non-Coding Rna Hotair in Protein Ubiquitination. Nat. Commun. 2013, 4, 2939. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Long, W.; Yang, L.; Zhao, Y.; Wu, X.; Li, M.; Du, F.; Chen, Y.; Yang, Z.; Wen, Q.; et al. Functional Peptides Encoded by Long Non-Coding RNAs in Gastrointestinal Cancer. Front. Oncol. 2021, 11, 777374. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liu, H.; Jiang, W.; Wang, L. LncRNA-Encoded Peptide: Functions and Predicting Methods. Front. Oncol. 2020, 10, 622294. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lim, K.H.; Kim, S.H.; Joo, J.Y. Molecular landscape of long noncoding RNAs in brain disorders. Mol. Psychiatry 2021, 26, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Mably, J.D.; Wang, D.Z. Long non-coding RNAs in cardiac hypertrophy and heart failure: Functions, mechanisms and clinical prospects. Nat. Rev. Cardiol. 2023, 21, 326–345. [Google Scholar] [CrossRef]
- Alam, T.; Agrawal, S.; Severin, J.; Young, R.S.; Andersson, R.; Arner, E.; Hasegawa, A.; Lizio, M.; Ramilowski, J.A.; Abugessaisa, I.; et al. Comparative transcriptomics of primary cells in vertebrates. Genome Res. 2020, 30, 951–961. [Google Scholar] [CrossRef]
- Carninci, P.; Kasukawa, T.; Katayama, S.; Gough, J.; Frith, M.C.; Maeda, N.; Oyama, R.; Ravasi, T.; Lenhard, B.; Wells, C.; et al. The transcriptional landscape of the mammalian genome. Science 2005, 309, 1559–1563. [Google Scholar] [CrossRef]
- Cheng, J.; Kapranov, P.; Drenkow, J.; Dike, S.; Brubaker, S.; Patel, S.; Long, J.; Stern, D.; Tammana, H.; Helt, G.; et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science 2005, 308, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Chung, H.; Basu, U. Regulation of long non-coding RNAs and genome dynamics by the RNA surveillance machinery. Nat. Rev. Mol. Cell Biol. 2020, 21, 123–136. [Google Scholar] [CrossRef]
- Beydon, M.; McCoy, S.; Nguyen, Y.; Sumida, T.; Mariette, X.; Seror, R. Epidemiology of Sjogren syndrome. Nat. Rev. Rheumatol. 2024, 20, 158–169. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Wang, B.; Chen, S.; Zheng, Q.; Li, Y.; Zhang, X.; Xuan, J.; Liu, Y.; Shi, G. Early diagnosis and treatment for Sjogren’s syndrome: Current challenges, redefined disease stages and future prospects. J. Autoimmun. 2021, 117, 102590. [Google Scholar] [CrossRef]
- Khatri, B.; Tessneer, K.L.; Rasmussen, A.; Aghakhanian, F.; Reksten, T.R.; Adler, A.; Alevizos, I.; Anaya, J.-M.; Aqrawi, L.A.; Baecklund, E.; et al. Genome-wide association study identifies Sjogren’s risk loci with functional implications in immune and glandular cells. Nat. Commun. 2022, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Shimizu, T.; Kawakami, A. Role of Viral Infections in the Pathogenesis of Sjogren’s Syndrome: Different Characteristics of Epstein-Barr Virus and HTLV-1. J. Clin. Med. 2020, 9, 1459. [Google Scholar] [CrossRef] [PubMed]
- Toussirot, E.; Roudier, J. Epstein-Barr virus in autoimmune diseases. Best. Pract. Res. Clin. Rheumatol. 2008, 22, 883–896. [Google Scholar] [CrossRef]
- Manganelli, P.; Fietta, P. Apoptosis and Sjogren syndrome. Semin. Arthritis Rheum. 2003, 33, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Polihronis, M.; Tapinos, N.I.; Theocharis, S.E.; Economou, A.; Kittas, C.; Moutsopoulos, H.M. Modes of epithelial cell death and repair in Sjogren’s syndrome (SS). Clin. Exp. Immunol. 1998, 114, 485–490. [Google Scholar] [CrossRef]
