Pathogenesis of Pulmonary Manifestations in ANCA-Associated Vasculitis and Goodpasture Syndrome
Abstract
1. Introduction
2. Pulmonary Manifestations of Small-Vessel Vasculitis
2.1. GPA and MPA
2.2. EGPA
2.3. Goodpasture Syndrome
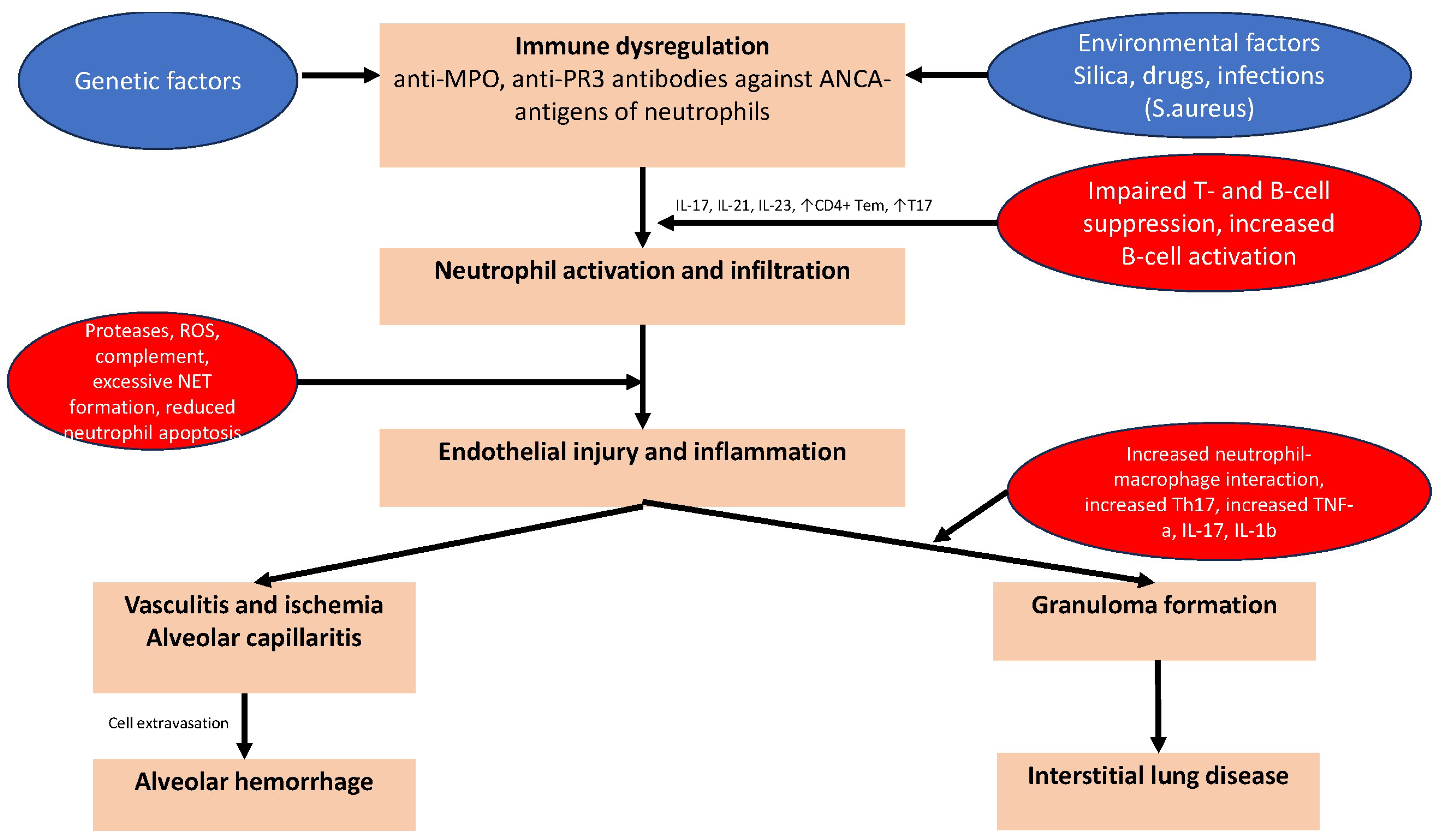
3. Pathogenetic Mechanisms of Pulmonary Manifestations of AAV
3.1. Genetic and Epigenetic Factors
3.2. The Role of Infections and Microbiome
3.3. Common Pathogenetic Mechanisms in AAV
3.4. Granuloma Formation in GPA
3.5. EGPA Pathogenesis
3.6. The Role of Drugs in AAV
4. Pathogenetic Mechanisms of Goodpasture Syndrome
5. Treatment of Pulmonary Manifestations in AAV
6. Perspectives in the Management of Pulmonary Manifestations of AAV
- -
- Early diagnosis: Advances in diagnostic methods and prognostic biomarkers may enable earlier detection of vasculitis and its flare-ups, thereby facilitating the timely initiation of treatment and prevention of organ damage.
- -
- Targeted therapies: Identification of the pathways involved in the pathogenesis of AAV and anti-GBM disease may lead to the development of targeted therapies that can selectively inhibit disease-causing mechanisms while leaving normal immune function unaffected. Recently tested medications, such as avacopan, targeting the complement cascade have demonstrated efficacy in reducing overall toxicity and minimizing the need for steroid therapy [135].
- -
- Relapse prevention: Prolonged maintenance therapy may improve long-term outcomes and reduce the burden of disease on patients by lowering the risk of relapse.
