Prurigo Nodularis: Pathogenesis and the Horizon of Potential Therapeutics
Abstract
:1. Introduction
2. Epidemiology
3. Etiology and Pathogenesis
3.1. Immune Dysregulation
3.2. Neural Dysregulation
3.3. Fibrotic Response
4. Clinical Features, Diagnosis, and Comorbidities
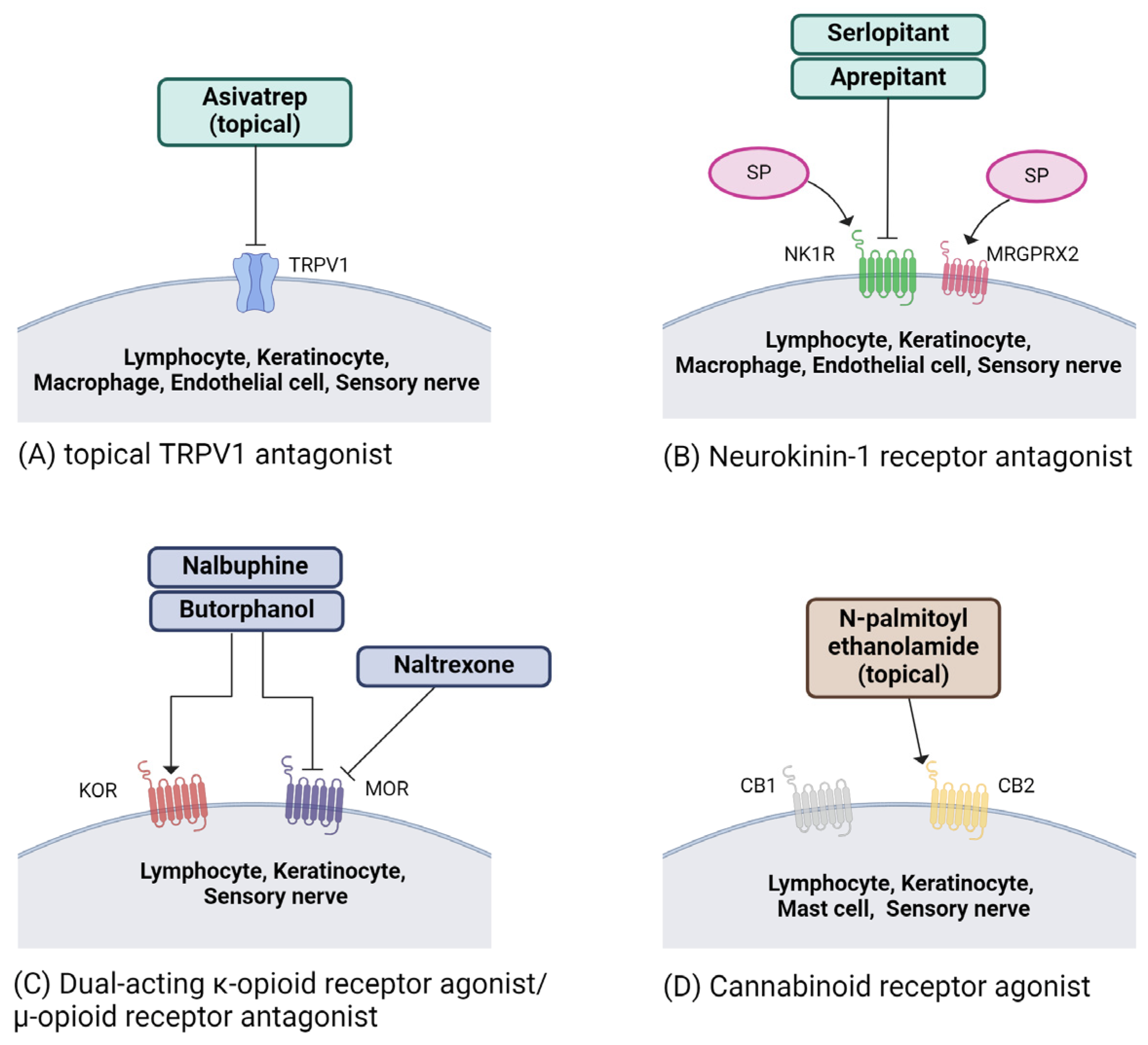
5. Treatment and Potential Therapeutics
5.1. Topical Therapy
5.2. Systemic Therapy
5.2.1. Phototherapy
5.2.2. Systemic Immunomodulating Agents
- Immunosuppressants
- 2.
- Targeting Th2 polarization (Dupilumab, Tralokinumab)
- 3.
- JAK inhibitors
5.2.3. Systemic Neuromodulating Agents
- Antidepressant
- 2.
- Gabapentinoids
- 3.
- Neurokinin-1 receptor antagonists (aprepitant, serlopitant)
- 4.
- Thalidomide
- 5.
- Targeting opioid receptor (Nalbuphine, Butorphanol, Naltrexone)
- 6.
- Cannabinoids
5.3. Up-and-Coming Therapeutic Agents
- Targeting IL-31 (Nemolizumab)
- 2.
- Targeting Oncostatin M beta receptor (Vixarelimab)
- 3.
- Targeting IL-5 (Benralizumab)
- 4.
- Targeting KIT (Barzolvolimab)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misery, L. Chronic prurigo. Br. J. Dermatol. 2022, 187, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Akiyama, T.; Nattkemper, L.A.; van Laarhoven, A.; Elberling, J.; Yosipovitch, G.; Arendt-Nielsen, L. Alloknesis and hyperknesis-mechanisms, assessment methodology, and clinical implications of itch sensitization. Pain 2018, 159, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Steinke, S.; Zeidler, C.; Forner, C.; Riepe, C.; Augustin, M.; Bobko, S.; Dalgard, F.; Elberling, J.; Garcovich, S.; et al. European academy of dermatology and venereology European prurigo project: Expert consensus on the definition, classification and terminology of chronic prurigo. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, C.; Pereira, M.P.; Stander, S. Chronic Prurigo: Similar Clinical Profile and Burden Across Clinical Phenotypes. Front. Med. 2021, 8, 649332. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.; Kim, B.; Berger, T.; Chisolm, S.; Kwatra, S.G.; Mollanazar, N.; Yosipovitch, G. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J. Am. Acad. Dermatol. 2021, 84, 747–760. [Google Scholar] [CrossRef]
- Kolkhir, P.; Akdis, C.A.; Akdis, M.; Bachert, C.; Bieber, T.; Canonica, G.W.; Guttman-Yassky, E.; Metz, M.; Mullol, J.; Palomares, O.; et al. Type 2 chronic inflammatory diseases: Targets, therapies and unmet needs. Nat. Rev. Drug Discov. 2023, 22, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Pereira, M.P.; Berger, T.; Zeidler, C.; Augustin, M.; Bobko, S.; Brenaut, E.; Chen, S.C.; Chisolm, S.; Dalgard, F.J.; et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch 2020, 5, e42. [Google Scholar] [CrossRef]
- Satoh, T.; Yokozeki, H.; Murota, H.; Tokura, Y.; Kabashima, K.; Takamori, K.; Shiohara, T.; Morita, E.; Aiba, S.; Aoyama, Y.; et al. 2020 guidelines for the diagnosis and treatment of prurigo. J. Dermatol. 2021, 48, e414–e431. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Williams, K.A.; Kwatra, S.G. Prurigo nodularis: Epidemiology and clinical features. J. Am. Acad. Dermatol. 2020, 83, 1559–1565. [Google Scholar] [CrossRef]
- Kwatra, S.G. Breaking the Itch-Scratch Cycle in Prurigo Nodularis. N. Engl. J. Med. 2020, 382, 757–758. [Google Scholar] [CrossRef]
- Schuhknecht, B.; Marziniak, M.; Wissel, A.; Phan, N.Q.; Pappai, D.; Dangelmaier, J.; Metze, D.; Stander, S. Reduced intraepidermal nerve fibre density in lesional and nonlesional prurigo nodularis skin as a potential sign of subclinical cutaneous neuropathy. Br. J. Dermatol. 2011, 165, 85–91. [Google Scholar] [CrossRef]
- Williams, K.A.; Huang, A.H.; Belzberg, M.; Kwatra, S.G. Prurigo nodularis: Pathogenesis and management. J. Am. Acad. Dermatol. 2020, 83, 1567–1575. [Google Scholar] [CrossRef]
- Huang, A.H.; Canner, J.K.; Khanna, R.; Kang, S.; Kwatra, S.G. Real-World Prevalence of Prurigo Nodularis and Burden of Associated Diseases. J. Investig. Dermatol. 2020, 140, 480–483.e4. [Google Scholar] [CrossRef] [PubMed]
- Whang, K.A.; Mahadevan, V.; Bakhshi, P.R.; Williams, K.A.; Gabriel, S.; Chavda, R.; Kwatra, S.G. Prevalence of prurigo nodularis in the United States. J. Allergy Clin. Immunol. Pract. 2020, 8, 3240–3241. [Google Scholar] [CrossRef] [PubMed]
- Wongvibulsin, S.; Sutaria, N.; Williams, K.A.; Huang, A.H.; Choi, J.; Roh, Y.S.; Hong, M.; Kelley, D.; Pahalyants, V.; Murphy, W.; et al. A Nationwide Study of Prurigo Nodularis: Disease Burden and Healthcare Utilization in the United States. J. Investig. Dermatol. 2021, 141, 2530–2533.e1. [Google Scholar] [CrossRef]
- Boozalis, E.; Tang, O.; Patel, S.; Semenov, Y.R.; Pereira, M.P.; Stander, S.; Kang, S.; Kwatra, S.G. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J. Am. Acad. Dermatol. 2018, 79, 714–719.e3. [Google Scholar] [CrossRef]
- Sutaria, N.; Adawi, W.; Brown, I.; Parthasarathy, V.; Roh, Y.S.; Choi, J.; Bordeaux, Z.A.; Trinh, P.; Le, T.K.; Deng, J.; et al. Racial disparities in mortality among patients with prurigo nodularis: A multi-center cohort study. J. Am. Acad. Dermatol. 2022, 86, 487–490. [Google Scholar] [CrossRef]
- Whang, K.A.; Khanna, R.; Thomas, J.; Aguh, C.; Kwatra, S.G. Racial and Gender Differences in the Presentation of Pruritus. Medicines 2019, 6, 98. [Google Scholar] [CrossRef]
- Augustin, M.; Garbe, C.; Hagenstrom, K.; Petersen, J.; Pereira, M.P.; Stander, S. Prevalence, incidence and presence of comorbidities in patients with prurigo and pruritus in Germany: A population-based claims data analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2270–2276. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J.; Ahn, H.S. Impact of Chronic Kidney Disease Severity on the Risk of Prurigo Nodularis: A Population-Based Cohort Study. Acta Derm. Venereol. 2022, 102, adv00781. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Wang, S.; Sohn, K.A.; Kim, H.S. Epidemiology, Comorbidities, and Prescription Patterns of Korean Prurigo Nodularis Patients: A Multi-Institution Study. J. Clin. Med. 2021, 11, 95. [Google Scholar] [CrossRef]
- Whang, K.A.; Kang, S.; Kwatra, S.G. Inpatient Burden of Prurigo Nodularis in the United States. Medicines 2019, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Ryczek, A.; Reich, A. Prevalence of Prurigo Nodularis in Poland. Acta Derm. Venereol. 2020, 100, adv00155. [Google Scholar] [CrossRef]
