Exploring the Influence of Cold Plasma on Epidermal Melanogenesis In Situ and In Vitro
Abstract
:1. Introduction
2. Results
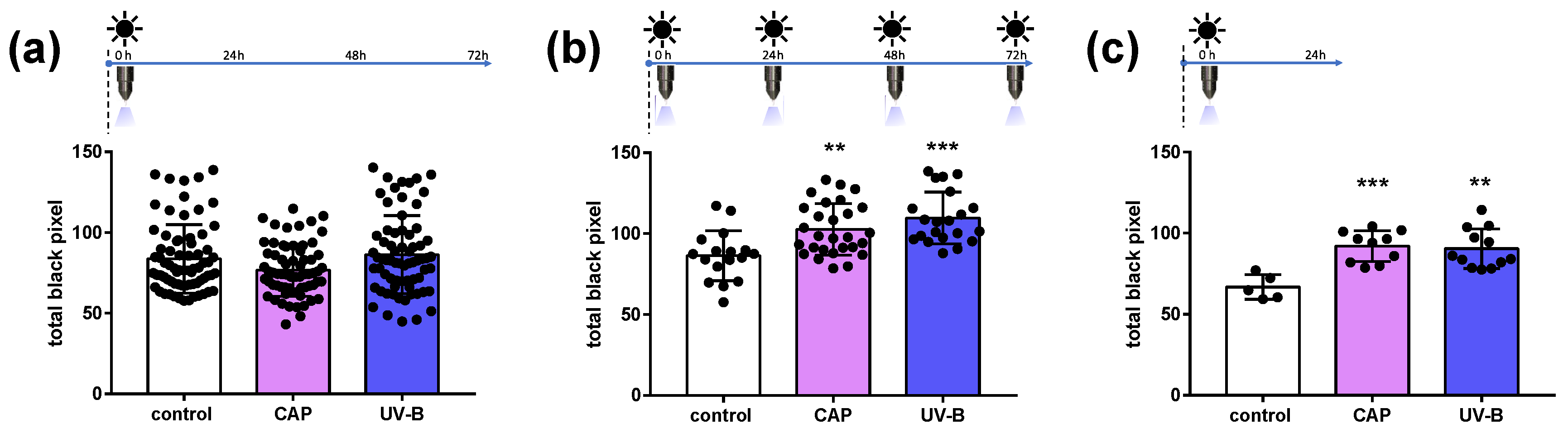
2.1. Melanin Content in Human Skin Samples after CAP Exposure
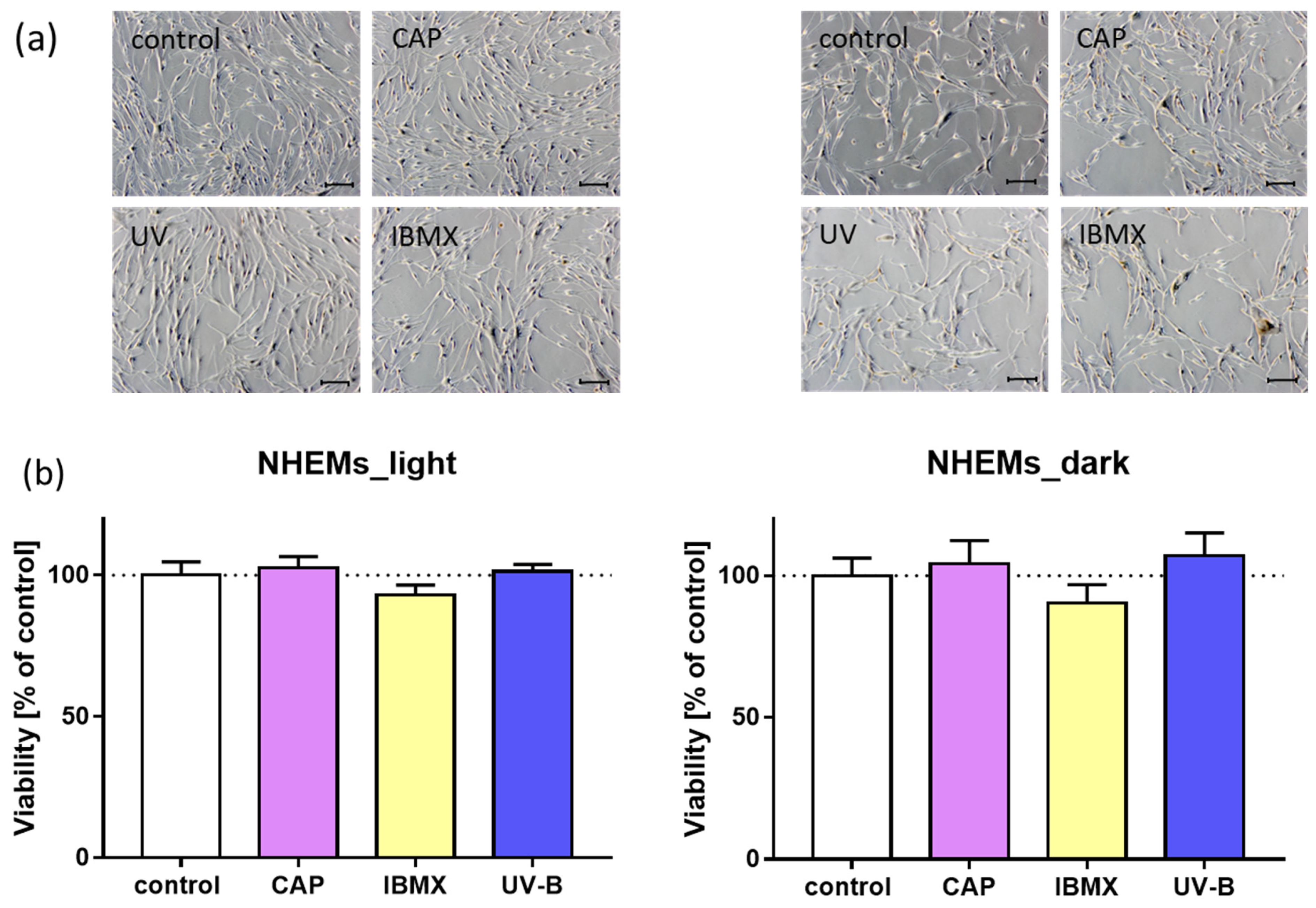
2.2. Morphology and Viability of Cultured Melanocytes after Treatment with CAP in Comparison to UV and IBMX
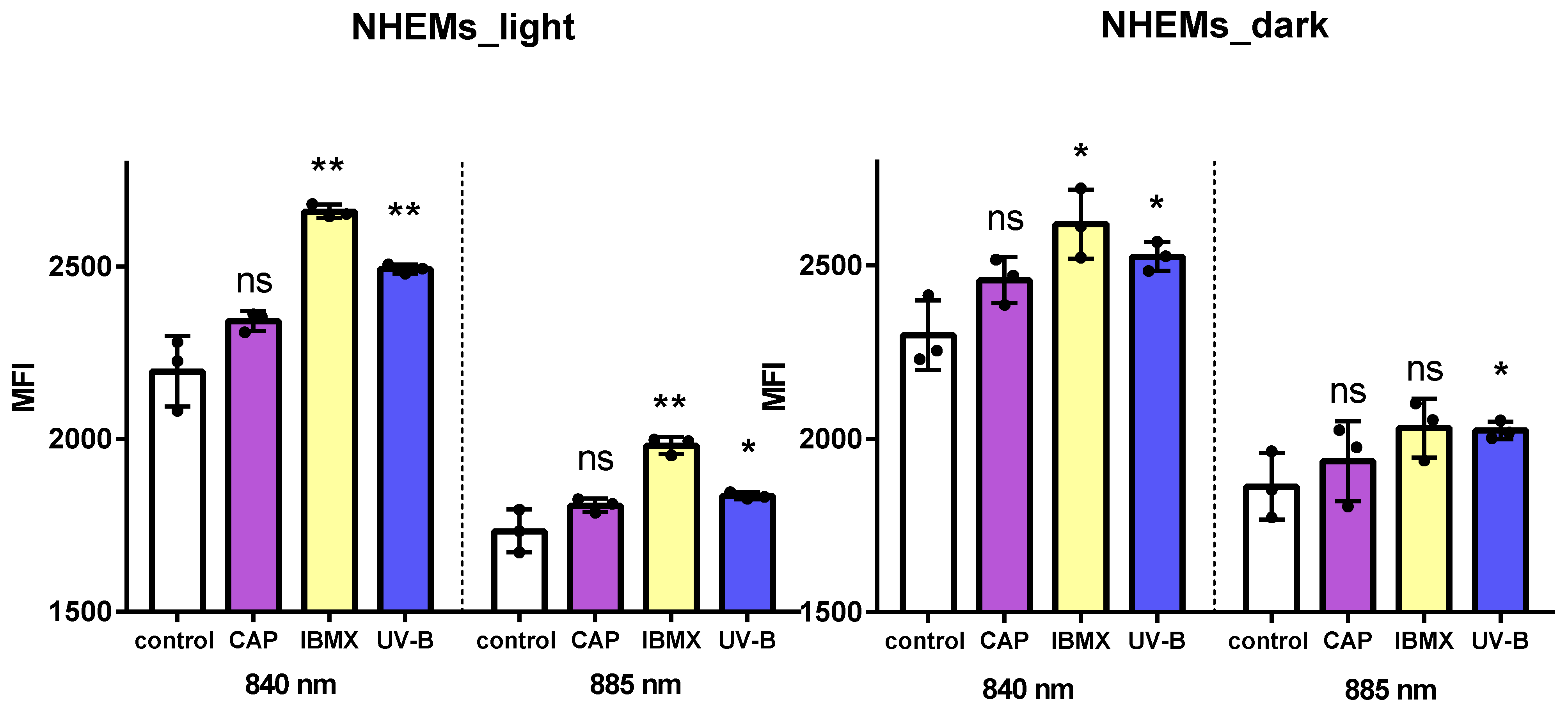
2.3. Flow Cytometry for Total Melanin Content in Cultured Human Melanocytes
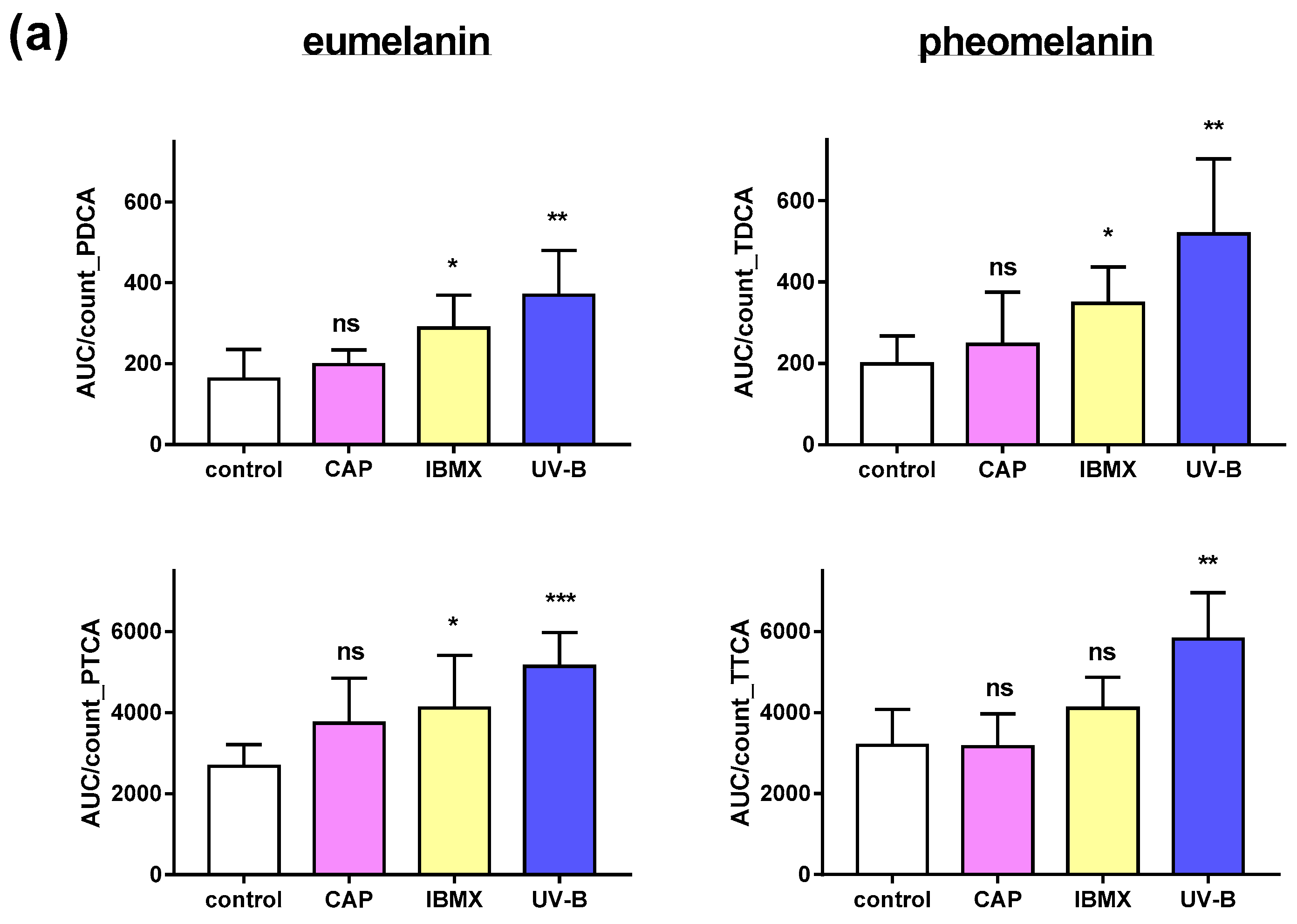
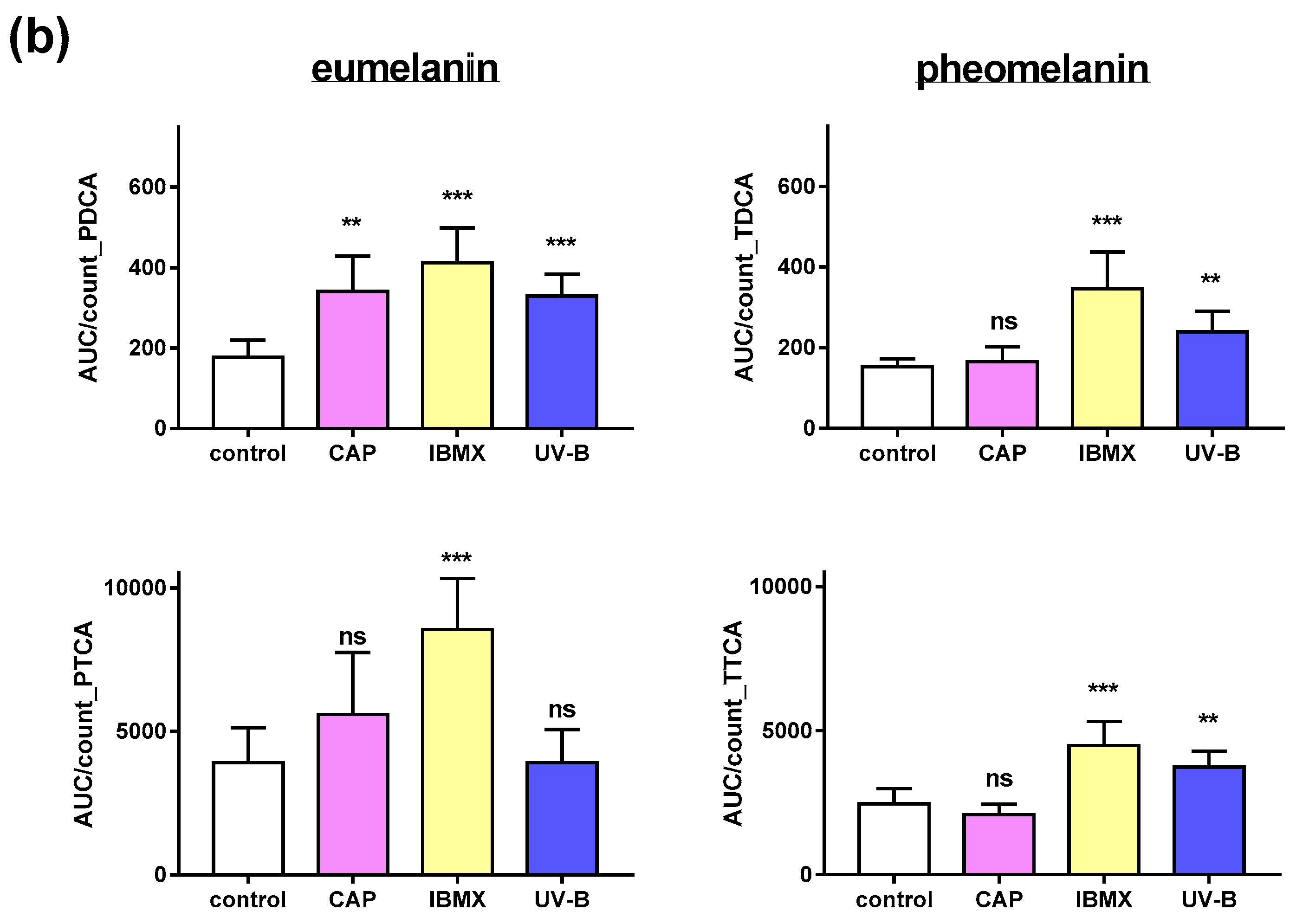
2.4. Pheomelanin and Eumelanin in Cultured Melanocytes after Treatment with CAP, UV and IBMX
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Exposure to CAP and UV
4.3. Skin Samples and Treatment Scheme
4.4. Melanin Staining and Image Analyses
4.5. Cell Culture and Treatment Scheme
4.6. Cell Viability and Cell Count
4.7. Flow Cytometry
4.8. Sample Preparation and Melanin Degradation
4.9. LC-MS Conditions
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schallreuter, K.U. A review of recent advances on the regulation of pigmentation in the human epidermis. Cell. Mol. Biol. 1999, 45, 943–949. [Google Scholar] [PubMed]
- Schallreuter, K.U.; Kothari, S.; Chavan, B.; Spencer, J.D. Regulation of melanogenesis—Controversies and new concepts. Exp. Dermatol. 2008, 17, 395–404. [Google Scholar] [CrossRef]
- D‘Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Dabas, G.; Vinay, K.; Parsad, D.; Kumar, A.; Kumaran, M.S. Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Kruger, C.; Schallreuter, K.U. Stigmatisation, Avoidance Behaviour and Difficulties in Coping are Common among Adult Patients with Vitiligo. Acta Derm.-Venereol. 