- -
- Personalized medicine: The development of personalised treatment plans based on the disease phenotype, genomics, immunological and biomarker profile of each patient may be the result of advances in our understanding of the pathogenesis of AAV-and anti-GBM-disease.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Savage, C.O.; Harper, L.; Cockwell, P.; Adu, D.; Howie, A.J. ABC of arterial and vascular disease: Vasculitis. BMJ 2000, 320, 1325–1328. [Google Scholar] [CrossRef]
- Watts, R.A.; Robson, J. Introduction, epidemiology and classification of vasculitis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 3–20. [Google Scholar] [CrossRef]
- Saadoun, D.; Vautier, M.; Cacoub, P. Medium- and Large-Vessel Vasculitis. Circulation 2021, 143, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Feragalli, B.; Mantini, C.; Sperandeo, M.; Galluzzo, M.; Belcaro, G.; Tartaro, A.; Cotroneo, A.R. The lung in systemic vasculitis: Radiological patterns and differential diagnosis. Br. J. Radiol. 2016, 89, 20150992. [Google Scholar] [CrossRef]
- Yaseen, K.; Mandell, B.F. ANCA associated vasculitis (AAV): A review for internists. Postgrad. Med. 2023, 135, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers 2020, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, F.; L’Imperio, V.; Calatroni, M.; Pagni, F.; Sinico, R.A. Goodpasture syndrome and anti-glomerular basement membrane disease. Clin. Exp. Rheumatol. 2023, 41, 964–974. [Google Scholar] [CrossRef]
- Shavit, E.; Alavi, A.; Sibbald, R.G. Vasculitis-What Do We Have to Know? A Review of Literature. Int. J. Low. Extrem. Wounds 2018, 17, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Fraticelli, P.; Benfaremo, D.; Gabrielli, A. Diagnosis and management of leukocytoclastic vasculitis. Intern. Emerg. Med. 2021, 16, 831–841. [Google Scholar] [CrossRef]
- Kerr, G.S.; Hallahan, C.W.; Giordano, J.; Leavitt, R.Y.; Fauci, A.S.; Rottem, M.; Hoffman, G.S. Takayasu arteritis. Ann. Intern. Med. 1994, 120, 919–929. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.U.; Oostdam, D.A.H.; Abud-Mendoza, C. Diffuse Alveolar Hemorrhage in Autoimmune Diseases. Curr. Rheumatol. Rep. 2017, 19, 27. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Nagai, S.; Sharma, O.P. Pulmonary hypertension and granulomatous vasculitis in sarcoidosis. Curr. Opin. Pulm. Med. 2007, 13, 434–438. [Google Scholar] [CrossRef]
- Piggott, K.; Biousse, V.; Newman, N.J.; Goronzy, J.J.; Weyand, C.M. Vascular damage in giant cell arteritis. Autoimmunity 2009, 42, 596–604. [Google Scholar] [CrossRef][Green Version]
- Okada, H. Multiple Thromboembolic Cerebral Infarctions from the Aorta in a Patient with Churg-Strauss Syndrome. J. Stroke Cerebrovasc. Dis. 2017, 26, e32–e33. [Google Scholar] [CrossRef]
- Adams, T.N.; Zhang, D.; Batra, K.; Fitzgerald, J.E. Pulmonary manifestations of large, medium, and variable vessel vasculitis. Respir. Med. 2018, 145, 182–191. [Google Scholar] [CrossRef]
- Walsh, M.; Merkel, P.A.; Peh, C.A.; Szpirt, W.M.; Puéchal, X.; Fujimoto, S.; Hawley, C.M.; Khalidi, N.; Floßmann, O.; Wald, R.; et al. Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis. N. Engl. J. Med. 2020, 382, 622–631. [Google Scholar] [CrossRef]
- Kronbichler, A.; Lee, K.H.; Denicolò, S.; Choi, D.; Lee, H.; Ahn, D.; Kim, K.H.; Lee, J.H.; Kim, H.; Hwang, M.; et al. Immunopathogenesis of ANCA-Associated Vasculitis. Int. J. Mol. Sci. 2020, 21, 7319. [Google Scholar] [CrossRef]
- Comarmond, C.; Cacoub, P. Granulomatosis with polyangiitis (Wegener): Clinical aspects and treatment. Autoimmun. Rev. 2014, 13, 1121–1125. [Google Scholar] [CrossRef]
- Chung, S.A.; Seo, P. Microscopic polyangiitis. Rheum. Dis. Clin. N. Am. 2010, 36, 545–558. [Google Scholar] [CrossRef]
- Emmi, G.; Bettiol, A.; Gelain, E.; Bajema, I.M.; Berti, A.; Burns, S.; Cid, M.C.; Cohen Tervaert, J.W.; Cottin, V.; Durante, E.; et al. Evidence-Based Guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis. Nat. Rev. Rheumatol. 2023, 19, 378–393. [Google Scholar] [CrossRef]
- Greco, A.; Marinelli, C.; Fusconi, M.; Macri, G.F.; Gallo, A.; De Virgilio, A.; Zambetti, G.; de Vincentiis, M. Clinic manifestations in granulomatosis with polyangiitis. Int. J. Immunopathol. Pharmacol. 2016, 29, 151–159. [Google Scholar] [CrossRef]
- Csernok, E.; Gross, W.L. Current understanding of the pathogenesis of granulomatosis with polyangiitis (Wegener’s). Expert Rev. Clin. Immunol. 2013, 9, 641–648. [Google Scholar] [CrossRef]
- Zimba, O.; Doskaliuk, B.; Yatsyshyn, R.; Bahrii, M.; Hrytsevych, M. Challenges in diagnosis of limited granulomatosis with polyangiitis. Rheumatol. Int. 2021, 41, 1337–1345. [Google Scholar] [CrossRef]
- Masiak, A.; Zdrojewski, Z.; Pęksa, R.; Smoleńska, Ż.; Czuszyńska, Z.; Siemińska, A.; Kowalska, B.; Stankiewicz, C.; Rutkowski, B.; Bułło-Piontecka, B. The usefulness of histopathological examinations of non-renal biopsies in the diagnosis of granulomatosis with polyangiitis. Rheumatologiay 2017, 55, 230–236. [Google Scholar] [CrossRef]