- Amer, A.; Fischer, H. Prurigo nodularis in a 9-year-old girl. Clin. Pediatr. (Phila) 2009, 48, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Rowland Payne, C.M.; Wilkinson, J.D.; McKee, P.H.; Jurecka, W.; Black, M.M. Nodular prurigo--a clinicopathological study of 46 patients. Br. J. Dermatol. 1985, 113, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Tey, H.L. Extensive prurigo nodularis: Characterization and etiology. Dermatology 2014, 228, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, C.; Tsianakas, A.; Pereira, M.; Stander, H.; Yosipovitch, G.; Stander, S. Chronic Prurigo of Nodular Type: A Review. Acta Derm. Venereol. 2018, 98, 173–179. [Google Scholar] [CrossRef]
- Croce, E.A.; Levy, M.L.; Adamson, A.S.; Matsui, E.C. Reframing racial and ethnic disparities in atopic dermatitis in Black and Latinx populations. J. Allergy Clin. Immunol. 2021, 148, 1104–1111. [Google Scholar] [CrossRef]
- Al-Obaydi, S.; Craig, T.J.; Al-Shaikhly, T. Racial and ethnic disparities in the treatment of patients with atopic dermatitis in the United States: A retrospective matched cohort study. J. Allergy Clin. Immunol. Pract. 2023, 11, 2602–2604.e1. [Google Scholar] [CrossRef]
- Pereira, M.P.; Zeidler, C.; Stander, S. Clinical characteristics of chronic nodular prurigo are independent of Fitzpatrick skin type: A European prospective cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e225–e227. [Google Scholar] [CrossRef]
- Vasavda, C.; Wan, G.; Szeto, M.D.; Marani, M.; Sutaria, N.; Rajeh, A.; Lu, C.; Lee, K.K.; Nguyen, N.T.T.; Adawi, W.; et al. A Polygenic Risk Score for Predicting Racial and Genetic Susceptibility to Prurigo Nodularis. J. Investig. Dermatol. 2023, 143, 2416–2426.e1. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.A.; Rojo, J.; Nieto, P.M.; Pinto, F.M.; Hernandez, M.; Martin, J.D.; Candenas, M.L. Tachykinins and tachykinin receptors: Structure and activity relationships. Curr. Med. Chem. 2004, 11, 2045–2081. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, C.; Yosipovitch, G.; Stander, S. Prurigo Nodularis and Its Management. Dermatol. Clin. 2018, 36, 189–197. [Google Scholar] [CrossRef]
- Raap, U.; Gunther, C. Pathogenesis of prurigo nodularis. Hautarzt 2014, 65, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Johansson, O.; Liang, Y.; Marcusson, J.A.; Reimert, C.M. Eosinophil cationic protein- and eosinophil-derived neurotoxin/eosinophil protein X-immunoreactive eosinophils in prurigo nodularis. Arch. Dermatol. Res. 2000, 292, 371–378. [Google Scholar] [CrossRef]
- Lee, M.R.; Shumack, S. Prurigo nodularis: A review. Australas. J. Dermatol. 2005, 46, 211–218, quiz 219–220. [Google Scholar] [CrossRef]
- Liang, Y.; Marcusson, J.A.; Jacobi, H.H.; Haak-Frendscho, M.; Johansson, O. Histamine-containing mast cells and their relationship to NGFr-immunoreactive nerves in prurigo nodularis: A reappraisal. J. Cutan. Pathol. 1998, 25, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nattkemper, L.A.; Kim, H.S.; Kursewicz, C.D.; Fowler, E.; Shah, S.M.; Nanda, S.; Fayne, R.A.; Paolini, J.F.; Romanelli, P.; et al. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp. Dermatol. 2021, 30, 804–810. [Google Scholar] [CrossRef]
- Grimstad, O.; Sawanobori, Y.; Vestergaard, C.; Bilsborough, J.; Olsen, U.B.; Gronhoj-Larsen, C.; Matsushima, K. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: A model of atopic dermatitis. Exp. Dermatol. 2009, 18, 35–43. [Google Scholar] [CrossRef]
- Sutaria, N.; Alphonse, M.P.; Roh, Y.S.; Choi, J.; Parthasarathy, V.; Deng, J.; Bordeaux, Z.A.; Taylor, M.T.; Pritchard, T.; Kim, N.; et al. Cutaneous Transcriptomics Identifies Fibroproliferative and Neurovascular Gene Dysregulation in Prurigo Nodularis Compared with Psoriasis and Atopic Dermatitis. J. Investig. Dermatol. 2022, 142, 2537–2540. [Google Scholar] [CrossRef]
- Park, K.; Mori, T.; Nakamura, M.; Tokura, Y. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur. J. Dermatol. 2011, 21, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, V.; Cravero, K.; Deng, J.; Sun, Z.; Engle, S.M.; Auxier, A.N.; Hahn, N.; Sims, J.T.; Okragly, A.J.; Alphonse, M.P.; et al. Circulating plasma IL-13 and periostin are dysregulated type 2 inflammatory biomarkers in prurigo nodularis: A cluster analysis. Front. Med. 2022, 9, 1011142. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, D.; Zhu, Y.; Xiao, Z.; Jin, T.; Peng, L.; Shen, Y.; Tang, H. Molecular mechanisms of pruritus in prurigo nodularis. Front. Immunol. 2023, 14, 1301817. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, H.M. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor. Rev. 2015, 26, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Harris, M.B.; Rothman, P. IL-4/IL-13 signaling beyond JAK/STAT. J. Allergy Clin. Immunol. 2000, 105 Pt 1, 1063–1070. [Google Scholar] [CrossRef]
- Philips, R.L.; Wang, Y.; Cheon, H.; Kanno, Y.; Gadina, M.; Sartorelli, V.; Horvath, C.M.; Darnell, J.E., Jr.; Stark, G.R.; O’Shea, J.J. The JAK-STAT pathway at 30: Much learned, much more to do. Cell 2022, 185, 3857–3876. [Google Scholar] [CrossRef]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Wang, H.; Byfield, G.; Jiang, Y.; Smith, G.W.; McCloskey, M.; Hartnett, M.E. VEGF-mediated STAT3 activation inhibits retinal vascularization by down-regulating local erythropoietin expression. Am. J. Pathol. 2012, 180, 1243–1253. [Google Scholar] [CrossRef]
- Agrawal, D.; Sardana, K.; Mathachan, S.R.; Bhardwaj, M.; Ahuja, A.; Jain, S. A prospective study examining the expression of STAT 1, 3, 6 in prurigo nodularis lesions with its immunopathogenic and therapeutic implications. J. Cosmet. Dermatol. 2022, 21, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.; Sardana, K.; Mathachan, S.R.; Bhardwaj, M.; Ahuja, A.; Jain, S. A Prospective Study Examining the Effect of Selected Topical and Systemic Drugs on Pruritus Grading System Score and STAT 6 Expression in Patients of Prurigo Nodularis. Indian J. Dermatol. 2021, 66, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, S.; Yamasaki, K.; Aiba, S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br. J. Dermatol. 2011, 165, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Maurelli, M.; Gori, N.; Argenziano, G.; De Simone, C.; Calabrese, G.; Girolomoni, G.; Peris, K. Dupilumab improves clinical manifestations, symptoms, and quality of life in adult patients with chronic nodular prurigo. J. Am. Acad. Dermatol. 2020, 83, 39–45. [Google Scholar] [CrossRef]
- Campion, M.; Smith, L.; Gatault, S.; Metais, C.; Buddenkotte, J.; Steinhoff, M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp. Dermatol. 2019, 28, 1501–1504. [Google Scholar] [CrossRef]
