Real-World Biomarkers for Pediatric Takayasu Arteritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
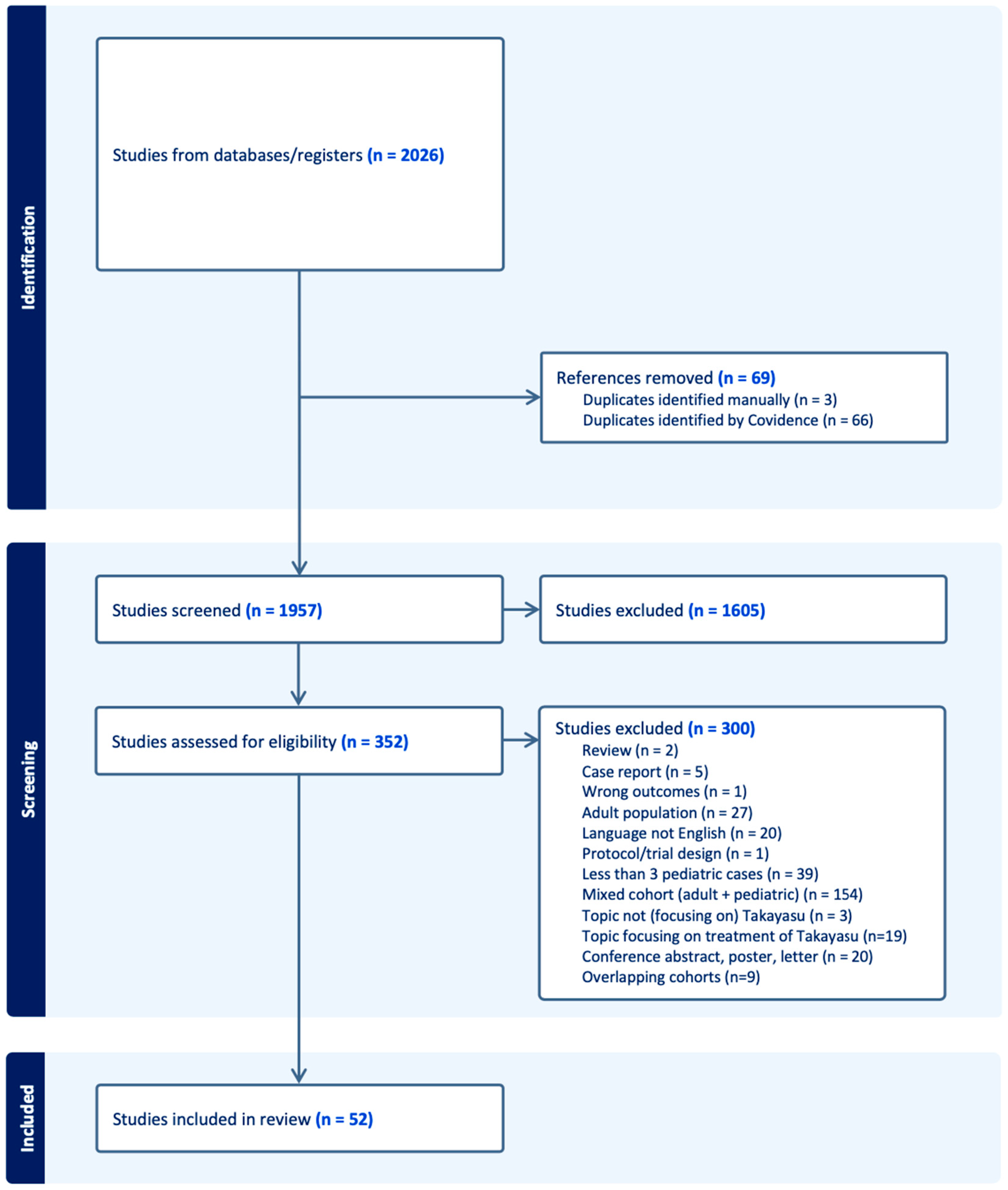