- Devaney, K.O.; Travis, W.D.; Hoffman, G.; Leavitt, R.; Lebovics, R.; Fauci, A.S. Interpretation of head and neck biopsies in Wegener’s granulomatosis. A pathologic study of 126 biopsies in 70 patients. Am. J. Surg. Pathol. 1990, 14, 555–564. [Google Scholar] [CrossRef]
- Sacoto, G.; Boukhlal, S.; Specks, U.; Flores-Suárez, L.F.; Cornec, D. Lung involvement in ANCA-associated vasculitis. Presse Med. 2020, 49, 104039. [Google Scholar] [CrossRef]
- Polychronopoulos, V.S.; Prakash, U.B.; Golbin, J.M.; Edell, E.S.; Specks, U. Airway involvement in Wegener’s granulomatosis. Rheum. Dis. Clin. N. Am. 2007, 33, 755–775, vi. [Google Scholar] [CrossRef]
- Gluth, M.B.; Shinners, P.A.; Kasperbauer, J.L. Subglottic stenosis associated with Wegener’s granulomatosis. Laryngoscope 2003, 113, 1304–1307. [Google Scholar] [CrossRef]
- Girard, C.; Charles, P.; Terrier, B.; Bussonne, G.; Cohen, P.; Pagnoux, C.; Cottin, V.; Cordier, J.F.; Guillevin, L. Tracheobronchial Stenoses in Granulomatosis with Polyangiitis (Wegener’s): A Report on 26 Cases. Medicne 2015, 94, e1088. [Google Scholar] [CrossRef]
- Nasser, M.; Cottin, V. Alveolar Hemorrhage in Vasculitis (Primary and Secondary). Semin. Respir. Crit. Care Med. 2018, 39, 482–493. [Google Scholar] [CrossRef]
- Smith, J.W. Diffuse Alveolar Hemorrhage; Springer: Cham, Switzerland, 2017; p. xxvi. 805p. [Google Scholar]
- Karras, A. Microscopic Polyangiitis: New Insights into Pathogenesis, Clinical Features and Therapy. Semin. Respir. Crit. Care Med. 2018, 39, 459–464. [Google Scholar] [CrossRef]
- Park, J.A. Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis. Int. J. Mol. Sci. 2021, 22, 793. [Google Scholar] [CrossRef]
- Lara, A.R.; Schwarz, M.I. Diffuse alveolar hemorrhage. Chest 2010, 137, 1164–1171. [Google Scholar] [CrossRef]
- Alexandre, A.T.; Vale, A.; Gomes, T. Diffuse alveolar hemorrhage: How relevant is etiology? Sarcoidosis Vasc. Diffus. Lung Dis. 2019, 36, 47–52. [Google Scholar] [CrossRef]
- Koslow, M.; Edell, E.S.; Midthun, D.E.; Mullon, J.J.; Kern, R.M.; Nelson, D.R.; Sakata, K.K.; Moua, T.; Roden, A.C.; Yi, E.S.; et al. Bronchoscopic Cryobiopsy and Forceps Biopsy for the Diagnostic Evaluation of Diffuse Parenchymal Lung Disease in Clinical Practice. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 565–574. [Google Scholar] [CrossRef]
- Escuissato, D.L.; Warszawiak, D.; Marchiori, E. Differential diagnosis of diffuse alveolar haemorrhage in immunocompromised patients. Curr. Opin. Infect. Dis. 2015, 28, 337–342. [Google Scholar] [CrossRef]
- Sun, X.; Peng, M.; Zhang, T.; Li, Z.; Song, L.; Li, M.; Shi, J. Clinical features and long-term outcomes of interstitial lung disease with anti-neutrophil cytoplasmic antibody. BMC Pulm. Med. 2021, 21, 88. [Google Scholar] [CrossRef]
- Schirmer, J.H.; Wright, M.N.; Vonthein, R.; Herrmann, K.; Nölle, B.; Both, M.; Henes, F.O.; Arlt, A.; Gross, W.L.; Schinke, S.; et al. Clinical presentation and long-term outcome of 144 patients with microscopic polyangiitis in a monocentric German cohort. Rheumatology 2016, 55, 71–79. [Google Scholar] [CrossRef]
- Paolini, M.V.; Ruffino, J.P.; Fernández Romero, D.S. Anti-neutrophil cytoplasmic antibody-associated vasculitis. Clinical aspects and treatment. Medcine 2013, 73, 119–126. [Google Scholar]
- Yamakawa, H.; Sato, S.; Nakamura, T.; Nishizawa, T.; Kawabe, R.; Oba, T.; Horikoshi, M.; Akasaka, K.; Amano, M.; Kuwano, K.; et al. Prognostic value of radiological findings indeterminate for UIP pattern and anterior upper lobe honeycomb-like lesion in chronic fibrosing interstitial lung disease associated with MPO-ANCA. BMC Pulm. Med. 2021, 21, 346. [Google Scholar] [CrossRef]
- Sakamoto, S.; Suzuki, A.; Homma, S.; Usui, Y.; Shimizu, H.; Sekiya, M.; Miyoshi, S.; Nakamura, Y.; Urabe, N.; Isshiki, T.; et al. Outcomes and prognosis of progressive pulmonary fibrosis in patients with antineutrophil cytoplasmic antibody-positive interstitial lung disease. Sci. Rep. 2023, 13, 17616. [Google Scholar] [CrossRef] [PubMed]
- Libra, A.; Muscato, G.; Ielo, G.; Spicuzza, L.; Palmucci, S.; Fagone, E.; Fruciano, M.; Gili, E.; Sambataro, G.; Vancheri, C. Clinical and Prognostic Significance of p-ANCA Positivity in Idiopathic Pulmonary Fibrosis: A Retrospective Observational Study. Diagnostics 2023, 13, 1882. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Ventura, I.B.; Achtar-Zadeh, N.; Elicker, B.M.; Jones, K.D.; Wolters, P.J.; Collard, H.R.; Adegunsoye, A.; Strek, M.E.; Ley, B. Prevalence and Clinical Significance of Antineutrophil Cytoplasmic Antibodies in North American Patients with Idiopathic Pulmonary Fibrosis. Chest 2019, 156, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Miyazaki, E.; Ishii, T.; Mukai, Y.; Yamasue, M.; Fujisaki, H.; Ito, T.; Nureki, S.; Kumamoto, T. Incidence of myeloperoxidase anti-neutrophil cytoplasmic antibody positivity and microscopic polyangitis in the course of idiopathic pulmonary fibrosis. Respir. Med. 2013, 107, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Kagiyama, N.; Takayanagi, N.; Kanauchi, T.; Ishiguro, T.; Yanagisawa, T.; Sugita, Y. Antineutrophil cytoplasmic antibody-positive conversion and microscopic polyangiitis development in patients with idiopathic pulmonary fibrosis. BMJ Open Respir. Res. 2015, 2, e000058. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, H.; Enomoto, N.; Oyama, Y.; Kono, M.; Fujisawa, T.; Inui, N.; Nakamura, Y.; Suda, T. Clinical Implication of Proteinase-3-antineutrophil Cytoplasmic Antibody in Patients with Idiopathic Interstitial Pneumonias. Lung 2016, 194, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, R.; Robson, J.C.; Grayson, P.C.; Ponte, C.; Craven, A.; Khalid, S.; Judge, A.; Hutchings, A.; Merkel, P.A.; Luqmani, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 2022, 81, 321–326. [Google Scholar] [CrossRef]
- Homma, S.; Suzuki, A.; Sato, K. Pulmonary involvement in ANCA-associated vasculitis from the view of the pulmonologist. Clin. Exp. Nephrol. 2013, 17, 667–671. [Google Scholar] [CrossRef]
- White, J.; Dubey, S. Eosinophilic granulomatosis with polyangiitis: A review. Autoimmun. Rev. 2023, 22, 103219. [Google Scholar] [CrossRef]
- Groh, M.; Pagnoux, C.; Baldini, C.; Bel, E.; Bottero, P.; Cottin, V.; Dalhoff, K.; Dunogué, B.; Gross, W.; Holle, J.; et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur. J. Intern. Med. 2015, 26, 545–553. [Google Scholar] [CrossRef]
- Jakes, R.W.; Kwon, N.; Nordstrom, B.; Goulding, R.; Fahrbach, K.; Tarpey, J.; Van Dyke, M.K. Burden of illness associated with eosinophilic granulomatosis with polyangiitis: A systematic literature review and meta-analysis. Clin. Rheumatol. 2021, 40, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Trivioli, G.; Terrier, B.; Vaglio, A. Eosinophilic granulomatosis with polyangiitis: Understanding the disease and its management. Rheumatology 2020, 59, iii84–iii94. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Rizzo, M.I.; De Virgilio, A.; Gallo, A.; Fusconi, M.; Ruoppolo, G.; Altissimi, G.; De Vincentiis, M. Churg-Strauss syndrome. Autoimmun. Rev. 2015, 14, 341–348. [Google Scholar] [CrossRef]
- Wu, E.Y.; Hernandez, M.L.; Jennette, J.C.; Falk, R.J. Eosinophilic Granulomatosis with Polyangiitis: Clinical Pathology Conference and Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 1496–1504. [Google Scholar] [CrossRef]
- Hellmark, T.; Segelmark, M. Diagnosis and classification of Goodpasture’s disease (anti-GBM). J. Autoimmun. 2014, 48–49, 108–112. [Google Scholar] [CrossRef]
- Salama, A.D.; Levy, J.B.; Lightstone, L.; Pusey, C.D. Goodpasture’s disease. Lancet 2001, 358, 917–920. [Google Scholar] [CrossRef]
- Ball, J.A.; Young, K.R., Jr. Pulmonary manifestations of Goodpasture’s syndrome. Antiglomerular basement membrane disease and related disorders. Clin. Chest Med. 1998, 19, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Goligher, E.C.; Detsky, A.S. Migratory pulmonary infiltrates. Goodpasture syndrome. CMAJ Can. Med. Assoc. J. 2009, 180, 75–77. [Google Scholar] [CrossRef]
- Jagiello, P.; Gencik, M.; Arning, L.; Wieczorek, S.; Kunstmann, E.; Csernok, E.; Gross, W.L.; Epplen, J.T. New genomic region for Wegener’s granulomatosis as revealed by an extended association screen with 202 apoptosis-related genes. Hum. Genet. 2004, 114, 468–477. [Google Scholar] [CrossRef]
- Lyons, P.A.; Rayner, T.F.; Trivedi, S.; Holle, J.U.; Watts, R.A.; Jayne, D.R.; Baslund, B.; Brenchley, P.; Bruchfeld, A.; Chaudhry, A.N.; et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 2012, 367, 214–223. [Google Scholar] [CrossRef]
- Merkel, P.A.; Xie, G.; Monach, P.A.; Ji, X.; Ciavatta, D.J.; Byun, J.; Pinder, B.D.; Zhao, A.; Zhang, J.; Tadesse, Y.; et al. Identification of Functional and Expression Polymorphisms Associated with Risk for Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis. Arthritis Rheumatol. 2017, 69, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.A.; Peters, J.E.; Alberici, F.; Liley, J.; Coulson, R.M.R.; Astle, W.; Baldini, C.; Bonatti, F.; Cid, M.C.; Elding, H.; et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat. Commun. 2019, 10, 5120. [Google Scholar] [CrossRef] [PubMed]
- Sablé-Fourtassou, R.; Cohen, P.; Mahr, A.; Pagnoux, C.; Mouthon, L.; Jayne, D.; Blockmans, D.; Cordier, J.F.; Delaval, P.; Puechal, X.; et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann. Intern. Med. 2005, 143, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, D.; Shida, H.; Tomaru, U.; Yoshida, M.; Nishio, S.; Atsumi, T.; Ishizu, A. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J. Am. Soc. Nephrol. 2014, 25, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Lavine, N.; Ohayon, A.; Mahroum, N. Renal autoimmunity: The role of bacterial and viral infections, an extensive review. Autoimmun. Rev. 2022, 21, 103073. [Google Scholar] [CrossRef] [PubMed]