- Jonsson, R.; Theander, E.; Sjöström, B.; Brokstad, K.; Henriksson, G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA 2013, 310, 1854–1855. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Pringle, S.; Bootsma, H.; Kroese, F.G.M. Epithelial-immune cell interplay in primary Sjogren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 2021, 17, 333–348. [Google Scholar] [CrossRef]
- Nocturne, G.; Mariette, X. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. [Google Scholar] [CrossRef]
- Mariette, X.; Criswell, L.A. Primary Sjogren’s Syndrome. N. Engl. J. Med. 2018, 379, 97. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, T.; Yu, X.; Xue, Z.; Shen, N. The role of long non-coding RNAs in rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E. The importance of sex. Nature 2021, 595, S51–S53. [Google Scholar] [CrossRef]
- Liu, K.; Kurien, B.T.; Zimmerman, S.L.; Kaufman, K.M.; Taft, D.H.; Kottyan, L.C.; Lazaro, S.; Weaver, C.A.; Ice, J.A.; Adler, A.J.; et al. X Chromosome Dose and Sex Bias in Autoimmune Diseases: Increased Prevalence of 47,XXX in Systemic Lupus Erythematosus and Sjogren’s Syndrome. Arthritis Rheumatol. 2016, 68, 1290–1300. [Google Scholar] [CrossRef]
- Harris, V.M.; Sharma, R.; Cavett, J.; Kurien, B.T.; Liu, K.; Koelsch, K.A.; Rasmussen, A.; Radfar, L.; Lewis, D.; Stone, D.U.; et al. Klinefelter’s syndrome (47,XXY) is in excess among men with Sjogren’s syndrome. Clin. Immunol. 2016, 168, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harris, V.M.; Cavett, J.; Kurien, B.T.; Liu, K.; Koelsch, K.A.; Fayaaz, A.; Chaudhari, K.S.; Radfar, L.; Lewis, D.; et al. Rare X Chromosome Abnormalities in Systemic Lupus Erythematosus and Sjogren’s Syndrome. Arthritis Rheumatol. 2017, 69, 2187–2192. [Google Scholar] [CrossRef]
- Miquel, C.H.; Faz-Lopez, B.; Guery, J.C. Influence of X chromosome in sex-biased autoimmune diseases. J. Autoimmun. 2023, 137, 102992. [Google Scholar] [CrossRef]
- Loda, A.; Collombet, S.; Heard, E. Gene regulation in time and space during X-chromosome inactivation. Nat. Rev. Mol. Cell Biol. 2022, 23, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.R.; Zhao, Y.; Belk, J.A.; Zhao, Y.; Casey, K.M.; Chen, D.C.; Li, R.; Yu, B.; Srinivasan, S.; Abe, B.T.; et al. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell 2024, 187, 733–749.e716. [Google Scholar] [CrossRef]
- Shaw, T.M.; Zhang, W.; McCoy, S.S.; Pagenkopf, A.; Carp, D.M.; Garg, S.; Parker, M.H.; Qiu, X.; Scofield, R.H.; Galipeau, J.; et al. X-linked genes exhibit miR6891-5p-regulated skewing in Sjogren’s syndrome. J. Mol. Med. 2022, 100, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Vallejo, M.; Guillen, F.; Simon, J.A.; Arena, E.; Reyes, P.A. Gender impact in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2004, 22, 713–721. [Google Scholar]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis—Common Origins, Divergent Mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef]
- Özbalkan, Z.; Baǧışlar, S.; Kiraz, S.; Akyerli, C.B.; Özer, H.T.E.; Yavuz, S.; Birlik, A.M.; Çalgüneri, M.; Özçelik, T. Skewed X chromosome inactivation in blood cells of women with scleroderma. Arthritis Rheum. 2005, 52, 1564–1570. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, A.; Tesmer, L.; Ray, D.; Yousif, N.; Richardson, B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J. Immunol. 2007, 179, 6352–6358. [Google Scholar] [CrossRef] [PubMed]
- Chabchoub, G.; Uz, E.; Maalej, A.; A Mustafa, C.; Rebai, A.; Mnif, M.; Bahloul, Z.; Farid, N.R.; Ozcelik, T.; Ayadi, H. Analysis of skewed X-chromosome inactivation in females with rheumatoid arthritis and autoimmune thyroid diseases. Arthritis Res. Ther. 2009, 11, R106. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, Q.C.; da Rocha, S.T.; Flynn, R.A.; Bharadwaj, M.; Calabrese, J.M.; Magnuson, T.; Heard, E.; Chang, H.Y. Systematic discovery of Xist RNA binding proteins. Cell 2015, 161, 404–416. [Google Scholar] [CrossRef]