2015, 95, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Heinlin, J.; Morfill, G.; Landthaler, M.; Stolz, W.; Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Karrer, S. Plasma medicine: Possible applications in dermatology. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2010, 8, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, T.; Semmler, M.L.; Schafer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxidative Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef] [PubMed]
- Heinlin, J.; Isbary, G.; Stolz, W.; Morfill, G.; Landthaler, M.; Shimizu, T.; Steffes, B.; Nosenko, T.; Zimmermann, J.; Karrer, S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. JEADV 2011, 25, 1–11. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Gorlitz, A.; Simon, D.; Schon, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 148–155. [Google Scholar] [CrossRef]
- Chuangsuwanich, A.; Assadamongkol, T.; Boonyawan, D. The Healing Effect of Low-Temperature Atmospheric-Pressure Plasma in Pressure Ulcer: A Randomized Controlled Trial. Int. J. Low. Extrem. Wounds 2016, 15, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, S.; Fathollah, S.; Mansouri, P.; Larijani, B.; Ghoranneviss, M.; Mohajeri Tehrani, M.; Amini, M.R. Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: A randomized clinical trial. Sci. Rep. 2020, 10, 10440. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients with Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Strohal, R.; Dietrich, S.; Mittlbock, M.; Hammerle, G. Chronic wounds treated with cold atmospheric plasmajet versus best practice wound dressings: A multicenter, randomized, non-inferiority trial. Sci. Rep. 2022, 12, 3645. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Shimizu, T.; Li, Y.F.; Stolz, W.