- Hutton, H.L.; Holdsworth, S.R.; Kitching, A.R. ANCA-Associated Vasculitis: Pathogenesis, Models, and Preclinical Testing. Semin. Nephrol. 2017, 37, 418–435. [Google Scholar] [CrossRef]
- Bonaci-Nikolic, B.; Andrejevic, S.; Pavlovic, M.; Dimcic, Z.; Ivanovic, B.; Nikolic, M. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: Diagnostic and therapeutic challenge. Clin. Rheumatol. 2010, 29, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Dekkema, G.J.; Rutgers, A.; Sanders, J.S.; Stegeman, C.A.; Heeringa, P. The Nasal Microbiome in ANCA-Associated Vasculitis: Picking the Nose for Clues on Disease Pathogenesis. Curr. Rheumatol. Rep. 2021, 23, 54. [Google Scholar] [CrossRef]
- Stegeman, C.A.; Tervaert, J.W.; Sluiter, W.J.; Manson, W.L.; de Jong, P.E.; Kallenberg, C.G. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann. Intern. Med. 1994, 120, 12–17. [Google Scholar] [CrossRef]
- Ooi, J.D.; Jiang, J.H.; Eggenhuizen, P.J.; Chua, L.L.; van Timmeren, M.; Loh, K.L.; O’Sullivan, K.M.; Gan, P.Y.; Zhong, Y.; Tsyganov, K.; et al. A plasmid-encoded peptide from Staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat. Commun. 2019, 10, 3392. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, C.A.; Tervaert, J.W.; de Jong, P.E.; Kallenberg, C.G. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N. Engl. J. Med. 1996, 335, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Krol, R.M.; Remmelts, H.H.F.; Klaasen, R.; Frima, A.; Hagen, E.C.; Kamalski, D.M.A.; Heijstek, M.W.; Spierings, J. Systemic and Local Medical or Surgical Therapies for Ear, Nose and/or Throat Manifestations in ANCA-Associated Vasculitis: A Systematic Literature Review. J. Clin. Med. 2023, 12, 3173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, S. Gut microbiota and immune mediation: A Mendelian randomization study on granulomatosis with polyangiitis. Front. Immunol. 2023, 14, 1296016. [Google Scholar] [CrossRef] [PubMed]
- Talotta, R.; Atzeni, F.; Ditto, M.C.; Gerardi, M.C.; Sarzi-Puttini, P. The Microbiome in Connective Tissue Diseases and Vasculitides: An Updated Narrative Review. J. Immunol. Res. 2017, 2017, 6836498. [Google Scholar] [CrossRef]
- Nakazawa, D.; Masuda, S.; Tomaru, U.; Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2019, 15, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Walulik, A.; Łysak, K.; Błaszkiewicz, M.; Górecki, I.; Gomułka, K. The Role of Neutrophils in ANCA-Associated Vasculitis: The Pathogenic Role and Diagnostic Utility of Autoantibodies. Int. J. Mol. Sci. 2023, 24, 17217. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Nonokawa, M.; Futamata, E.; Nishibata, Y.; Iwasaki, S.; Tsuji, T.; Hatanaka, Y.; Nakazawa, D.; Tanaka, S.; Tomaru, U.; et al. Formation and Disordered Degradation of Neutrophil Extracellular Traps in Necrotizing Lesions of Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis. Am. J. Pathol. 2019, 189, 839–846. [Google Scholar] [CrossRef]
- Nakazawa, D.; Tomaru, U.; Suzuki, A.; Masuda, S.; Hasegawa, R.; Kobayashi, T.; Nishio, S.; Kasahara, M.; Ishizu, A. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: Implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012, 64, 3779–3787. [Google Scholar] [CrossRef]
- Falk, R.J.; Terrell, R.S.; Charles, L.A.; Jennette, J.C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl. Acad. Sci. USA 1990, 87, 4115–4119. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Dolff, S.; Witzke, O.; Wilde, B. Th17 cells in renal inflammation and autoimmunity. Autoimmun. Rev. 2019, 18, 129–136. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Xiao, H.; Jennette, J.C.; Schneider, W.; Luft, F.C.; Kettritz, R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 2009, 20, 289–298. [Google Scholar] [CrossRef]
- Huang, Y.M.; Wang, H.; Wang, C.; Chen, M.; Zhao, M.H. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol. 2015, 67, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Gou, S.J.; Huang, J.; Hao, J.; Chen, M.; Zhao, M.H. C5a and its receptors in human anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Arthritis Res. Ther. 2012, 14, R140. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, S.; Tripodo, C.; Chiodoni, C.; Guarnotta, C.; Cappetti, B.; Casalini, P.; Piconese, S.; Parenza, M.; Guiducci, C.; Vitali, C.; et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 2012, 120, 3007–3018. [Google Scholar] [CrossRef]
- Abdulahad, W.H.; Stegeman, C.A.; van der Geld, Y.M.; Doornbos-van der Meer, B.; Limburg, P.C.; Kallenberg, C.G. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2007, 56, 2080–2091. [Google Scholar] [CrossRef]
- Wilde, B.; Thewissen, M.; Damoiseaux, J.; Knippenberg, S.; Hilhorst, M.; van Paassen, P.; Witzke, O.; Cohen Tervaert, J.W. Regulatory B cells in ANCA-associated vasculitis. Ann. Rheum. Dis. 2013, 72, 1416–1419. [Google Scholar] [CrossRef]