- Nguyen, J.K.; Austin, E.; Huang, A.; Mamalis, A.; Jagdeo, J. The IL-4/IL-13 axis in skin fibrosis and scarring: Mechanistic concepts and therapeutic targets. Arch. Dermatol. Res. 2020, 312, 81–92. [Google Scholar] [CrossRef]
- Muller, S.; Bieber, T.; Stander, S. Therapeutic potential of biologics in prurigo nodularis. Expert. Opin. Biol. Ther. 2022, 22, 47–58. [Google Scholar] [CrossRef]
- Wang, F.; Yang, T.B.; Kim, B.S. The Return of the Mast Cell: New Roles in Neuroimmune Itch Biology. J. Investig. Dermatol. 2020, 140, 945–951. [Google Scholar] [CrossRef]
- Kolkhir, P.; Pyatilova, P.; Ashry, T.; Jiao, Q.; Abad-Perez, A.T.; Altrichter, S.; Vera Ayala, C.E.; Church, M.K.; He, J.; Lohse, K.; et al. Mast cells, cortistatin, and its receptor, MRGPRX2, are linked to the pathogenesis of chronic prurigo. J. Allergy Clin. Immunol. 2022, 149, 1998–2009.e5. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Muller, M.; Carter, S.; Hofer, M.J.; Campbell, I.L. Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity--a tale of conflict and conundrum. Neuropathol. Appl. Neurobiol. 2010, 36, 368–387. [Google Scholar] [CrossRef]
- Yang, H.; Chen, W.; Zhu, R.; Wang, J.; Meng, J. Critical Players and Therapeutic Targets in Chronic Itch. Int. J. Mol. Sci. 2022, 23, 9935. [Google Scholar] [CrossRef]
- Walsh, C.M.; Hill, R.Z.; Schwendinger-Schreck, J.; Deguine, J.; Brock, E.C.; Kucirek, N.; Rifi, Z.; Wei, J.; Gronert, K.; Brem, R.B.; et al. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. Elife 2019, 8, 48448. [Google Scholar] [CrossRef]
- Agelopoulos, K.; Renkhold, L.; Wiegmann, H.; Dugas, M.; Suer, A.; Zeidler, C.; Schmelz, M.; Pereira, M.P.; Stander, S. Transcriptomic, Epigenomic, and Neuroanatomic Signatures Differ in Chronic Prurigo, Atopic Dermatitis, and Brachioradial Pruritus. J. Investig. Dermatol. 2023, 143, 264–272.e3. [Google Scholar] [CrossRef]
- Pereira, M.P.; Pogatzki-Zahn, E.; Snels, C.; Vu, T.H.; Uceyler, N.; Loser, K.; Sommer, C.; Evers, A.W.M.; van Laarhoven, A.I.M.; Agelopoulos, K.; et al. There is no functional small-fibre neuropathy in prurigo nodularis despite neuroanatomical alterations. Exp. Dermatol. 2017, 26, 969–971. [Google Scholar] [CrossRef]
- Bobko, S.; Zeidler, C.; Osada, N.; Riepe, C.; Pfleiderer, B.; Pogatzki-Zahn, E.; Lvov, A.; Stander, S. Intraepidermal Nerve Fibre Density is Decreased in Lesional and Inter-lesional Prurigo Nodularis and Reconstitutes on Healing of Lesions. Acta Derm. Venereol. 2016, 96, 404–406. [Google Scholar] [CrossRef]
- Hashimoto, T.; Okuno, S.; Okuzawa, M.; Satoh, T. Increased sensitivity to touch-evoked itch (punctate hyperknesis) in prurigo nodularis and type 2 inflammation: A cross-sectional pilot study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e789–e791. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Pereira, M.P.; Cremer, A.; Zeidler, C.; Dreyer, T.; Riepe, C.; Wempe, C.; Lotts, T.; Segelcke, D.; Ringkamp, M.; et al. Peripheral Sensitization and Loss of Descending Inhibition Is a Hallmark of Chronic Pruritus. J. Investig. Dermatol. 2020, 140, 203–211.e4. [Google Scholar] [CrossRef]
- Choragudi, S.; Yosipovitch, G. Prurigo nodularis is highly linked with neural sensitization disorders of pain among hospitalized adults in the United States—National Inpatient Sample 2016–2019. Br. J. Dermatol. 2023, 189, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Agelopoulos, K.; Kollner, J.; Neufang, G.; Schmelz, M.; Stander, S. Selective Nerve Fibre Activation in Patients with Generalized Chronic Pruritus: Hint for Central Sensitization? Acta Derm. Venereol. 2019, 99, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Kabata, H.; Artis, D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Investig. 2019, 129, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Ohanyan, T.; Schoepke, N.; Eirefelt, S.; Hoey, G.; Koopmann, W.; Hawro, T.; Maurer, M.; Metz, M. Role of Substance P and Its Receptor Neurokinin 1 in Chronic Prurigo: A Randomized, Proof-of-Concept, Controlled Trial with Topical Aprepitant. Acta Derm. Venereol. 2018, 98, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Capellino, S.; Phan, N.Q.; Bohm, M.; Luger, T.A.; Straub, R.H.; Stander, S. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J. Dermatol. Sci. 2010, 58, 193–197. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Nakashima, C.; Ishida, Y.; Kitoh, A.; Otsuka, A.; Kabashima, K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp. Dermatol. 2019, 28, 1405–1411. [Google Scholar] [CrossRef]
- Misery, L.; Pierre, O.; Le Gall-Ianotto, C.; Lebonvallet, N.; Chernyshov, P.V.; Le Garrec, R.; Talagas, M. Basic mechanisms of itch. J. Allergy Clin. Immunol. 2023, 152, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Vander Does, A.; Ju, T.; Mohsin, N.; Chopra, D.; Yosipovitch, G. How to get rid of itching. Pharmacol. Ther. 2023, 243, 108355. [Google Scholar] [CrossRef]
- Sutaria, N.; Adawi, W.; Goldberg, R.; Roh, Y.S.; Choi, J.; Kwatra, S.G. Itch: Pathogenesis and treatment. J. Am. Acad. Dermatol. 2022, 86, 17–34. [Google Scholar] [CrossRef]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef]
- Stander, S.; Moormann, C.; Schumacher, M.; Buddenkotte, J.; Artuc, M.; Shpacovitch, V.; Brzoska, T.; Lippert, U.; Henz, B.M.; Luger, T.A.; et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp. Dermatol. 2004, 13, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Wheeler, J.J.; Pitake, S.; Ding, H.; Jiang, C.; Fukuyama, T.; Paps, J.S.; Ralph, P.; Coyne, J.; Parkington, M.; et al. Periostin Activation of Integrin Receptors on Sensory Neurons Induces Allergic Itch. Cell Rep. 2020, 31, 107472. [Google Scholar] [CrossRef] [PubMed]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef]
- Oh, M.H.; Oh, S.Y.; Lu, J.; Lou, H.; Myers, A.C.; Zhu, Z.; Zheng, T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J. Immunol. 2013, 191, 5371–5382. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ji, Q.; Ji, W. Role of transient receptor potential ankyrin subfamily member 1 in pruritus induced by endothelin-1. Neurosci. Lett. 2011, 492, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Hacini-Rachinel, F.; Fogel, P.; Rousseau, F.; Xing, X.; Patrick, M.T.; Billi, A.C.; Berthier, C.C.; Kahlenberg, J.M.; Lazzari, A.; et al. Transcriptomic characterization of prurigo nodularis and the therapeutic response to nemolizumab. J. Allergy Clin. Immunol. 2022, 149, 1329–1339. [Google Scholar] [CrossRef]