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L. Cold atmospheric plasma devices for medical issues. Expert Rev. Med. Devices 2013, 10, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Laroussi, M.; Gherardi, M. Foundations of plasmas for medical applications. Plasma Sources Sci. Technol. 2022, 31, 054002. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wazir, U.; Kothari, S.; Gibbons, N.C.; Moore, J.; Wood, J.M. Human phenylalanine hydroxylase is activated by H2O2: A novel mechanism for increasing the L-tyrosine supply for melanogenesis in melanocytes. Biochem. Biophys. Res. Commun. 2004, 322, 88–92. [Google Scholar] [CrossRef]
- Wood, J.M.; Chavan, B.; Hafeez, I.; Schallreuter, K.U. Regulation of tyrosinase by tetrahydropteridines and H2O2. Biochem. Biophys. Res. Commun. 2004, 325, 1412–1417. [Google Scholar] [CrossRef]
- Mishima, Y. New Technic for Comprehensive Demonstration of Melanin, Premelanin, and Tyrosinase Sites—Combined Dopa-Premelanin Reaction. J. Investig. Dermatol. 1960, 34, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Jimbow, K. Quantitative Analysis of Eumelanin and Pheomelanin in Hair and Melanomas. J. Investig. Dermatol. 1983, 80, 268–272. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Quantitative Analysis of Eumelanin and Pheomelanin in Humans, Mice, and Other Animals: A Comparative Review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.; Stoffels, I.; Dissemond, J.; Schadendorf, D.; Roesch, A. Actinic keratoses treated with cold atmospheric plasma. J. Eur. Acad. Dermatol. 2018, 32, E37–E39. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; et al. Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Advanced Chemical Methods in Melanin Determination. Pigment Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Gan, L.; Nie, L.; Sun, F.; Lu, X.; He, G. On the penetration of reactive oxygen and nitrogen species generated by a plasma jet into and through mice skin with/without stratum corneum. Phys. Plasmas 2019, 26, 043504. [Google Scholar] [CrossRef]
- Lademann, O.; Richter, H.; Kramer, A.; Patzelt, A.; Meinke, M.C.; Graf, C.; Gao, Q.; Korotianskiy, E.; Rühl, E.; Weltmann, K.D.; et al. Stimulation of the penetration of particles into the skin by plasma tissue interaction. Laser Phys. Lett. 2011, 8, 758–764. [Google Scholar] [CrossRef]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doring, F.; Normann, L.; Diedrich, S.; Weiss, F.U.; Evert, M.; Hubner, N.O.; Gunther, C.; et al. Tissue Tolerable Plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Hasse, S.; Duong Tran, T.; Hahn, O.; Kindler, S.; Metelmann, H.R.; von Woedtke, T.; Masur, K. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clin. Exp. Dermatol. 2016, 41, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Morita, A. Mechanisms of ultraviolet (UV) B and UVA phototherapy. J. Investig. Dermatology. Symp. Proc. 1999, 4, 70–72. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Szili, E.J.; Bradley, J.W.; Short, R.D. A ‘tissue model’ to study the plasma delivery of reactive oxygen species. J. Phys. D Appl. Phys. 2014, 47, 152002. [Google Scholar] [CrossRef]
- Magina, S.; Esteves-Pinto, C.; Moura, E.; Serrao, M.P.; Moura, D.; Petrosino, S.; Di Marzo, V.; Vieira-Coelho, M.A. Inhibition of basal and ultraviolet B-induced melanogenesis by cannabinoid CB(1) receptors: A keratinocyte-dependent effect. Arch. Dermatol. Res. 2011, 303, 201–210. [Google Scholar] [CrossRef]
