CA-125 KELIM as an Alternative Predictive Tool to Identify Which Patients Can Benefit from PARPi in High-Grade Serous Advanced Ovarian Cancer: A Retrospective Pilot Diagnostic Accuracy Study
Abstract
1. Introduction
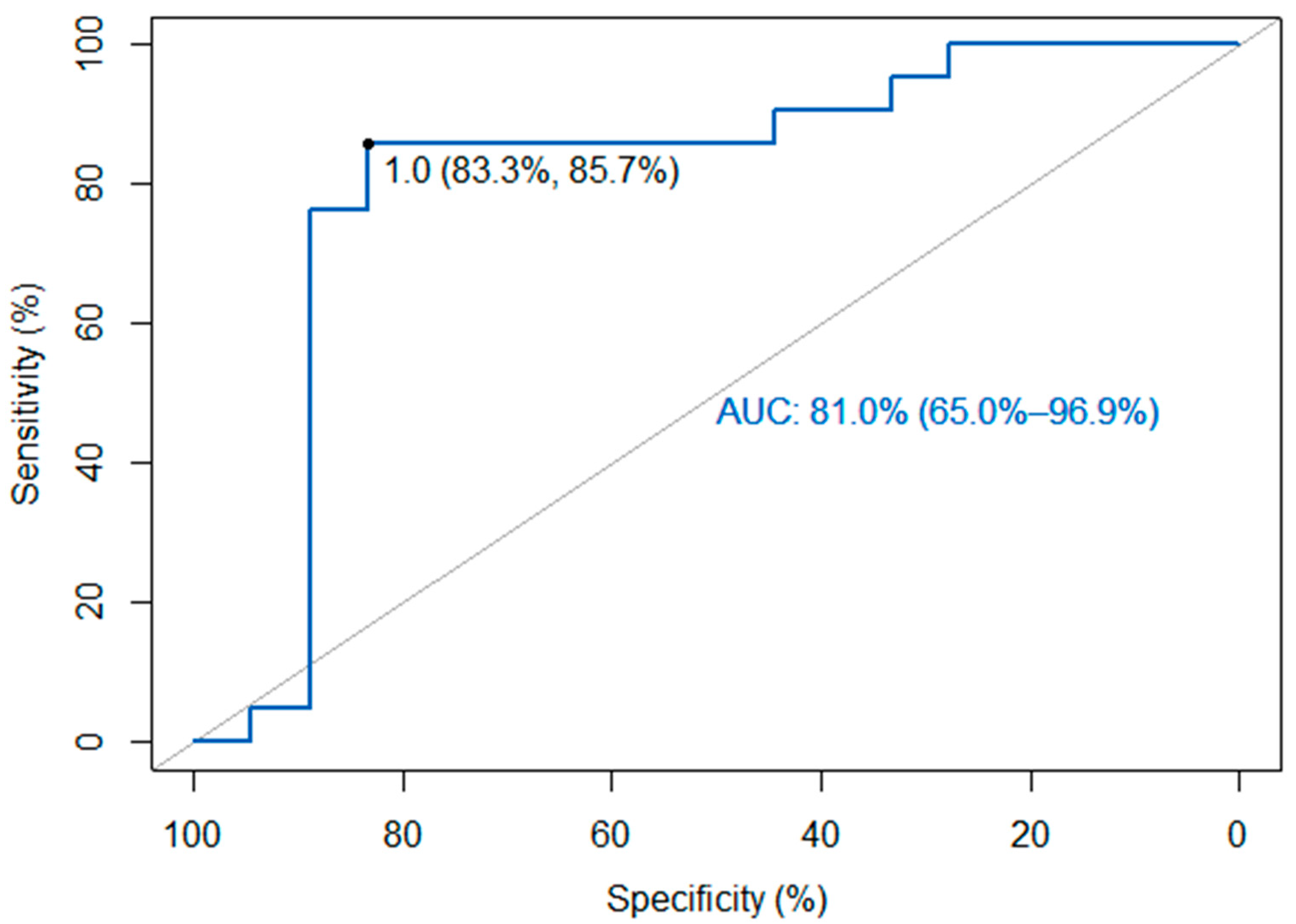
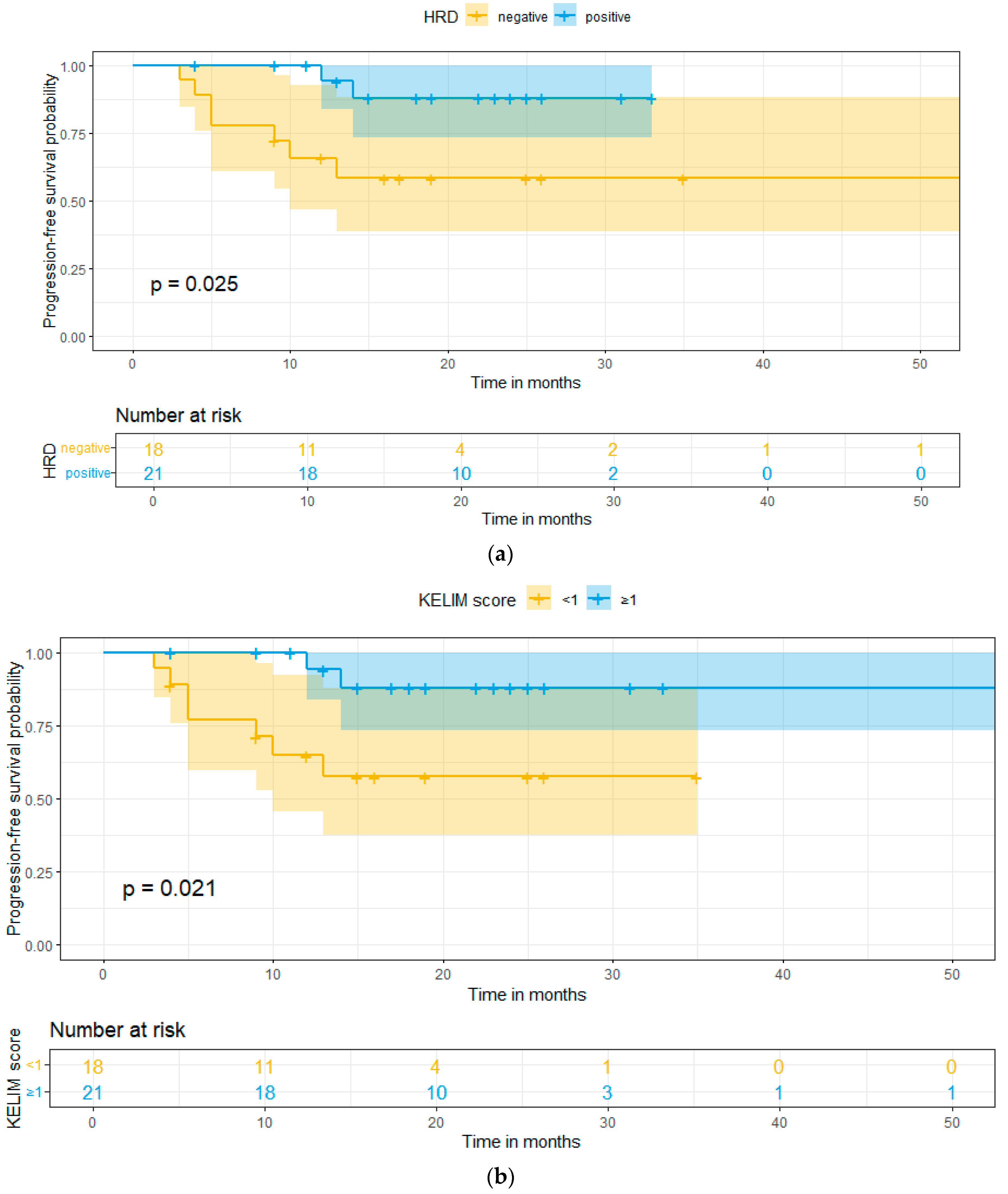
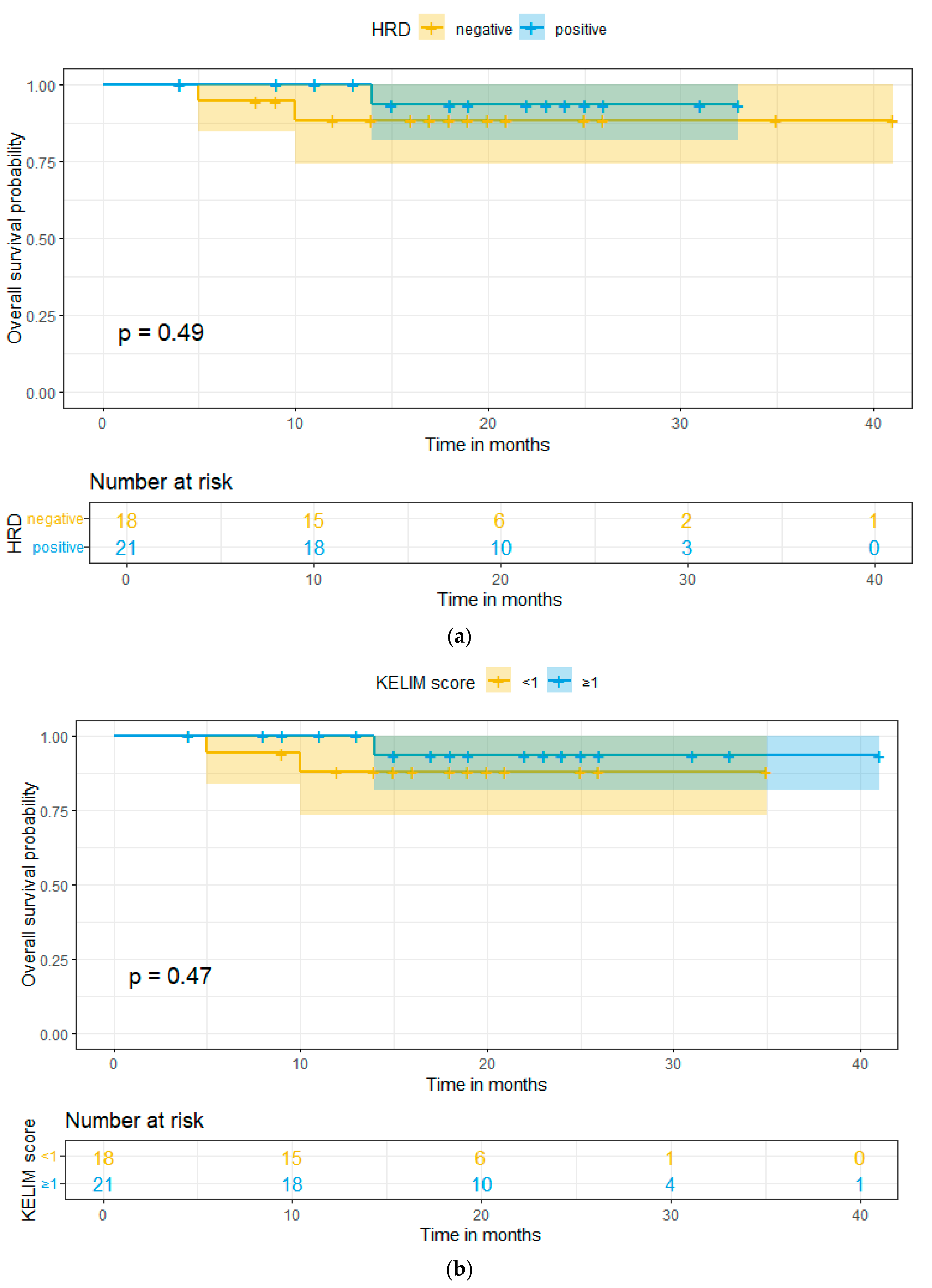