- Routsias, J.G.; Touloupi, E.; Dotsika, E.; Moulia, A.; Tsikaris, V.; Sakarellos, C.; Sakarellos-Daitsiotis, M.; Moutsopoulos, H.M.; Tzioufas, A.G. Unmasking the anti-La/SSB response in sera from patients with Sjogren’s syndrome by specific blocking of anti-idiotypic antibodies to La/SSB antigenic determinants. Mol. Med. 2002, 8, 293–305. [Google Scholar] [CrossRef]
- Bergh, K.V.D.; Hooijkaas, H.; Blockmans, D.; Westhovens, R.; Op De Beeck, K.; Verschueren, P.; Dufour, D.; van de Merwe, J.P.; Fijak, M.; Klug, J.; et al. Heterogeneous nuclear ribonucleoprotein h1, a novel nuclear autoantigen. Clin. Chem. 2009, 55, 946–954. [Google Scholar] [CrossRef]
- Farh, K.K.-H.; Marson, A.; Zhu, J.; Kleinewietfeld, M.; Housley, W.J.; Beik, S.; Shoresh, N.; Whitton, H.; Ryan, R.J.H.; Shishkin, A.A.; et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 2015, 518, 337–343. [Google Scholar] [CrossRef]
- Ding, M.; Liu, Y.; Liao, X.; Zhan, H.; Liu, Y.; Huang, W. Enhancer RNAs (eRNAs): New Insights into Gene Transcription and Disease Treatment. J. Cancer 2018, 9, 2334–2340. [Google Scholar] [CrossRef]
- Aune, T.M.; Crooke, P.S.; Patrick, A.E., 3rd; Tossberg, J.T.; Olsen, N.J.; Spurlock, C.F., 3rd. Expression of long non-coding RNAs in autoimmunity and linkage to enhancer function and autoimmune disease risk genetic variants. J. Autoimmun. 2017, 81, 99–109. [Google Scholar] [CrossRef]
- Cross, T.; Haug, K.B.F.; Brusletto, B.S.; Ommundsen, S.K.; Trøseid, A.-M.S.; Aspelin, T.; Olstad, O.K.; Aass, H.C.D.; Galtung, H.K.; Utheim, T.P.; et al. Non-Coding RNA in Salivary Extracellular Vesicles: A New Frontier in Sjogren’s Syndrome Diagnostics? Int. J. Mol. Sci. 2023, 24, 13409. [Google Scholar] [CrossRef]
- Tarn, J.R.; Howard-Tripp, N.; Lendrem, D.W.; Mariette, X.; Saraux, A.; Devauchelle-Pensec, V.; Seror, R.; Skelton, A.J.; James, K.; McMeekin, P.; et al. Symptom-based stratification of patients with primary Sjogren’s syndrome: Multi-dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumatol. 2019, 1, e85–e94. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.; Nocturne, G.; Henry, J.; Ng, W.-F.; Belkhir, R.; Desmoulins, F.; Bergé, E.; Morel, J.; Perdriger, A.; Dernis, E.; et al. Identification of distinct subgroups of Sjogren’s disease by cluster analysis based on clinical and biological manifestations: Data from the cross-sectional Paris-Saclay and the prospective ASSESS cohorts. Lancet Rheumatol. 2024, 6, e216–e225. [Google Scholar] [CrossRef] [PubMed]
- Soret, P.; Le Dantec, C.; Desvaux, E.; Foulquier, N.; Chassagnol, B.; Hubert, S.; Jamin, C.; Barturen, G.; Desachy, G.; Devauchelle-Pensec, V.; et al. A new molecular classification to drive precision treatment strategies in primary Sjogren’s syndrome. Nat. Commun. 2021, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Lendrem, D.; Wason, J.; Tarn, J.; Howard-Tripp, N.; Bodewes, I.; Versnel, M.A.; Gottenberg, J.-E.; Seror, R.; Mariette, X.; et al. Revisiting the JOQUER trial: Stratification of primary Sjogren’s syndrome and the clinical and interferon response to hydroxychloroquine. Rheumatol. Int. 2021, 41, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.C.; Hu, Y.; Guan, S.Y.; Ye, D.Q.; Pan, H.F. Differential Plasma Expression Profiles of Long Non-Coding RNAs Reveal Potential Biomarkers for Systemic Lupus Erythematosus. Biomolecules 2019, 9, 206. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zu, B.; Liu, J.; Sun, L.; Ding, C.; Wang, D.; Cheng, X.; Yang, D.; Niu, G. Identification of Long Noncoding RNAs lnc-DC in Plasma as a New Biomarker for Primary Sjögren’s Syndrome. J. Immunol. Res. 2020, 2020, 9236234. [Google Scholar] [CrossRef]