- Duval, C.; Régnier, M.; Schmidt, R. Distinct Melanogenic Response of Human Melanocytes in Mono-culture, in Co-Culture with Keratinocytes and in Reconstructed Epidermis, to UV Exposure. Pigment Cell Res. 2001, 14, 348–355. [Google Scholar] [CrossRef]
- Schmidt, A.; von Woedtke, T.; Vollmar, B.; Hasse, S.; Bekeschus, S. Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing. Theranostics 2019, 9, 1066–1084. [Google Scholar] [CrossRef]
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxidative Med. Cell. Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef]
- Kim, J.E.; Finlay, G.J.; Baguley, B.C. The Role of the Hippo Pathway in Melanocytes and Melanoma. Front. Oncol. 2013, 3, 123. [Google Scholar] [CrossRef]
- Marrot, L.; Jones, C.; Perez, P.; Meunier, J.R. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008, 21, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishitsuka, Y. NRF2 in the Epidermal Pigmentary System. Biomolecules 2022, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kim, M.Y.; Sohn, K.C.; Jung, S.Y.; Lee, H.E.; Lim, J.W.; Kim, S.; Lee, Y.H.; Im, M.; Seo, Y.J.; et al. Nrf2 negatively regulates melanogenesis by modulating PI3K/Akt signaling. PLoS ONE 2014, 9, e96035. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Li, K.; Song, P.; Zhu, G.; Zhu, L.; Cui, T.; Liu, B.; Tang, L.; Wang, X.; Wang, G.; et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: A possible mechanism for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2014, 134, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Yamaguchi, Y.; Batzer, J.; Coelho, S.G.; Zmudzka, B.Z.; Miller, S.A.; Wolber, R.; Beer, J.Z.; Hearing, V.J. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Investig. Dermatol. 