- Belzberg, M.; Alphonse, M.P.; Brown, I.; Williams, K.A.; Khanna, R.; Ho, B.; Wongvibulsin, S.; Pritchard, T.; Roh, Y.S.; Sutaria, N.; et al. Prurigo Nodularis Is Characterized by Systemic and Cutaneous T Helper 22 Immune Polarization. J. Investig. Dermatol. 2021, 141, 2208–2218.e14. [Google Scholar] [CrossRef]
- Brunner, P.M.; Pavel, A.B.; Khattri, S.; Leonard, A.; Malik, K.; Rose, S.; Jim On, S.; Vekaria, A.S.; Traidl-Hoffmann, C.; Singer, G.K.; et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 2019, 143, 142–154. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Brunner, P.M.; Neumann, A.U.; Khattri, S.; Pavel, A.B.; Malik, K.; Singer, G.K.; Baum, D.; Gilleaudeau, P.; Sullivan-Whalen, M.; et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 2018, 78, 872–881.e6. [Google Scholar] [CrossRef]
- Kou, K.; Okawa, T.; Yamaguchi, Y.; Ono, J.; Inoue, Y.; Kohno, M.; Matsukura, S.; Kambara, T.; Ohta, S.; Izuhara, K.; et al. Periostin levels correlate with disease severity and chronicity in patients with atopic dermatitis. Br. J. Dermatol. 2014, 171, 283–291. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, Y.; Xiao, Z.; Shen, Y.; Dai, B.; Tang, H.; Wang, D. RNA sequencing reveals the transcriptome profile of the atopic prurigo nodularis with severe itching. Exp. Dermatol. 2023, 32, 30–40. [Google Scholar] [CrossRef]
- Sims, J.T.; Chang, C.Y.; Higgs, R.E.; Engle, S.M.; Liu, Y.; Sissons, S.E.; Rodgers, G.H.; Simpson, E.L.; Silverberg, J.I.; Forman, S.B.; et al. Insights into adult atopic dermatitis heterogeneity derived from circulating biomarker profiling in patients with moderate-to-severe disease. Exp. Dermatol. 2021, 30, 1650–1661. [Google Scholar] [CrossRef]
- Alkon, N.; Assen, F.P.; Arnoldner, T.; Bauer, W.M.; Medjimorec, M.A.; Shaw, L.E.; Rindler, K.; Holzer, G.; Weber, P.; Weninger, W.; et al. Single-cell RNA sequencing defines disease-specific differences between chronic nodular prurigo and atopic dermatitis. J. Allergy Clin. Immunol. 2023, 152, 420–435. [Google Scholar] [CrossRef]
- Ma, F.; Gharaee-Kermani, M.; Tsoi, L.C.; Plazyo, O.; Chaskar, P.; Harms, P.; Patrick, M.T.; Xing, X.; Hile, G.; Piketty, C.; et al. Single-cell profiling of prurigo nodularis demonstrates immune-stromal crosstalk driving profibrotic responses and reversal with nemolizumab. J. Allergy Clin. Immunol. 2024, 153, 146–160. [Google Scholar] [CrossRef]
- Conrad, C.; Schlapbach, C. Prurigo nodularis forecast: Light type 2 inflammation with high chances of fibrosis. J. Allergy Clin. Immunol. 2024, 153, 93–94. [Google Scholar] [CrossRef]
- Patel, J.R.; Joel, M.Z.; Lee, K.K.; Kambala, A.; Cornman, H.; Oladipo, O.; Taylor, M.; Deng, J.; Parthasarathy, V.; Cravero, K.; et al. Single-cell RNA sequencing reveals dysregulated fibroblast subclusters in prurigo nodularis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kwon, C.D.; Khanna, R.; Williams, K.A.; Kwatra, M.M.; Kwatra, S.G. Diagnostic Workup and Evaluation of Patients with Prurigo Nodularis. Medicines 2019, 6, 97. [Google Scholar] [CrossRef]
- Fostini, A.C.; Girolomoni, G.; Tessari, G. Prurigo nodularis: An update on etiopathogenesis and therapy. J. Dermatol. Treat. 2013, 24, 458–462. [Google Scholar] [CrossRef]
- Kang, S.; Amagai, M.; Bruckner, A.L.; Enk, A.H.; Margolis, D.J.; McMichael, A.J.; Orringer, J.S. Fitzpatrick’s Dermatology, 9th ed.; McaGraw-Hill: New York, NY, USA, 2019. [Google Scholar]
- Weigelt, N.; Metze, D.; Stander, S. Prurigo nodularis: Systematic analysis of 58 histological criteria in 136 patients. J. Cutan. Pathol. 2010, 37, 578–586. [Google Scholar] [CrossRef]
- Zeidler, C.; Raap, U.; Witte, F.; Stander, S. Clinical aspects and management of chronic itch. J. Allergy Clin. Immunol. 2023, 152, 1–10. [Google Scholar] [CrossRef]
- Stander, S.; Zeidler, C.; Riepe, C.; Steinke, S.; Fritz, F.; Bruland, P.; Soto-Rey, I.; Storck, M.; Agner, T.; Augustin, M.; et al. European EADV network on assessment of severity and burden of Pruritus (PruNet): First meeting on outcome tools. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1144–1147. [Google Scholar] [CrossRef]
- Storck, M.; Sandmann, S.; Bruland, P.; Pereira, M.P.; Steinke, S.; Riepe, C.; Soto-Rey, I.; Garcovich, S.; Augustin, M.; Blome, C.; et al. Pruritus Intensity Scales across Europe: A prospective validation study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1176–1185. [Google Scholar] [CrossRef]
- Pereira, M.P.; Stander, S. Assessment of severity and burden of pruritus. Allergol. Int. 2017, 66, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, K.M.; Egeberg, A.; Gislason, G.H.; Skov, L.; Thyssen, J.P. Anxiety, depression and suicide in patients with prurigo nodularis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e106–e107. [Google Scholar] [CrossRef]
- Pereira, M.P.; Zeidler, C.; Wallengren, J.; Halvorsen, J.A.; Weisshaar, E.; Garcovich, S.; Misery, L.; Brenaut, E.; Savk, E.; Potekaev, N.; et al. Chronic Nodular Prurigo: A European Cross-sectional Study of Patient Perspectives on Therapeutic Goals and Satisfaction. Acta Derm. Venereol. 2021, 101, adv00403. [Google Scholar] [CrossRef]
- Zeidler, C.; Pereira, M.P.; Dugas, M.; Augustin, M.; Storck, M.; Weyer-Elberich, V.; Schneider, G.; Stander, S. The burden in chronic prurigo: Patients with chronic prurigo suffer more than patients with chronic pruritus on non-lesional skin: A comparative, retrospective, explorative statistical analysis of 4484 patients in a real-world cohort. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 738–743. [Google Scholar] [CrossRef]
- Schneider, G.; Hockmann, J.; Stander, S.; Luger, T.A.; Heuft, G. Psychological factors in prurigo nodularis in comparison with psoriasis vulgaris: Results of a case-control study. Br. J. Dermatol. 2006, 154, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.B.; Cerci, F.B.; Miracle, J.; Pokharel, A.; Chen, S.C.; Chan, Y.H.; Wilkin, A.; Yosipovitch, G. Chronic pruritus in HIV-positive patients in the southeastern United States: Its prevalence and effect on quality of life. J. Am. Acad. Dermatol. 2014, 70, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Magand, F.; Nacher, M.; Cazorla, C.; Cambazard, F.; Marie, D.S.; Couppie, P. Predictive values of prurigo nodularis and herpes zoster for HIV infection and immunosuppression requiring HAART in French Guiana. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 401–404. [Google Scholar] [CrossRef]
- Matthews, S.N.; Cockerell, C.J. Prurigo nodularis in HIV-infected individuals. Int. J. Dermatol. 1998, 37, 401–409. [Google Scholar] [CrossRef]