- Joachims, M.L.; Khatri, B.; Li, C.; Tessneer, K.L.; A Ice, J.; Stolarczyk, A.M.; Means, N.; Grundahl, K.M.; Glenn, S.B.; A Kelly, J.; et al. Dysregulated long non-coding RNA in Sjögren’s disease impacts both interferon and adaptive immune responses. RMD Open 2022, 8, e002672. [Google Scholar] [CrossRef] [PubMed]
- Bettacchioli, E.; Saraux, A.; Tison, A.; Cornec, D.; Dueymes, M.; Foulquier, N.; Hillion, S.; Roguedas-Contios, A.-M.; Benyoussef, A.-A.; Alarcon-Riquelme, M.E.; et al. Association of Combined Anti-Ro52/TRIM21 and Anti-Ro60/SSA Antibodies With Increased Sjogren Disease Severity Through Interferon Pathway Activation. Arthritis Rheumatol. 2023, 76, 751–762. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.; Tian, J.; Ma, J.; Tang, X.; Rui, K.; Tian, X.; Mao, C.; Lu, L.; Xu, H.; et al. The Long Noncoding RNA IFNG-AS1 Promotes T Helper Type 1 Cells Response in Patients with Hashimoto’s Thyroiditis. Sci. Rep. 2015, 5, 17702. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, B.; Servaas, N.H.; Rossato, M.; Tamassia, N.; Cassatella, M.A.; Cossu, M.; Beretta, L.; van der Kroef, M.; Radstake, T.R.D.J.; Bazzoni, F. The Long Non-coding RNA NRIR Drives IFN-Response in Monocytes: Implication for Systemic Sclerosis. Front. Immunol. 2019, 10, 100. [Google Scholar] [CrossRef]
- Barturen, G.; Babaei, S.; Català-Moll, F.; Martínez-Bueno, M.; Makowska, Z.; Martorell-Marugán, J.; Carmona-Sáez, P.; Toro-Domínguez, D.; Carnero-Montoro, E.; Teruel, M.; et al. Integrative Analysis Reveals a Molecular Stratification of Systemic Autoimmune Diseases. Arthritis Rheumatol. 2021, 73, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Satpathy, A.T.; Chang, H.Y. Gene regulation in the immune system by long noncoding RNAs. Nat. Immunol. 2017, 18, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cao, N.; Pu, Y.; Xie, L.; Zheng, L.; Yu, C. Long non-coding RNA expression profile in minor salivary gland of primary Sjögren’s syndrome. Arthritis Res. Ther. 2016, 18, 109. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, J.; Chen, R.; Shibata, Y.; Tanaka, R.; Wang, J.; Zhang, J. Predicted Disease-Specific Immune Infiltration Patterns Decode the Potential Mechanisms of Long Non-Coding RNAs in Primary Sjogren’s Syndrome. Front. Immunol. 2021, 12, 624614. [Google Scholar] [CrossRef] [PubMed]
- Colafrancesco, S.; Priori, R.; Smith, C.G.; Minniti, A.; Iannizzotto, V.; Pipi, E.; Lucchesi, D.; Pontarini, E.; Nayar, S.; Campos, J.; et al. CXCL13 as biomarker for histological involvement in Sjogren’s syndrome. Rheumatology 2020, 59, 165–170. [Google Scholar] [CrossRef]
- Dolcino, M.; Tinazzi, E.; Vitali, C.; Del Papa, N.; Puccetti, A.; Lunardi, C. Long Non-Coding RNAs Modulate Sjögren’s Syndrome Associated Gene Expression and Are Involved in the Pathogenesis of the Disease. J. Clin. Med. 2019, 8, 1349. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Q.; Du, Y.; Liu, L.; Wu, H. Differential long non-coding RNA expression profile and function analysis in primary Sjogren’s syndrome. BMC Immunol. 2021, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Luo, X.; Chen, Y.; Peng, L.; Deng, C.; Fei, Y.; Zhang, W.; Zhao, Y. LncRNA and mRNA expression profile of peripheral blood mononuclear cells in primary Sjögren’s syndrome patients. Sci. Rep. 2020, 10, 19629. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Shi, H.; Wang, B.; Zhan, T.; Shao, Y.; Ye, L.; Wu, S.; Yu, C.; Zheng, L. LncRNA PVT1 links Myc to glycolytic metabolism upon CD4(+) T cell activation and Sjögren’s syndrome-like autoimmune response. J. Autoimmun. 2020, 107, 102358. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Shi, H.; Yu, C.; Fu, J.; Chen, C.; Wu, S.; Zhan, T.; Wang, B.; Zheng, L. LncRNA Neat1 positively regulates MAPK signaling and is involved in the pathogenesis of Sjögren’s syndrome. Int. Immunopharmacol. 2020, 88, 106992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, L.; Qian, J.; Qu, B.; Xia, S.; La, T.; Wu, Y.; Ma, J.; Zeng, J.; Guo, Q.; et al. Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. J. Autoimmun. 2016, 75, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, H.; Tian, J.; Ma, J.; Tang, X.; Rui, K.; Tian, X.; Wang, Y.; Chen, J.; Lu, L.; et al. Upregulation of long noncoding RNA TMEVPG1 enhances T helper type 1 cell response in patients with Sjögren syndrome. Immunol. Res. 2016, 64, 489–496. [Google Scholar] [CrossRef]
- Peng, H.; Ren, S.; Liu, Y.; Zhou, H.; Tang, X.; Yang, J.; Tian, J.; Xu, P.; Xu, H.; Wang, S. Elevated Expression of the Long Noncoding RNA IFNG-AS1 in the Peripheral Blood from Patients with Rheumatoid Arthritis. J. Immunol. Res. 2020, 2020, 6401978. [Google Scholar] [CrossRef] [PubMed]
- Inamo, J.; Suzuki, K.; Takeshita, M.; Kassai, Y.; Takiguchi, M.; Kurisu, R.; Okuzono, Y.; Tasaki, S.; Yoshimura, A.; Takeuchi, T. Identification of novel genes associated with dysregulation of B cells in patients with primary Sjögren’s syndrome. Arthritis Res. Ther. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Petri, A.; Dybkær, K.; Bøgsted, M.; Thrue, C.A.; Hagedorn, P.H.; Schmitz, A.; Bødker, J.S.; Johnsen, H.E.; Kauppinen, S. Long Noncoding RNA Expression during Human B-Cell Development. PLoS ONE 2015, 10, e0138236. [Google Scholar] [CrossRef]
- Amezcua-Guerra, L.M.; Sánchez-Muñoz, F.; Pichardo-Ontiveros, E.; González-Ramírez, J.; Martínez-Martínez, L.A.; Juárez-Vicuña, Y. Interferon-alpha regulates expression of lncRNA MALAT1 and interferon-stimulated genes, as well as chemokine production, in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2022, 40, 2275–2282. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Liu, L.; Yang, Z.; Liu, S.; Ma, Z.; Liu, Y.; Ma, Y.; Zhang, L.; Zhang, X.; et al. LncRNA Malat1 inhibition of TDP43 cleavage suppresses IRF3-initiated antiviral innate immunity. Proc. Natl. Acad. Sci. USA 2020, 117, 23695–23706. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Tarpley, T.M.; Jr Anderson, L.G.; White, C.L. Minor salivary gland involvement in Sjogren’s syndrome. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 64–74. [Google Scholar] [CrossRef] [PubMed]
| SjD | SLE | RA | SSc | |
|---|---|---|---|---|
| Prevalence female/male | 14:1 [21] | 9:1 [43] | 3:1 [44] | 3–8:1 [45] |
| Skewed XCI | present [42] | present [46] | present [47] | present [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastva, O.; Klein, K. Long Non-Coding RNAs in Sjögren’s Disease. Int. J. Mol. Sci. 2024, 25, 5162. https://doi.org/10.3390/ijms25105162
Pastva O, Klein K. Long Non-Coding RNAs in Sjögren’s Disease. International Journal of Molecular Sciences. 2024; 25(10):5162. https://doi.org/10.3390/ijms25105162
Chicago/Turabian StylePastva, Ondřej, and Kerstin Klein. 2024. "Long Non-Coding RNAs in Sjögren’s Disease" International Journal of Molecular Sciences 25, no. 10: 5162. https://doi.org/10.3390/ijms25105162