2005, 124, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, G.; Bolasco, G.; Aspite, N.; Lucania, G.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J. Investig. Dermatol. 2008, 128, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.G.; Nair, N.; Begum, G.; Joshi, N.B.; Sinkar, V.P.; Vora, S. Melanocyte-keratinocyte interaction induces calcium signalling and melanin transfer to keratinocytes. Pigm. Cell Res. 2007, 20, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Schallreuter, K.U.; Thody, A.J.; Gummer, C.; Tobin, D.J. Regulation of human epidermal melanocyte biology by β-endorphin. J. Investig. Dermatol. 2003, 120, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ashraf, Z.; Kumar, N.; Rafiq, M.; Jabeen, F.; Park, J.H.; Choi, K.H.; Lee, S.; Seo, S.Y.; Choi, E.H.; et al. Influence of plasma-activated compounds on melanogenesis and tyrosinase activity. Sci. Rep. 2016, 6, 21779. [Google Scholar] [CrossRef]
- Fernandes, B.; Matama, T.; Guimaraes, D.; Gomes, A.; Cavaco-Paulo, A. Fluorescent quantification of melanin. Pigment Cell Melanoma Res. 2016, 29, 707–712. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, H.; Hamzavi, I.; Alajlan, A.; Tan, E.; McLean, D.I.; Lui, H. Cutaneous melanin exhibiting fluorescence emission under near-infrared light excitation. J. Biomed. Opt. 2006, 11, 34010. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Zhao, J.; Zeng, H.; McLean, D.; Kollias, N.; Lui, H. Melanin quantification by in vitro and in vivo analysis of near-infrared fluorescence. Pigment Cell Melanoma Res. 2018, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.; Swope, V.B.; Pallas, J.; Kriug, K.; Nordlund, J.J. Mitogenic, Melanogenie, and cAMP Responses of Cultured Neonatal Human Melanocytes to Commonly Used Mitogens. J. Cell. Physiol. 