- Motegi, S.; Kato, M.; Uchiyama, A.; Yamada, K.; Shimizu, A.; Amano, H.; Ishikawa, O. Persistent prurigo nodularis in HIV-infected patient responsive to antiretroviral therapy with raltegravir. J. Dermatol. 2014, 41, 272–273. [Google Scholar] [CrossRef]
- Unemori, P.; Leslie, K.S.; Maurer, T. Persistent prurigo nodularis responsive to initiation of combination therapy with raltegravir. Arch. Dermatol. 2010, 146, 682–683. [Google Scholar] [CrossRef]
- Weisshaar, E.; Stander, S. Prurigo nodularis in hepatitis C infection: Result of an occupational disease? Acta Derm. Venereol. 2012, 92, 532–533. [Google Scholar] [CrossRef]
- Neri, S.; Raciti, C.; D’Angelo, G.; Ierna, D.; Bruno, C.M. Hyde’s prurigo nodularis and chronic HCV hepatitis. J. Hepatol. 1998, 28, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Yaoita, H.; Tsuda, F.; Murata, K.; Okamoto, H. Association of prurigo with hepatitis C virus infection. Arch. Dermatol. 1995, 131, 852–853. [Google Scholar] [CrossRef]
- Francesco Stefanini, G.; Resta, F.; Marsigli, L.; Gaddoni, G.; Baldassarri, L.; Caprio, G.P.; Degli Azzi, I.; Giuseppe Foschi, F.; Gasbarrini, G. Prurigo nodularis (Hyde’s prurigo) disclosing celiac disease. Hepatogastroenterology 1999, 46, 2281–2284. [Google Scholar]
- McKenzie, A.W.; Stubbing, D.G.; Elvy, B.L. Prurigo nodularis and gluten enteropathy. Br. J. Dermatol. 1976, 95, 89–92. [Google Scholar] [PubMed]
- Goodwin, P.G. Nodular prurigo assiciated with gluten enteropathy. Proc. R. Soc. Med. 1977, 70, 140–141. [Google Scholar] [CrossRef]
- Akarsu, S.; Ozbagcivan, O.; Ilknur, T.; Semiz, F.; Inci, B.B.; Fetil, E. Xerosis cutis and associated co-factors in women with prurigo nodularis. An. Bras. Dermatol. 2018, 93, 671–679. [Google Scholar] [CrossRef]
- Iking, A.; Grundmann, S.; Chatzigeorgakidis, E.; Phan, N.Q.; Klein, D.; Stander, S. Prurigo as a symptom of atopic and non-atopic diseases: Aetiological survey in a consecutive cohort of 108 patients. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 550–557. [Google Scholar] [CrossRef]
- Xu, Q.; Li, C.; Zhang, J.; Ling, B.; Yu, H.; Yao, Z. Generalized eruptive keratoacanthoma with vitiligo followed by the development of prurigo nodularis: A case report and published work review. J. Dermatol. 2018, 45, 211–215. [Google Scholar] [CrossRef]
- Wu, T.P.; Miller, K.; Cohen, D.E.; Stein, J.A. Keratoacanthomas arising in association with prurigo nodules in pruritic, actinically damaged skin. J. Am. Acad. Dermatol. 2013, 69, 426–430. [Google Scholar] [CrossRef]
- Roenigk, R.K.; Dahl, M.V. Bullous pemphigoid and prurigo nodularis. J. Am. Acad. Dermatol. 1986, 14 Pt 2, 944–947. [Google Scholar] [CrossRef]
- Torchia, D.; Caproni, M.; Del Bianco, E.; Cozzani, E.; Ketabchi, S.; Fabbri, P. Linear IgA disease presenting as prurigo nodularis. Br. J. Dermatol. 2006, 155, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Schweda, K.; Hainz, M.; Loquai, C.; Grabbe, S.; Saloga, J.; Tuettenberg, A. Prurigo nodularis as index symptom of (non-Hodgkin) lymphoma: Ultrasound as a helpful diagnostic tool in dermatological disorders of unknown origin. Int. J. Dermatol. 2015, 54, 462–464. [Google Scholar] [CrossRef]
- Shelnitz, L.S.; Paller, A.S. Hodgkin’s disease manifesting as prurigo nodularis. Pediatr. Dermatol. 1990, 7, 136–139. [Google Scholar] [CrossRef]
- Rubenstein, M.; Duvic, M. Cutaneous manifestations of Hodgkin’s disease. Int. J. Dermatol. 2006, 45, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Larson, V.A.; Tang, O.; Stander, S.; Miller, L.S.; Kang, S.; Kwatra, S.G. Association between prurigo nodularis and malignancy in middle-aged adults. J. Am. Acad. Dermatol. 2019, 81, 1198–1201. [Google Scholar] [CrossRef]
- Jerkovic Gulin, S.; Ceovic, R.; Loncaric, D.; Ilic, I.; Radman, I. Nodular Prurigo Associated with Mycosis Fungoides—Case Report. Acta Dermatovenerol. Croat. 2015, 23, 203–207. [Google Scholar]
- Funaki, M.; Ohno, T.; Dekio, S.; Jidoi, J.; Nakagawa, C.; Kin, S.; Tamura, K. Prurigo nodularis associated with advanced gastric cancer: Report of a case. J. Dermatol. 1996, 23, 703–707. [Google Scholar] [CrossRef]
- Winhoven, S.M.; Gawkrodger, D.J. Nodular prurigo: Metabolic diseases are a common association. Clin. Exp. Dermatol. 2007, 32, 224–225. [Google Scholar] [CrossRef]
- Kowalski, E.H.; Kneiber, D.; Valdebran, M.; Patel, U.; Amber, K.T. Treatment-resistant prurigo nodularis: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2019, 12, 163–172. [Google Scholar] [CrossRef]
- Swarna, S.S.; Aziz, K.; Zubair, T.; Qadir, N.; Khan, M. Pruritus Associated With Chronic Kidney Disease: A Comprehensive Literature Review. Cureus 2019, 11, e5256. [Google Scholar] [CrossRef]
- Shirazian, S.; Aina, O.; Park, Y.; Chowdhury, N.; Leger, K.; Hou, L.; Miyawaki, N.; Mathur, V.S. Chronic kidney disease-associated pruritus: Impact on quality of life and current management challenges. Int. J. Nephrol. Renov. Dis. 2017, 10, 11–26. [Google Scholar] [CrossRef]
- Combs, S.A.; Teixeira, J.P.; Germain, M.J. Pruritus in Kidney Disease. Semin. Nephrol. 2015, 35, 383–391. [Google Scholar] [CrossRef]
- Mettang, T. Pruritus in Renal Disease. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Prodanovich, S.; Kirsner, R.S.; Kravetz, J.D.; Ma, F.; Martinez, L.; Federman, D.G. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch. Dermatol. 2009, 145, 700–703. [Google Scholar] [CrossRef]
- Saco, M.; Cohen, G. Prurigo nodularis: Picking the right treatment. J. Fam. Pract. 2015, 64, 221–226. [Google Scholar]
- Stander, H.F.; Elmariah, S.; Zeidler, C.; Spellman, M.; Stander, S. Diagnostic and treatment algorithm for chronic nodular prurigo. J. Am. Acad. Dermatol. 2020, 82, 460–468. [Google Scholar] [CrossRef]
- Huang, A.H.; Canner, J.K.; Kang, S.; Kwatra, S.G. Analysis of real-world treatment patterns in patients with prurigo nodularis. J. Am. Acad. Dermatol. 2020, 82, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Saraceno, R.; Chiricozzi, A.; Nistico, S.P.; Tiberti, S.; Chimenti, S. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J. Dermatol. Treat. 2010, 21, 363–366. [Google Scholar] [CrossRef]
- Richards, R.N. Update on intralesional steroid: Focus on dermatoses. J. Cutan. Med. Surg. 2010, 14, 19–23. [Google Scholar] [CrossRef]
- Siepmann, D.; Lotts, T.; Blome, C.; Braeutigam, M.; Phan, N.Q.; Butterfass-Bahloul, T.; Augustin, M.; Luger, T.A.; Stander, S. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: Results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology 2013, 227, 353–360. [Google Scholar] [CrossRef]
- Stander, S.; Luger, T.; Metze, D. Treatment of prurigo nodularis with topical capsaicin. J. Am. Acad. Dermatol. 2001, 44, 471–478. [Google Scholar] [CrossRef]