- Kallenberg, C.G.; Stegeman, C.A.; Abdulahad, W.H.; Heeringa, P. Pathogenesis of ANCA-associated vasculitis: New possibilities for intervention. Am. J. Kidney Dis. 2013, 62, 1176–1187. [Google Scholar] [CrossRef]
- Kantari, C.; Pederzoli-Ribeil, M.; Amir-Moazami, O.; Gausson-Dorey, V.; Moura, I.C.; Lecomte, M.C.; Benhamou, M.; Witko-Sarsat, V. Proteinase 3, the Wegener autoantigen, is externalized during neutrophil apoptosis: Evidence for a functional association with phospholipid scramblase 1 and interference with macrophage phagocytosis. Blood 2007, 110, 4086–4095. [Google Scholar] [CrossRef]
- Millet, A.; Martin, K.R.; Bonnefoy, F.; Saas, P.; Mocek, J.; Alkan, M.; Terrier, B.; Kerstein, A.; Tamassia, N.; Satyanarayanan, S.K.; et al. Proteinase 3 on apoptotic cells disrupts immune silencing in autoimmune vasculitis. J. Clin. Investig. 2015, 125, 4107–4121. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Hamour, S.; Sawant, D.; Henderson, S.; Mansfield, N.; Chavele, K.M.; Pusey, C.D.; Salama, A.D. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol. Dial. Transpl. 2010, 25, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Milne, M.E.; Kimball, J.; Tarrant, T.K.; Al-Rohil, R.N.; Leverenz, D.L. The Role of T Helper Type 2 (Th2) Cytokines in the Pathogenesis of Eosinophilic Granulomatosis with Polyangiitis (eGPA): An Illustrative Case and Discussion. Curr. Allergy Asthma Rep. 2022, 22, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Ueki, S.; Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int. 2020, 69, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Bieche, I.; Maisonobe, T.; Laurendeau, I.; Rosenzwajg, M.; Kahn, J.E.; Diemert, M.C.; Musset, L.; Vidaud, M.; Sene, D.; et al. Interleukin-25: A cytokine linking eosinophils and adaptive immunity in Churg-Strauss syndrome. Blood 2010, 116, 4523–4531. [Google Scholar] [CrossRef]
- Tsurikisawa, N.; Oshikata, C.; Watanabe, M.; Tsuburai, T.; Kaneko, T.; Saito, H. Innate immune response reflects disease activity in eosinophilic granulomatosis with polyangiitis. Clin. Exp. Allergy 2018, 48, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Dion, J.; Van Dyken, S.; Ricardo-Gonzalez, R.R.; Danel, C.J.; Taille, C.; Mouthon, L.; Locksley, R.M.; Terrier, B. A role for IL-33-activated ILC2s in eosinophilic vasculitis. JCI Insight 2021, 6, e143366. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Hashimoto, M. Eosinophilic Granulomatosis with Polyangiitis: Latest Findings and Updated Treatment Recommendations. J. Clin. Med. 2023, 12, 5996. [Google Scholar] [CrossRef]
- Fukuchi, M.; Miyabe, Y.; Furutani, C.; Saga, T.; Moritoki, Y.; Yamada, T.; Weller, P.F.; Ueki, S. How to detect eosinophil ETosis (EETosis) and extracellular traps. Allergol. Int. 2021, 70, 19–29. [Google Scholar] [CrossRef]
- Fagni, F.; Bello, F.; Emmi, G. Eosinophilic Granulomatosis With Polyangiitis: Dissecting the Pathophysiology. Front. Med. 2021, 8, 627776. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Salapow, M.A.; Breen, R.; Broide, D.H. Eosinophil peroxidase differs from neutrophil myeloperoxidase in its ability to bind antineutrophil cytoplasmic antibodies reactive with myeloperoxidase. Int. Arch. Allergy Immunol. 1994, 105, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Gioffredi, A.; Maritati, F.; Oliva, E.; Buzio, C. Eosinophilic granulomatosis with polyangiitis: An overview. Front. Immunol. 2014, 5, 549. [Google Scholar] [CrossRef]
- Okubo, K.; Kamiya, M.; Urano, Y.; Nishi, H.; Herter, J.M.; Mayadas, T.; Hirohama, D.; Suzuki, K.; Kawakami, H.; Tanaka, M.; et al. Lactoferrin Suppresses Neutrophil Extracellular Traps Release in Inflammation. EBioMedicine 2016, 10, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Shida, H.; Nakazawa, D.; Tateyama, Y.; Miyoshi, A.; Kusunoki, Y.; Hattanda, F.; Masuda, S.; Tomaru, U.; Kawakami, T.; Atsumi, T.; et al. The Presence of Anti-Lactoferrin Antibodies in a Subgroup of Eosinophilic Granulomatosis with Polyangiitis Patients and Their Possible Contribution to Enhancement of Neutrophil Extracellular Trap Formation. Front. Immunol. 2016, 7, 636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, Y.; Zhao, M.H. Review article: Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology 2009, 14, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Liu, Z.C. Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Chin. Med. J. 2019, 132, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin. Exp. Nephrol. 2013, 17, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Bensiradj, F.; Hignard, M.; Nakkash, R.; Proux, A.; Massy, N.; Kadri, N.; Doucet, J.; Landrin, I. Benzylthiouracil-induced ANCA-associated Vasculitis: A Case Report and Literature Review. Eur. J. Case Rep. Intern. Med. 2019, 6, 001283. [Google Scholar] [CrossRef]
- Sokumbi, O.; Wetter, D.A.; Makol, A.; Warrington, K.J. Vasculitis associated with tumor necrosis factor-α inhibitors. Mayo Clin. Proc. 2012, 87, 739–745. [Google Scholar] [CrossRef]