1992, 150, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Hasse, S.; Meder, T.; Freund, E.; von Woedtke, T.; Bekeschus, S. Plasma Treatment Limits Human Melanoma Spheroid Growth and Metastasis Independent of the Ambient Gas Composition. Cancers 2020, 12, 2570. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, A.; Oh, C.; Diffey, B.; Wakamatsu, K.; Ito, S.; Rees, J. Eumelanin and pheomelanin concentrations in human epidermis before and after UVB irradiation. Pigment Cell Res. 2005, 18, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Thody, A.J.; Higgins, E.M.; Wakamatsu, K.; Ito, S.; Burchill, S.A.; Marks, J.M. Pheomelanin as well as eumelanin is present in human epidermis. J. Investig. Dermatol. 1991, 97, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: Application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Affenzeller, S.; Frauendorf, H.; Licha, T.; Jackson, D.J.; Wolkenstein, K. Quantitation of eumelanin and pheomelanin markers in diverse biological samples by HPLC-UV-MS following solid-phase extraction. PLoS ONE 2019, 14, e0223552. [Google Scholar] [CrossRef]
- Prota, G. Recent Advances in the Chemistry of Melanogenesis in Mammals. J. Investig. Dermatol. 1980, 75, 122–127. [Google Scholar] [CrossRef]
- Benathan, M.; Virador, V.; Furumura, M.; Kobayashi, N.; Panizzon, R.G.; Hearing, V.J. Co-regulation of melanin precursors and tyrosinase in human pigment cells: Roles of cysteine and glutathione. Cell. Mol. Biol. 1999, 45, 981–990. [Google Scholar] [PubMed]