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Characteristics
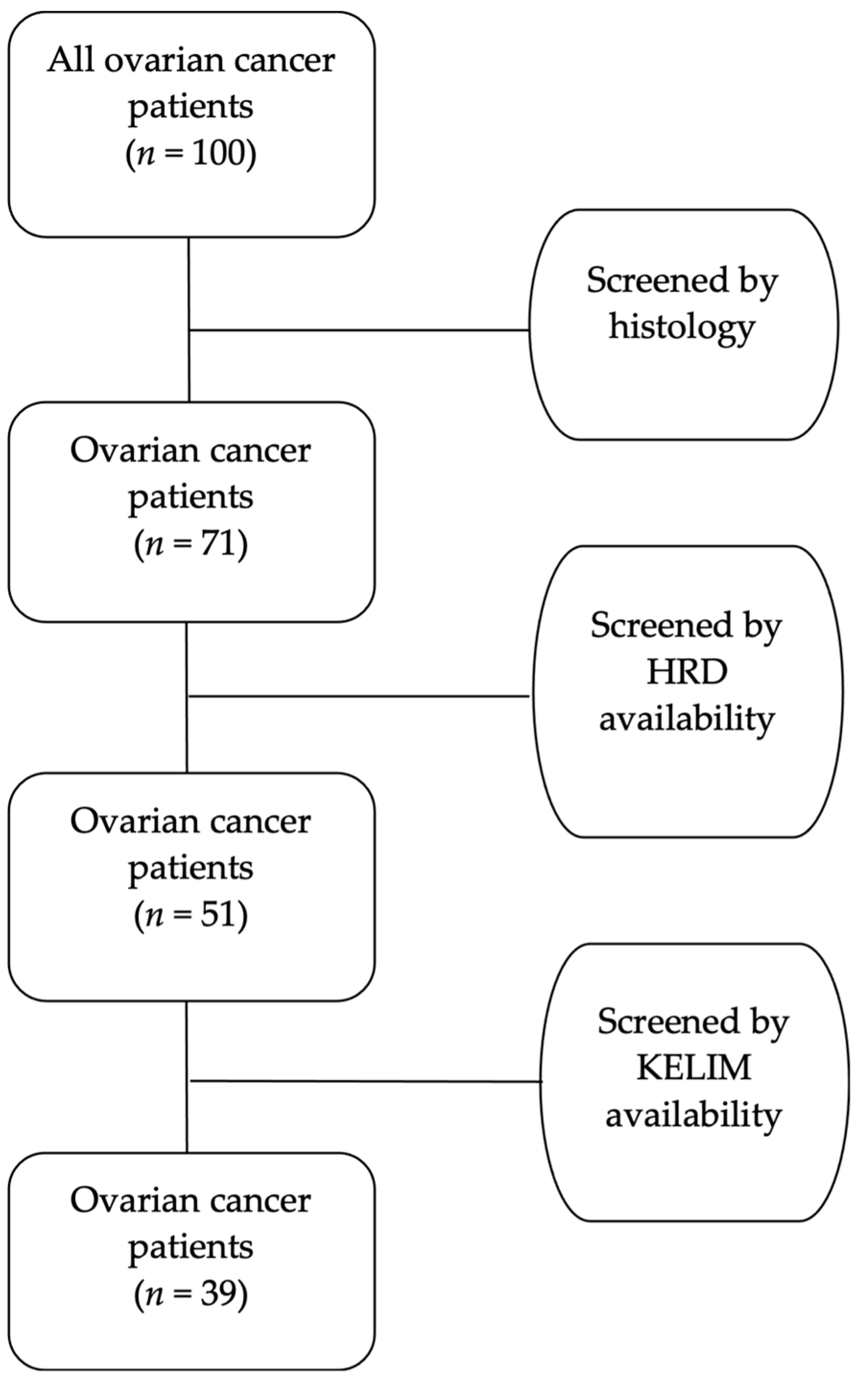
4.2. Patients
- Histological confirmation of ovarian cancer.
- Surgical treatment at our Gynecological–Oncology Unit.
- Any other histological type except high-grade serous.
- Patients not tested for HRD.
- Missing important registry data of CA-125 values to calculate KELIM.