- Patowary, P.; Pathak, M.P.; Zaman, K.; Raju, P.S.; Chattopadhyay, P. Research progress of capsaicin responses to various pharmacological challenges. Biomed. Pharmacother. 2017, 96, 1501–1512. [Google Scholar] [CrossRef]
- Lee, Y.W.; Won, C.H.; Jung, K.; Nam, H.J.; Choi, G.; Park, Y.H.; Park, M.; Kim, B. Efficacy and safety of PAC-14028 cream—A novel, topical, nonsteroidal, selective TRPV1 antagonist in patients with mild-to-moderate atopic dermatitis: A phase IIb randomized trial. Br. J. Dermatol. 2019, 180, 1030–1038. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, B.J.; Lee, Y.W.; Won, C.; Park, C.O.; Chung, B.Y.; Lee, D.H.; Jung, K.; Nam, H.J.; Choi, G.; et al. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: Phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). J. Allergy Clin. Immunol. 2022, 149, 1340–1347.e4. [Google Scholar] [CrossRef]
- Hammes, S.; Hermann, J.; Roos, S.; Ockenfels, H.M. UVB 308-nm excimer light and bath PUVA: Combination therapy is very effective in the treatment of prurigo nodularis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 799–803. [Google Scholar] [CrossRef]
- Spring, P.; Gschwind, I.; Gilliet, M. Prurigo nodularis: Retrospective study of 13 cases managed with methotrexate. Clin. Exp. Dermatol. 2014, 39, 468–473. [Google Scholar] [CrossRef]
- Klejtman, T.; Beylot-Barry, M.; Joly, P.; Richard, M.A.; Debarbieux, S.; Misery, L.; Wolkenstein, P.; Chosidow, O.; Ingen-Housz-Oro, S. Treatment of prurigo with methotrexate: A multicentre retrospective study of 39 cases. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 437–440. [Google Scholar] [CrossRef]
- Siepmann, D.; Luger, T.A.; Stander, S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: Results of a case series. J. Dtsch. Dermatol. Ges. 2008, 6, 941–946. [Google Scholar] [CrossRef]
- Wiznia, L.E.; Callahan, S.W.; Cohen, D.E.; Orlow, S.J. Rapid improvement of prurigo nodularis with cyclosporine treatment. J. Am. Acad. Dermatol. 2018, 78, 1209–1211. [Google Scholar] [CrossRef]
- Lear, J.T.; English, J.S.; Smith, A.G. Nodular prurigo responsive to azathioprine. Br. J. Dermatol. 1996, 134, 1151. [Google Scholar] [CrossRef]
- Maley, A.; Swerlick, R.A. Azathioprine treatment of intractable pruritus: A retrospective review. J. Am. Acad. Dermatol. 2015, 73, 439–443. [Google Scholar] [CrossRef]
- Zhai, L.L.; Savage, K.T.; Qiu, C.C.; Jin, A.; Valdes-Rodriguez, R.; Mollanazar, N.K. Chronic Pruritus Responding to Dupilumab-A Case Series. Medicines 2019, 6, 72. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Elgash, M.; Weaver, L.; Valdes-Rodriguez, R.; Hsu, S. Reduced Itch Associated With Dupilumab Treatment In 4 Patients With Prurigo Nodularis. JAMA Dermatol. 2019, 155, 121–122. [Google Scholar] [CrossRef]
- Beck, K.M.; Yang, E.J.; Sekhon, S.; Bhutani, T.; Liao, W. Dupilumab Treatment for Generalized Prurigo Nodularis. JAMA Dermatol. 2019, 155, 118–120. [Google Scholar] [CrossRef]
- Napolitano, M.; Fabbrocini, G.; Scalvenzi, M.; Nistico, S.P.; Dastoli, S.; Patruno, C. Effectiveness of Dupilumab for the Treatment of Generalized Prurigo Nodularis Phenotype of Adult Atopic Dermatitis. Dermatitis 2020, 31, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Rambhia, P.H.; Levitt, J.O. Recalcitrant prurigo nodularis treated successfully with dupilumab. JAAD Case Rep. 2019, 5, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Mollanazar, N.; Stander, S.; Kwatra, S.G.; Kim, B.S.; Laws, E.; Mannent, L.P.; Amin, N.; Akinlade, B.; Staudinger, H.W.; et al. Dupilumab in patients with prurigo nodularis: Two randomized, double-blind, placebo-controlled phase 3 trials. Nat. Med. 2023, 29, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Fachler, T.; Maria Faitataziadou, S.; Molho-Pessach, V. Dupilumab for pediatric prurigo nodularis: A case report. Pediatr. Dermatol. 2021, 38, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.Y.; Lian, C.H. The effectiveness and safety of dupilumab in the management of refractory prurigo nodularis in 45 Chinese patients: A real-life observational study. J. Dermatol. 2023, 50, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Mori, F.; Oranges, T.; Ricci, S.; Barni, S.; Canessa, C.; Liccioli, G.; Lodi, L.; Sarti, L.; Novembre, E.; et al. Dupilumab treatment of prurigo nodularis in an adolescent. Eur. J. Dermatol. 2021, 31, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Husein-ElAhmed, H.; Steinhoff, M. Dupilumab in prurigo nodularis: A systematic review of current evidence and analysis of predictive factors to response. J. Dermatol. Treat. 2022, 33, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, J.R.; Croitoru, D.; Felfeli, T.; Alhusayen, R.; Lansang, P.; Shear, N.H.; Yeung, J.; Walsh, S. Long-term dupilumab treatment for chronic refractory generalized prurigo nodularis: A retrospective cohort study. J. Am. Acad. Dermatol. 2021, 85, 1049–1051. [Google Scholar] [CrossRef]
- Selvaraj, K.J.; Stewart, T.; Frew, J.W. Maintenance of response to dupilumab in prurigo nodularis: A retrospective cohort study. JAAD Int. 2023, 11, 143–144. [Google Scholar] [CrossRef]
- Pezzolo, E.; Gambardella, A.; Guanti, M.; Bianchelli, T.; Bertoldi, A.; Giacchetti, A.; Donini, M.; Argenziano, G.; Naldi, L. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: A multicenter, prospective, open-label case series study. J. Am. Acad. Dermatol. 2023, 89, 430–432. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78. [Google Scholar] [CrossRef]
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2019, 20, 181–192. [Google Scholar] [CrossRef]
- Bieber, T.; Simpson, E.L.; Silverberg, J.I.; Thaci, D.; Paul, C.; Pink, A.E.; Kataoka, Y.; Chu, C.Y.; DiBonaventura, M.; Rojo, R.; et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1101–1112. [Google Scholar] [CrossRef]
- Stolzl, D.; Sander, N.; Heratizadeh, A.; Haufe, E.; Harder, I.; Abraham, S.; Heinrich, L.; Kleinheinz, A.; Wollenberg, A.; Weisshaar, E.; et al. Real-world data on the effectiveness, safety and drug survival of dupilumab: An analysis from the TREATgermany registry. Br. J. Dermatol. 2022, 187, 1022–1024. [Google Scholar] [CrossRef]
- Simpson, E.L.; Papp, K.A.; Blauvelt, A.; Chu, C.Y.; Hong, H.C.; Katoh, N.; Calimlim, B.M.; Thyssen, J.P.; Chiou, A.S.; Bissonnette, R.; et al. Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis: Analysis of Follow-up Data From the Measure Up 1 and Measure Up 2 Randomized Clinical Trials. JAMA Dermatol. 2022, 158, 404–413. [Google Scholar] [CrossRef]