- Yaseen, K.; Nevares, A.; Tamaki, H. A Spotlight on Drug-Induced Vasculitis. Curr. Rheumatol. Rep. 2022, 24, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Rahmattulla, C.; Mooyaart, A.L.; van Hooven, D.; Schoones, J.W.; Bruijn, J.A.; Dekkers, O.M.; Bajema, I.M. Genetic variants in ANCA-associated vasculitis: A meta-analysis. Ann. Rheum. Dis. 2016, 75, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Ciavatta, D.J.; Yang, J.; Preston, G.A.; Badhwar, A.K.; Xiao, H.; Hewins, P.; Nester, C.M.; Pendergraft, W.F., 3rd; Magnuson, T.R.; Jennette, J.C.; et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J. Clin. Investig. 2010, 120, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.E.; Yang, J.; Muthigi, A.; Hogan, S.L.; Hu, Y.; Starmer, J.; Henderson, C.D.; Poulton, C.J.; Brant, E.J.; Pendergraft, W.F., 3rd; et al. Gene-Specific DNA Methylation Changes Predict Remission in Patients with ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2017, 28, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Cornacchia, E.; Golbus, J.; Maybaum, J.; Strahler, J.; Hanash, S.; Richardson, B. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J. Immunol. 1988, 140, 2197–2200. [Google Scholar] [CrossRef] [PubMed]
- Irizarry-Caro, J.A.; Carmona-Rivera, C.; Schwartz, D.M.; Khaznadar, S.S.; Kaplan, M.J.; Grayson, P.C. Brief Report: Drugs Implicated in Systemic Autoimmunity Modulate Neutrophil Extracellular Trap Formation. Arthritis Rheumatol. 2018, 70, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Segelmark, M.; Hellmark, T. Anti-glomerular basement membrane disease: An update on subgroups, pathogenesis and therapies. Nephrol. Dial. Transpl. 2019, 34, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, Y.; Cui, Z.; Yang, R.; Zhao, M.H. The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHalpha3(IV)NC1 is associated with disease severity. Hum. Immunol. 2009, 70, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.D.; Holdsworth, S.R.; Kitching, A.R. Advances in the pathogenesis of Goodpasture’s disease: From epitopes to autoantibodies to effector T cells. J. Autoimmun. 2008, 31, 295–300. [Google Scholar] [CrossRef]
- Salama, A.D.; Chaudhry, A.N.; Holthaus, K.A.; Mosley, K.; Kalluri, R.; Sayegh, M.H.; Lechler, R.I.; Pusey, C.D.; Lightstone, L. Regulation by CD25+ lymphocytes of autoantigen-specific T-cell responses in Goodpasture’s (anti-GBM) disease. Kidney Int. 2003, 64, 1685–1694. [Google Scholar] [CrossRef]
- Hunemorder, S.; Treder, J.; Ahrens, S.; Schumacher, V.; Paust, H.J.; Menter, T.; Matthys, P.; Kamradt, T.; Meyer-Schwesinger, C.; Panzer, U.; et al. TH1 and TH17 cells promote crescent formation in experimental autoimmune glomerulonephritis. J. Pathol. 2015, 237, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.D.; Chang, J.; O’Sullivan, K.M.; Pedchenko, V.; Hudson, B.G.; Vandenbark, A.A.; Fugger, L.; Holdsworth, S.R.; Kitching, A.R. The HLA-DRB1*15:01-restricted Goodpasture’s T cell epitope induces GN. J. Am. Soc. Nephrol. 2013, 24, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Carney, E.F. Role of infection and molecular mimicry in the pathogenesis of anti-GBM disease. Nat. Rev. Nephrol. 2020, 16, 430. [Google Scholar] [CrossRef]
- Shah, M.K. Outcomes in patients with Goodpasture’s syndrome and hydrocarbon exposure. Ren. Fail. 2002, 24, 545–555. [Google Scholar] [CrossRef]
- Luo, H.; Chen, M.; Cui, Z.; Yang, R.; Xu, P.C.; Zhou, X.J.; Zhao, M.H. The association of HLA-DQB1, -DQA1 and -DPB1 alleles with anti- glomerular basement membrane (GBM) disease in Chinese patients. BMC Nephrol. 2011, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Lv, J.C.; Zhao, M.H.; Zhang, H. Advances in the genetics of anti-glomerular basement membrane disease. Am. J. Nephrol. 2010, 32, 482–490. [Google Scholar] [CrossRef]
- Huynh, M.; Eggenhuizen, P.J.; Olson, G.L.; Rao, N.B.; Self, C.R.; Sun, Y.; Holdsworth, S.R.; Kitching, A.R.; Ooi, J.D. HLA-DR15-specific inhibition attenuates autoreactivity to the Goodpasture antigen. J. Autoimmun. 2019, 103, 102276. [Google Scholar] [CrossRef]
- Gu, Q.H.; Jia, X.Y.; Li, J.N.; Chen, F.J.; Cui, Z.; Zhao, M.H. The critical amino acids of a nephritogenic epitope on human Goodpasture autoantigen for binding to HLA-DRB1*1501. Mol. Immunol. 2017, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.B.; Hammad, T.; Coulthart, A.; Dougan, T.; Pusey, C.D. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004, 66, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Cui, Z.; Wang, J.; Hu, S.Y.; Jia, X.Y.; Guan, Z.; Chen, M.; Xie, C.; Zhao, M.H. Autoantibodies against Linear Epitopes of Myeloperoxidase in Anti-Glomerular Basement Membrane Disease. Clin. J. Am. Soc. Nephrol. 2016, 11, 568–575. [Google Scholar] [CrossRef]