4.3. Data Collection
- Patient’s identifiers:
- ○
- Name
- ○
- Hospital identification number
- Patient’s age
- Tumor marker CA-125 serial values during chemotherapy
- KELIM Score
- Homologous Recombination Deficiency (HRD) status
- Genomic Instability Score (GIS)
- Residual Disease after debulking surgery
- Chemotherapy:
- ○
- Adjuvant
- ○
- Neoadjuvant
- Maintenance therapy (PARPi)
- Time-related data:
- ○
- Date of surgery
- ○
- Date of recurrence or disease progression
- ○
- Date of last follow-up or death
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.S.; Miller, A.; Rungruang, B.; Richard, S.D.; Rodriguez, N.; Bookman, M.A.; Hamilton, C.A.; Krivak, T.C.; Maxwell, G.L. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: An analysis of GOG 182. J. Clin. Oncol. 2015, 33, 937–943. [Google Scholar] [CrossRef]
- Lauby, A.; Colomban, O.; Corbaux, P.; Peron, J.; Van Wagensveld, L.; Gertych, W.; Bakrin, N.; Descargues, P.; Lopez, J.; Kepenekian, V.; et al. The Increasing Prognostic and Predictive Roles of the Tumor Primary Chemosensitivity Assessed by CA-125 Elimination Rate Constant K (KELIM) in Ovarian Cancer: A Narrative Review. Cancers 2021, 14, 98. [Google Scholar] [CrossRef]
- Rutten, M.J.; van de Vrie, R.; Bruining, A.; Spijkerboer, A.M.; Mol, B.W.; Kenter, G.G.; Buist, M.R. Predicting surgical outcome in patients with International Federation of Gynecology and Obstetrics stage III or IV ovarian cancer using computed tomography: A systematic review of prediction models. Int. J. Gynecol. Cancer 2015, 25, 407–415. [Google Scholar] [CrossRef]
- Rustin, G.J.S.; Quinn, M.; Thigpen, T.; du Bois, A.; Pujade-Lauraine, E.; Jakobsen, A.; Eisenhauer, E.; Sagae, S.; Greven, K.; Vergote, I.; et al. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). J. Natl. Cancer Inst. 2004, 96, 487–488. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int. J. Gynecol. Cancer 2019, 30, 672–705. [Google Scholar]
- Kim, J.H.; Cho, H.-W.; Park, E.Y.; Han, K.-H.; Kim, E.T.; Lee, J.-K.; Park, S.-Y.; Armbrust, R.; Fotopoulou, C.; Lim, M.C. Prognostic value of CA125 kinetics, half-life, and nadir in the treatment of epithelial ovarian cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2023, 33, 1913–1920. [Google Scholar] [CrossRef]
- Xu, J.L.; Commins, J.; Partridge, E.; Riley, T.L.; Prorok, P.C.; Johnson, C.C.; Buys, S.S. Longitudinal evaluation of CA-125 velocity and prediction of ovarian cancer. Gynecol. Oncol. 2012, 125, 70–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almufti, R.; Wilbaux, M.; Oza, A.; Henin, E.; Freyer, G.; Tod, M.; Colomban, O.; You, B. A critical review of the analytical approaches for circulating tumor biomarker kinetics during treatment. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 41–56. [Google Scholar] [CrossRef]
- Riedinger, J.M.; Wafflart, J.; Ricolleau, G.; Eche, N.; Larbre, H.; Basuyau, J.P.; Dalifard, I.; Hacene, K.; Pichon, M.F. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: Results of a French multicentric study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006, 17, 1234–1238. [Google Scholar] [CrossRef]
- Lee, C.K.; Friedlander, M.; Brown, C.; Gebski, V.J.; Georgoulopoulos, A.; Vergote, I.; Pignata, S.; Donadello, N.; Schmalfeldt, B.; Delva, R.; et al. Early decline in cancer antigen 125 as a surrogate for progression-free survival in recurrent ovarian cancer. J. Natl. Cancer Inst. 2011, 103, 1338–1342. [Google Scholar] [CrossRef]