- Simpson, E.L.; Merola, J.F.; Silverberg, J.I.; Reich, K.; Warren, R.B.; Staumont-Salle, D.; Girolomoni, G.; Papp, K.; de Bruin-Weller, M.; Thyssen, J.P.; et al. Safety of tralokinumab in adult patients with moderate-to-severe atopic dermatitis: Pooled analysis of five randomized, double-blind, placebo-controlled phase II and phase III trials. Br. J. Dermatol. 2022, 187, 888–899. [Google Scholar] [CrossRef]
- Reich, K.; Thyssen, J.P.; Blauvelt, A.; Eyerich, K.; Soong, W.; Rice, Z.P.; Hong, H.C.; Katoh, N.; Valenzuela, F.; DiBonaventura, M.; et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: A randomised, double-blind, multicentre phase 3 trial. Lancet 2022, 400, 273–282. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Thyssen, J.P.; Silverberg, J.I.; Papp, K.A.; Paller, A.S.; Weidinger, S.; Chih-Ho Hong, H.; Hendrickson, B.; Dilley, D.; Tenorio, A.R.; et al. Safety of upadacitinib in moderate-to-severe atopic dermatitis: An integrated analysis of phase 3 studies. J. Allergy Clin. Immunol. 2023, 151, 172–181. [Google Scholar] [CrossRef]
- Yew, Y.W.; Yeo, P.M. Comparison between dupilumab and oral Janus kinase inhibitors in the treatment of prurigo nodularis with or without atopic dermatitis in a tertiary care center in Singapore. JAAD Int. 2023, 13, 13–14. [Google Scholar] [CrossRef]
- Peng, C.; Li, C.; Zhou, Y.; Wang, Q.; Xie, P.; Li, T.; Hao, P. Tofacitinib for Prurigo Nodularis: A Case Report. Clin. Cosmet. Investig. Dermatol. 2022, 15, 503–506. [Google Scholar] [CrossRef]
- Liu, T.; Chu, Y.; Wang, Y.; Zhong, X.; Yang, C.; Bai, J.; Fang, H.; Qiao, J. Successful treatment of prurigo nodularis with tofacitinib: The experience from a single center. Int. J. Dermatol. 2023, 62, e293–e295. [Google Scholar] [CrossRef]
- Molloy, O.E.; Kearney, N.; Byrne, N.; Kirby, B. Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin. Exp. Dermatol. 2020, 45, 918–920. [Google Scholar] [CrossRef]
- Gil-Lianes, J.; Morgado-Carrasco, D.; Riquelme-Mc Loughlin, C. Treatment of chronic prurigo with upadacitinib: A case series. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e106–e109. [Google Scholar] [CrossRef]
- Yin, M.; Wu, R.; Chen, J.; Dou, X. Successful treatment of refractory prurigo nodularis with baricitinib. Dermatol. Ther. 2022, 35, e15642. [Google Scholar] [CrossRef]
- Pereira, M.P.; Zeidler, C.; Stander, S. Improvement of chronic nodular prurigo with baricitinib. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e486–e488. [Google Scholar] [CrossRef]
- He, Y.; Ji, S.; Yu, Q. Effectiveness of baricitinib in prurigo-type atopic dermatitis: A case report. Dermatol. Ther. 2021, 34, e14878. [Google Scholar] [CrossRef]
- Agrawal, D.; Sardana, K.; Mathachan, S.R.; Ahuja, A. A Case of Recalcitrant Prurigo Nodularis with Heightened Expression of STAT 3 and STAT 6 and its Dramatic Response to Tofacitinib. Indian Dermatol. Online J. 2023, 14, 564–566. [Google Scholar] [CrossRef]
- Fakhraie, S.; Chovatiya, R. Janus Kinase Inhibition in the Treatment of Prurigo Nodularis. Dermatitis 2023. [Google Scholar] [CrossRef]
- Bonnekoh, H.; Butze, M.; Metz, M. Characterization of the effects on pruritus by novel treatments for atopic dermatitis. J. Dtsch. Dermatol. Ges. 2022, 20, 150–156. [Google Scholar] [CrossRef]
- Stander, S.; Bockenholt, B.; Schurmeyer-Horst, F.; Weishaupt, C.; Heuft, G.; Luger, T.A.; Schneider, G. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: Results of an open-labelled, two-arm proof-of-concept study. Acta Derm. Venereol. 2009, 89, 45–51. [Google Scholar] [CrossRef]
- Hashimoto, T.; Satoh, T.; Yokozeki, H. Prurigo successfully treated with duloxetine hydrochloride. Australas. J. Dermatol. 2019, 60, 237–239. [Google Scholar] [CrossRef]
- Griffin, J.R.; Davis, M.D. Amitriptyline/Ketamine as therapy for neuropathic pruritus and pain secondary to herpes zoster. J. Drugs Dermatol. 2015, 14, 115–118. [Google Scholar]
- Boozalis, E.; Khanna, R.; Zampella, J.G.; Kwatra, S.G. Tricyclic antidepressants for the treatment of chronic pruritus. J. Dermatol. Treat. 2021, 32, 124–126. [Google Scholar] [CrossRef]
- Matsuda, K.M.; Sharma, D.; Schonfeld, A.R.; Kwatra, S.G. Gabapentin and pregabalin for the treatment of chronic pruritus. J. Am. Acad. Dermatol. 2016, 75, 619–625.e6. [Google Scholar] [CrossRef]
- He, A.; Alhariri, J.M.; Sweren, R.J.; Kwatra, M.M.; Kwatra, S.G. Aprepitant for the Treatment of Chronic Refractory Pruritus. BioMed Res. Int. 2017, 2017, 4790810. [Google Scholar] [CrossRef]
- Stander, S.; Kwon, P.; Hirman, J.; Perlman, A.J.; Weisshaar, E.; Metz, M.; Luger, T.A.; Group, T.C.P.S. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 2019, 80, 1395–1402. [Google Scholar] [CrossRef]
- Stander, S.; Siepmann, D.; Herrgott, I.; Sunderkotter, C.; Luger, T.A. Targeting the neurokinin receptor 1 with aprepitant: A novel antipruritic strategy. PLoS ONE 2010, 5, e10968. [Google Scholar] [CrossRef]
- Ogasawara, H.; Furuno, M.; Edamura, K.; Noguchi, M. Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells. J. Leukoc. Biol. 2019, 106, 1069–1077. [Google Scholar] [CrossRef]
- Sharma, D.; Kwatra, S.G. Thalidomide for the treatment of chronic refractory pruritus. J. Am. Acad. Dermatol. 2016, 74, 363–369. [Google Scholar] [CrossRef]
- Taefehnorooz, H.; Truchetet, F.; Barbaud, A.; Schmutz, J.L.; Bursztejn, A.C. Efficacy of thalidomide in the treatment of prurigo nodularis. Acta Derm. Venereol. 2011, 91, 344–345. [Google Scholar] [CrossRef]
- Andersen, T.P.; Fogh, K. Thalidomide in 42 patients with prurigo nodularis Hyde. Dermatology 2011, 223, 107–112. [Google Scholar] [CrossRef]
- Kim, B.S.; Inan, S.; Stander, S.; Sciascia, T.; Szepietowski, J.C.; Yosipovitch, G. Role of kappa-opioid and mu-opioid receptors in pruritus: Peripheral and central itch circuits. Exp. Dermatol. 2022, 31, 1900–1907. [Google Scholar] [CrossRef]
- Dawn, A.G.; Yosipovitch, G. Butorphanol for treatment of intractable pruritus. J. Am. Acad. Dermatol. 2006, 54, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Metze, D.; Reimann, S.; Beissert, S.; Luger, T. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J. Am. Acad. Dermatol. 1999, 41, 533–539. [Google Scholar] [PubMed]
- Lee, J.; Shin, J.U.; Noh, S.; Park, C.O.; Lee, K.H. Clinical Efficacy and Safety of Naltrexone Combination Therapy in Older Patients with Severe Pruritus. Ann. Dermatol. 2016, 28, 159–163. [Google Scholar] [CrossRef]
- Hawi, A.; Alcorn, H., Jr.; Berg, J.; Hines, C.; Hait, H.; Sciascia, T. Pharmacokinetics of nalbuphine hydrochloride extended release tablets in hemodialysis patients with exploratory effect on pruritus. BMC Nephrol. 2015, 16, 47. [Google Scholar] [CrossRef]