- Ghasemi, E.; Nilforoushzadeh, M.A.; Khani, M.; Amirkhani, M.A.; Nouri, M.; Charipoor, P.; Eftekhari, M.; Izadpanah, S.; Shokri, B. The quantitative investigation of spark plasma on skin parameters with skin elasticity, thickness, density, and biometric characteristics. Sci. Rep. 2023, 13, 7738. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Xu, M.; Li, Q.; Guo, K.; Chen, H.; Kong, M.G.; Xia, Y. Successful Treatment of Vitiligo with Cold Atmospheric Plasma–Activated Hydrogel. J. Investig. Dermatol. 2021, 141, 2710–2719.e6. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Del Bino, S.; Hirobe, T.; Wakamatsu, K. Improved HPLC Conditions to Determine Eumelanin and Pheomelanin Contents in Biological Samples Using an Ion Pair Reagent. Int. J. Mol. Sci. 2020, 21, 5134. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasse, S.; Sommer, M.-C.; Guenther, S.; Schulze, C.; Bekeschus, S.; von Woedtke, T. Exploring the Influence of Cold Plasma on Epidermal Melanogenesis In Situ and In Vitro. Int. J. Mol. Sci. 2024, 25, 5186. https://doi.org/10.3390/ijms25105186
Hasse S, Sommer M-C, Guenther S, Schulze C, Bekeschus S, von Woedtke T. Exploring the Influence of Cold Plasma on Epidermal Melanogenesis In Situ and In Vitro. International Journal of Molecular Sciences. 2024; 25(10):5186. https://doi.org/10.3390/ijms25105186
Chicago/Turabian StyleHasse, Sybille, Marie-Christine Sommer, Sebastian Guenther, Christian Schulze, Sander Bekeschus, and Thomas von Woedtke. 2024. "Exploring the Influence of Cold Plasma on Epidermal Melanogenesis In Situ and In Vitro" International Journal of Molecular Sciences 25, no. 10: 5186. https://doi.org/10.3390/ijms25105186
APA StyleHasse, S., Sommer, M.-C., Guenther, S., Schulze, C., Bekeschus, S., & von Woedtke, T. (2024). Exploring the Influence of Cold Plasma on Epidermal Melanogenesis In Situ and In Vitro. International Journal of Molecular Sciences, 25(10), 5186. https://doi.org/10.3390/ijms25105186