- You, B.; Colomban, O.; Heywood, M.; Lee, C.; Davy, M.; Reed, N.; Pignata, S.; Varsellona, N.; Emons, G.; Rehman, K.; et al. The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: Data from CALYPSO trial (a GINECO-GCIG study). Gynecol. Oncol. 2013, 130, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Colomban, O.; Tod, M.; Leary, A.; Ray-Coquard, I.; Lortholary, A.; Hardy-Bessard, A.C.; Pfisterer, J.; Du Bois, A.; Kurzeder, C.; Burges, A.; et al. Early Modeled Longitudinal CA-125 Kinetics and Survival of Ovarian Cancer Patients: A GINECO AGO MRC CTU Study. Clin. Cancer Res. 2019, 25, 5342–5350. [Google Scholar] [CrossRef] [PubMed]
- Ducoulombier, S.; Golfier, F.; Colomban, O.; Benayoun, D.; Bolze, P.-A.; Tod, M.; You, B. Modeling CA-125 During Neoadjuvant Chemotherapy for Predicting Optimal Cytoreduction and Relapse Risk in Ovarian Cancer. Anticancer. Res. 2017, 37, 6879–6886. [Google Scholar] [PubMed]
- You, B.; Purdy, C.; Copeland, L.J.; Swisher, E.M.; Bookman, M.A.; Fleming, G.; Coleman, R.; Randall, L.M.; Tewari, K.S.; Monk, B.J.; et al. Identification of Patients With Ovarian Cancer Experiencing the Highest Benefit From Bevacizumab in the First-Line Setting on the Basis of Their Tumor-Intrinsic Chemosensitivity (KELIM): The GOG-0218 Validation Study. J. Clin. Oncol. 2022, 40, 3965–3974. [Google Scholar] [CrossRef]
- You, B.; Robelin, P.; Tod, M.; Louvet, C.; Lotz, J.-P.; Abadie-Lacourtoisie, S.; Fabbro, M.; Desauw, C.; Bonichon-Lamichhane, N.; Kurtz, J.-E.; et al. CA-125 ELIMination Rate Constant K (KELIM) Is a Marker of Chemosensitivity in Patients with Ovarian Cancer: Results from the Phase II CHIVA Trial. Clin. Cancer Res. 2020, 26, 4625–4632. [Google Scholar] [CrossRef]
- Corbaux, P.; You, B.; Glasspool, R.M.; Yanaihara, N.; Tinker, A.V.; Lindemann, K.; Ray-Coquard, I.L.; Mirza, M.R.; Subtil, F.; Colomban, O.; et al. Survival and modelled cancer antigen-125 ELIMination rate constant K score in ovarian cancer patients in first-line before poly(ADP-ribose) polymerase inhibitor era: A Gynaecologic Cancer Intergroup meta-analysis. Eur. J. Cancer 2023, 191, 112966. [Google Scholar] [CrossRef] [PubMed]
- Labidi-Galy, S.I.; de La Motte Rouge, T.; Derbel, O.; Wolfer, A.; Kalbacher, E.; Olivier, T.; Combes, J.D.; Heimgartner-Hu, K.; Tredan, O.; Guevara, H.; et al. Clinical factors associated with prolonged response and survival under olaparib as maintenance therapy in BRCA mutated ovarian cancers. Gynecol. Oncol. 2019, 155, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Yap, T.A.; Boss, D.S.; Carden, C.P.; Mergui-Roelvink, M.; Gourley, C.; De Greve, J.; Lubinski, J.; Shanley, S.; Messiou, C.; et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 2010, 28, 2512–2519. [Google Scholar] [CrossRef]
- Monk, B.J.; Parkinson, C.; Lim, M.C.; O’Malley, D.M.; Oaknin, A.; Wilson, M.K.; Coleman, R.L.; Lorusso, D.; Bessette, P.; Ghamande, S.; et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J. Clin. Oncol. 2022, 40, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Majdak, E.J.; Debniak, J.; Milczek, T.; Cornelisse, C.J.; Devilee, P.; Emerich, J.; Jassem, J.; De Bock, G.H. Prognostic impact of BRCA1 pathogenic and BRCA1/BRCA2 unclassified variant mutations in patients with ovarian carcinoma. Cancer 2005, 104, 1004–1012. [Google Scholar] [CrossRef]