- Weisshaar, E.; Szepietowski, J.C.; Bernhard, J.D.; Hait, H.; Legat, F.J.; Nattkemper, L.; Reich, A.; Sadoghi, B.; Sciascia, T.R.; Zeidler, C.; et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: Results of a phase 2 randomized controlled trial with an open-label extension phase. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Visse, K.; Blome, C.; Phan, N.Q.; Augustin, M.; Stander, S. Efficacy of Body Lotion Containing N-palmitoylethanolamine in Subjects with Chronic Pruritus due to Dry Skin: A Dermatocosmetic Study. Acta Derm. Venereol. 2017, 97, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Khanna, R.; Denny, G.; Kwatra, S.G. Cannabinoids for the treatment of chronic refractory pruritus. J. Dermatol. Treat. 2021, 32, 266–267. [Google Scholar] [CrossRef]
- Avila, C.; Massick, S.; Kaffenberger, B.H.; Kwatra, S.G.; Bechtel, M. Cannabinoids for the treatment of chronic pruritus: A review. J. Am. Acad. Dermatol. 2020, 82, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Massimini, M.; Dalle Vedove, E.; Bachetti, B.; Di Pierro, F.; Ribecco, C.; D’Addario, C.; Pucci, M. Polyphenols and Cannabidiol Modulate Transcriptional Regulation of Th1/Th2 Inflammatory Genes Related to Canine Atopic Dermatitis. Front. Vet. Sci. 2021, 8, 606197. [Google Scholar] [CrossRef]
- Todurga Seven, Z.G.; Cakir Gundogdu, A.; Ozyurt, R.; Ozyazgan, S. The Effects of Cannabinoid Agonist, Heat Shock Protein 90 and Nitric Oxide Synthase Inhibitors on Increasing IL-13 and IL-31 Levels in Chronic Pruritus. Immunol. Investig. 2022, 51, 1938–1949. [Google Scholar] [CrossRef]
- Haruna, T.; Soga, M.; Morioka, Y.; Hikita, I.; Imura, K.; Furue, Y.; Yamamoto, M.; Imura, C.; Ikeda, M.; Yamauchi, A.; et al. S-777469, a novel cannabinoid type 2 receptor agonist, suppresses itch-associated scratching behavior in rodents through inhibition of itch signal transmission. Pharmacology 2015, 95, 95–103. [Google Scholar] [CrossRef]
- Pulvirenti, N.; Nasca, M.R.; Micali, G. Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: A pilot study. Acta Dermatovenerol. Croat. 2007, 15, 80–83. [Google Scholar] [PubMed]
- Yuan, C.; Wang, X.M.; Guichard, A.; Tan, Y.M.; Qian, C.Y.; Yang, L.J.; Humbert, P. N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: Results of a randomized, double-blind, controlled study in 60 patients. Clin. Interv. Aging 2014, 9, 1163–1169. [Google Scholar] [CrossRef]
- Stander, S.; Reinhardt, H.W.; Luger, T.A. Topical cannabinoid agonists. An effective new possibility for treating chronic pruritus. Hautarzt 2006, 57, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Nemolizumab: First Approval. Drugs 2022, 82, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Mihara, R. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 2093. [Google Scholar] [CrossRef]
- Muller, S.; Zeidler, C.; Stander, S. Chronic Prurigo Including Prurigo Nodularis: New Insights and Treatments. Am. J. Clin. Dermatol. 2024, 25, 15–33. [Google Scholar] [CrossRef]
- Kwatra, S.G.; Yosipovitch, G.; Legat, F.J.; Reich, A.; Paul, C.; Simon, D.; Naldi, L.; Lynde, C.; De Bruin-Weller, M.S.; Nahm, W.K.; et al. Phase 3 Trial of Nemolizumab in Patients with Prurigo Nodularis. N. Engl. J. Med. 2023, 389, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Stander, S.; Yosipovitch, G.; Legat, F.J.; Lacour, J.P.; Paul, C.; Narbutt, J.; Bieber, T.; Misery, L.; Wollenberg, A.; Reich, A.; et al. Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis. N. Engl. J. Med. 2020, 382, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Stander, S.; Yosipovitch, G.; Lacour, J.P.; Legat, F.J.; Paul, C.; Reich, A.; Chaouche, K.; Ahmad, F.; Piketty, C. Nemolizumab efficacy in prurigo nodularis: Onset of action on itch and sleep disturbances. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Labib, A.; Ju, T.; Vander Does, A.; Yosipovitch, G. Immunotargets and Therapy for Prurigo Nodularis. Immunotargets Ther. 2022, 11, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sofen, H.; Bissonnette, R.; Yosipovitch, G.; Silverberg, J.I.; Tyring, S.; Loo, W.J.; Zook, M.; Lee, M.; Zou, L.; Jiang, G.L.; et al. Efficacy and safety of vixarelimab, a human monoclonal oncostatin M receptor beta antibody, in moderate-to-severe prurigo nodularis: A randomised, double-blind, placebo-controlled, phase 2a study. EClinicalMedicine 2023, 57, 101826. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Koenderman, L. Immunological and hematological effects of IL-5(Ralpha)-targeted therapy: An overview. Allergy 2018, 73, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M.; et al. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Singh, U.; Rao, M.B.; Berendts, K.; Zhang, X.; Mutasim, D. Benralizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 2020, 383, 1389–1391. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Singh, U.; Rao, M.B.; Berendts, K.; Zhang, X.; Mutasim, D. Treatment of chronic spontaneous urticaria with benralizumab: Report of primary endpoint per-protocol analysis and exploratory endpoints. Allergy 2021, 76, 1277–1280. [Google Scholar] [CrossRef]
- Zhang, S.; Sumpter, T.L.; Kaplan, D.H. Neuron–Mast Cell Cross-Talk in the Skin. J. Investig. Dermatol. 2022, 142 Pt B, 841–848. [Google Scholar] [CrossRef]
- Gupta, K.; Harvima, I.T. Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, D.; Maurer, M.; Gedrich, R.; Seibel, S.B.; Murphy, M.B.; Crew, L.; Goldstein, J.; Crocker, A.; Vitale, L.A.; Morani, P.A.; et al. Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study. Allergy 2022, 77, 2393–2403. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yook, H.J.; Lee, J.H. Prurigo Nodularis: Pathogenesis and the Horizon of Potential Therapeutics. Int. J. Mol. Sci. 2024, 25, 5164. https://doi.org/10.3390/ijms25105164
Yook HJ, Lee JH. Prurigo Nodularis: Pathogenesis and the Horizon of Potential Therapeutics. International Journal of Molecular Sciences. 2024; 25(10):5164. https://doi.org/10.3390/ijms25105164
Chicago/Turabian StyleYook, Hwa Jung, and Ji Hyun Lee. 2024. "Prurigo Nodularis: Pathogenesis and the Horizon of Potential Therapeutics" International Journal of Molecular Sciences 25, no. 10: 5164. https://doi.org/10.3390/ijms25105164
APA StyleYook, H. J., & Lee, J. H. (2024). Prurigo Nodularis: Pathogenesis and the Horizon of Potential Therapeutics. International Journal of Molecular Sciences, 25(10), 5164. https://doi.org/10.3390/ijms25105164