- Kronbichler, A.; Bajema, I.M.; Bruchfeld, A.; Mastroianni Kirsztajn, G.; Stone, J.H. Diagnosis and management of ANCA-associated vasculitis. Lancet 2024, 403, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.; St Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010, 363, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Tervaert, J.W.; Hauser, T.; Luqmani, R.; Morgan, M.D.; Peh, C.A.; Savage, C.O.; Segelmark, M.; Tesar, V.; van Paassen, P.; et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 2010, 363, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.R.W.; Merkel, P.A.; Schall, T.J.; Bekker, P.; Group, A.S. Avacopan for the Treatment of ANCA-Associated Vasculitis. N. Engl. J. Med. 2021, 384, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Merkel, P.A.; Niles, J.; Jimenez, R.; Spiera, R.F.; Rovin, B.H.; Bomback, A.; Pagnoux, C.; Potarca, A.; Schall, T.J.; Bekker, P.; et al. Adjunctive Treatment with Avacopan, an Oral C5a Receptor Inhibitor, in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. ACR Open Rheumatol. 2020, 2, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Roccatello, D.; Fenoglio, R.; Oddone, V.; Sciascia, S. How the Availability of Anti-C5a Agents Could Change the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Kidney Blood Press. Res. 2022, 47, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Chalkia, A.; Flossmann, O.; Jones, R.; Nair, J.R.; Simpson, T.; Smith, R.; Willcocks, L.; Jayne, D. Avacopan for ANCA-associated vasculitis with hypoxic pulmonary haemorrhage. Nephrol. Dial. Transpl. 2024, gfae020. [Google Scholar] [CrossRef]
- Guillevin, L.; Pagnoux, C.; Karras, A.; Khouatra, C.; Aumaitre, O.; Cohen, P.; Maurier, F.; Decaux, O.; Ninet, J.; Gobert, P.; et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N. Engl. J. Med. 2014, 371, 1771–1780. [Google Scholar] [CrossRef]
- De Groot, K.; Rasmussen, N.; Bacon, P.A.; Tervaert, J.W.; Feighery, C.; Gregorini, G.; Gross, W.L.; Luqmani, R.; Jayne, D.R. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005, 52, 2461–2469. [Google Scholar] [CrossRef]
- Yamakawa, H.; Toyoda, Y.; Baba, T.; Kishaba, T.; Fukuda, T.; Takemura, T.; Kuwano, K. Anti-Inflammatory and/or Anti-Fibrotic Treatment of MPO-ANCA-Positive Interstitial Lung Disease: A Short Review. J. Clin. Med. 2022, 11, 3835. [Google Scholar] [CrossRef]
- Collins, B.F.; Raghu, G. Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2019, 28, 190022. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Budinger, G.R.S.; Dematte, J.E. Advances in the management of idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. BMJ 2022, 377, e066354. [Google Scholar] [CrossRef] [PubMed]
- Maillet, T.; Goletto, T.; Beltramo, G.; Dupuy, H.; Jouneau, S.; Borie, R.; Crestani, B.; Cottin, V.; Blockmans, D.; Lazaro, E.; et al. Usual interstitial pneumonia in ANCA-associated vasculitis: A poor prognostic factor. J. Autoimmun. 2020, 106, 102338. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.D.; d’Ancona, G.; Fernandes, M.; Green, L.; Roxas, C.; Thomson, L.; Nanzer, A.M.; Kavanagh, J.; Agarwal, S.; Jackson, D.J. Oral corticosteroid-sparing effects of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020, 6, 00311-2019. [Google Scholar] [CrossRef] [PubMed]
- Manka, L.A.; Guntur, V.P.; Denson, J.L.; Dunn, R.M.; Dollin, Y.T.; Strand, M.J.; Wechsler, M.E. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann. Allergy Asthma Immunol. 2021, 126, 696–701.e1. [Google Scholar] [CrossRef] [PubMed]
- Guntur, V.P.; Manka, L.A.; Denson, J.L.; Dunn, R.M.; Dollin, Y.T.; Gill, M.; Kolakowski, C.; Strand, M.J.; Wechsler, M.E. Benralizumab as a Steroid-Sparing Treatment Option in Eosinophilic Granulomatosis with Polyangiitis. J. Allergy Clin. Immunol. Pract. 2021, 9, 1186–1193.e1. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Calatroni, M.; Moroni, G. Anti-glomerular basement membrane vasculitis. Autoimmun. Rev. 2023, 22, 103212. [Google Scholar] [CrossRef]
- Levy, J.B.; Turner, A.N.; Rees, A.J.; Pusey, C.D. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann. Intern. Med. 2001, 134, 1033–1042. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouka, E.; Drakopanagiotakis, F.; Steiropoulos, P. Pathogenesis of Pulmonary Manifestations in ANCA-Associated Vasculitis and Goodpasture Syndrome. Int. J. Mol. Sci. 2024, 25, 5278. https://doi.org/10.3390/ijms25105278
Fouka E, Drakopanagiotakis F, Steiropoulos P. Pathogenesis of Pulmonary Manifestations in ANCA-Associated Vasculitis and Goodpasture Syndrome. International Journal of Molecular Sciences. 2024; 25(10):5278. https://doi.org/10.3390/ijms25105278
Chicago/Turabian StyleFouka, Evangelia, Fotios Drakopanagiotakis, and Paschalis Steiropoulos. 2024. "Pathogenesis of Pulmonary Manifestations in ANCA-Associated Vasculitis and Goodpasture Syndrome" International Journal of Molecular Sciences 25, no. 10: 5278. https://doi.org/10.3390/ijms25105278
APA StyleFouka, E., Drakopanagiotakis, F., & Steiropoulos, P. (2024). Pathogenesis of Pulmonary Manifestations in ANCA-Associated Vasculitis and Goodpasture Syndrome. International Journal of Molecular Sciences, 25(10), 5278. https://doi.org/10.3390/ijms25105278