- Chetrit, A.; Hirsh-Yechezkel, G.; Ben-David, Y.; Lubin, F.; Friedman, E.; Sadetzki, S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: The national Israeli study of ovarian cancer. J. Clin. Oncol. 2008, 26, 20–25. [Google Scholar] [CrossRef]
- Yang, D.; Khan, S.; Sun, Y.; Hess, K.; Shmulevich, I.; Sood, A.K.; Zhang, W. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011, 306, 1557–1565. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, Y.; Hao, D. A genomic instability score in discriminating nonequivalent outcomes of BRCA1/2 mutations and in predicting outcomes of ovarian cancer treated with platinum-based chemotherapy. PLoS ONE 2014, 9, e113169. [Google Scholar] [CrossRef]
- Stoppa-Lyonnet, D. The biological effects and clinical implications of BRCA mutations: Where do we go from here? Eur. J. Hum. Genet. 2016, 24 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef]
- You, B.; Sehgal, V.; Hosmane, B.; Huang, X.; Ansell, P.J.; Dinh, M.H.; Bell-McGuinn, K.; Luo, X.; Fleming, G.F.; Friedlander, M.; et al. CA-125 KELIM as a Potential Complementary Tool for Predicting Veliparib Benefit: An Exploratory Analysis From the VELIA/GOG-3005 Study. J. Clin. Oncol. 2023, 41, 107–116. [Google Scholar] [CrossRef]
- Hannaway, N.; Kassaris, S.; Davies, J.M.; Smrke, A.; Tinker, A.; Drew, Y. Using chemotherapy response by KELIM score to predict response to first line maintenance PARP inhibitor therapy in non-BRCA mutant/homologous recombination deficiency (HRD) unknown high grade serous ovarian cancer (HGSOC). J. Clin. Oncol. 2023, 41 (Suppl. S16), e17547. [Google Scholar] [CrossRef]
- Colomban, O.; Swisher, E.M.; Kristeleit, R.; McNeish, I.; Shapira-Frommer, R.; Goble, S.; Lin, K.K.; Maloney, L.; Freyer, G.; You, B. Mathematical modeling of the early modeled CA-125 longitudinal kinetics (KELIM-PARP) as a pragmatic indicator of rucaparib efficacy in patients with recurrent ovarian carcinoma in ARIEL2 & STUDY 10. EBioMedicine 2023, 89, 104477. [Google Scholar]
- Piedimonte, S.; Kim, R.; Bernardini, M.Q.; Atenafu, E.G.; Clark, M.; Lheureux, S.; May, T. Validation of the KELIM score as a predictor of response to neoadjuvant treatment in patients with advanced high grade serous ovarian cancer. Gynecol. Oncol. 2022, 167, 417–422. [Google Scholar] [CrossRef]
- van Wagensveld, L.; Colomban, O.; van der Aa, M.A.; Freyer, G.; Sonke, G.S.; Kruitwagen, R.F.; You, B. Confirmation of the utility of the CA-125 elimination rate (KELIM) as an indicator of the chemosensitivity in advanced-stage ovarian cancer in a “real-life setting”. J. Gynecol. Oncol. 2024, 35, e34. [Google Scholar] [CrossRef]
- Bouvarel, B.; Colomban, O.; Frenel, J.-S.; Loaec, C.; Bourgin, C.; Berton, D.; Freyer, G.; You, B.; Classe, J.-M. Clinical impact of CA-125 ELIMination rate constant K (KELIM) on surgical strategy in advanced serous ovarian cancer patients. Int. J. Gynecol. Cancer 2024, 34, 574–580. [Google Scholar] [CrossRef]
- Zouzoulas, D.; Tsolakidis, D.; Tzitzis, P.; Sofianou, I.; Chatzistamatiou, K.; Theodoulidis, V.; Topalidou, M.; Timotheadou, E.; Grimbizis, G. The Use of CA-125 KELIM to Identify Which Patients Can Achieve Complete Cytoreduction after Neoadjuvant Chemotherapy in High-Grade Serous Advanced Ovarian Cancer. Cancers 2024, 16, 1266. [Google Scholar] [CrossRef]
- Arora, S.; Balasubramaniam, S.; Zhang, H.; Berman, T.; Narayan, P.; Suzman, D.; Bloomquist, E.; Tang, S.; Gong, Y.; Sridhara, R.; et al. FDA Approval Summary: Olaparib Monotherapy or in Combination with Bevacizumab for the Maintenance Treatment of Patients with Advanced Ovarian Cancer. Oncologist 2021, 26, e164–e172. [Google Scholar] [CrossRef]
- Tsantikidi, A.; Papazisis, K.; Floros, T.; Gazouli, M.; Papadopoulou, E.; Tsaousis, G.; Nasioulas, G.; Mester, A.; Milan, K.P.; Gozman, B.; et al. RediScore: Prospective validation of a pipeline for homologous recombination deficiency analysis. Oncol. Lett. 2023, 26, 480. [Google Scholar] [CrossRef]
- Liu, X. Classification accuracy and cut point selection. Stat. Med. 2012, 31, 2676–2686. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients (n) | Percentage (%) | ||
|---|---|---|---|
| Age (years) | mean: 60 | SD: 10 | |
| KELIM score | |||
| <1 | 18 | 46 | |
| ≥1 | 21 | 54 | |
| HRD status | |||
| Positive | 21 | 54 | |
| Negative | 18 | 46 | |
| Chemotherapy | |||
| Neoadjuvant | 22 | 56 | |
| Adjuvant | 17 | 44 | |
| Residual disease (cm) | |||
| 0 | 22 | 56 | |
| <1 | 11 | 28 | |
| ≥1 | 6 | 16 | |
| Point of Estimates | 95% CIs | |
|---|---|---|
| Sensitivity | 0.86 | (0.64–0.97) |
| Specificity | 0.83 | (0.59–0.96) |
| Positive Predictive Value | 0.86 | (0.64–0.97) |
| Negative Predictive Value | 0.83 | (0.59–0.96) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouzoulas, D.; Tsolakidis, D.; Tzitzis, P.; Chatzistamatiou, K.; Theodoulidis, V.; Sofianou, I.; Grimbizis, G.; Timotheadou, E. CA-125 KELIM as an Alternative Predictive Tool to Identify Which Patients Can Benefit from PARPi in High-Grade Serous Advanced Ovarian Cancer: A Retrospective Pilot Diagnostic Accuracy Study. Int. J. Mol. Sci. 2024, 25, 5230. https://doi.org/10.3390/ijms25105230
Zouzoulas D, Tsolakidis D, Tzitzis P, Chatzistamatiou K, Theodoulidis V, Sofianou I, Grimbizis G, Timotheadou E. CA-125 KELIM as an Alternative Predictive Tool to Identify Which Patients Can Benefit from PARPi in High-Grade Serous Advanced Ovarian Cancer: A Retrospective Pilot Diagnostic Accuracy Study. International Journal of Molecular Sciences. 2024; 25(10):5230. https://doi.org/10.3390/ijms25105230
Chicago/Turabian StyleZouzoulas, Dimitrios, Dimitrios Tsolakidis, Panagiotis Tzitzis, Kimon Chatzistamatiou, Vasilis Theodoulidis, Iliana Sofianou, Grigoris Grimbizis, and Eleni Timotheadou. 2024. "CA-125 KELIM as an Alternative Predictive Tool to Identify Which Patients Can Benefit from PARPi in High-Grade Serous Advanced Ovarian Cancer: A Retrospective Pilot Diagnostic Accuracy Study" International Journal of Molecular Sciences 25, no. 10: 5230. https://doi.org/10.3390/ijms25105230
APA StyleZouzoulas, D., Tsolakidis, D., Tzitzis, P., Chatzistamatiou, K., Theodoulidis, V., Sofianou, I., Grimbizis, G., & Timotheadou, E. (2024). CA-125 KELIM as an Alternative Predictive Tool to Identify Which Patients Can Benefit from PARPi in High-Grade Serous Advanced Ovarian Cancer: A Retrospective Pilot Diagnostic Accuracy Study. International Journal of Molecular Sciences, 25(10), 5230. https://doi.org/10.3390/ijms25105230