3.1.1. Included Studies
3.1.2. Characteristics of Included Studies
3.1.3. Quality Assessment
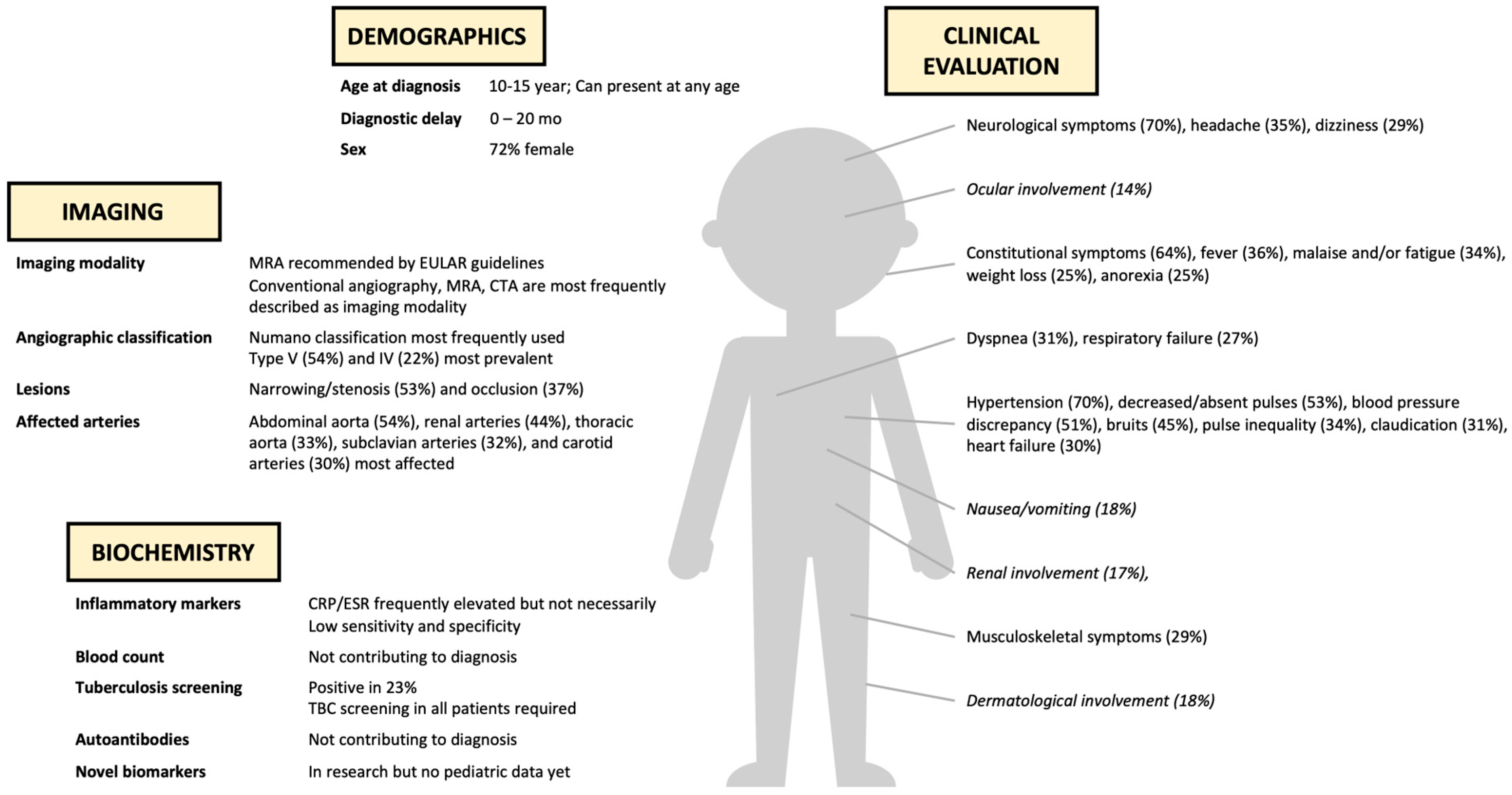
3.2. Demographics
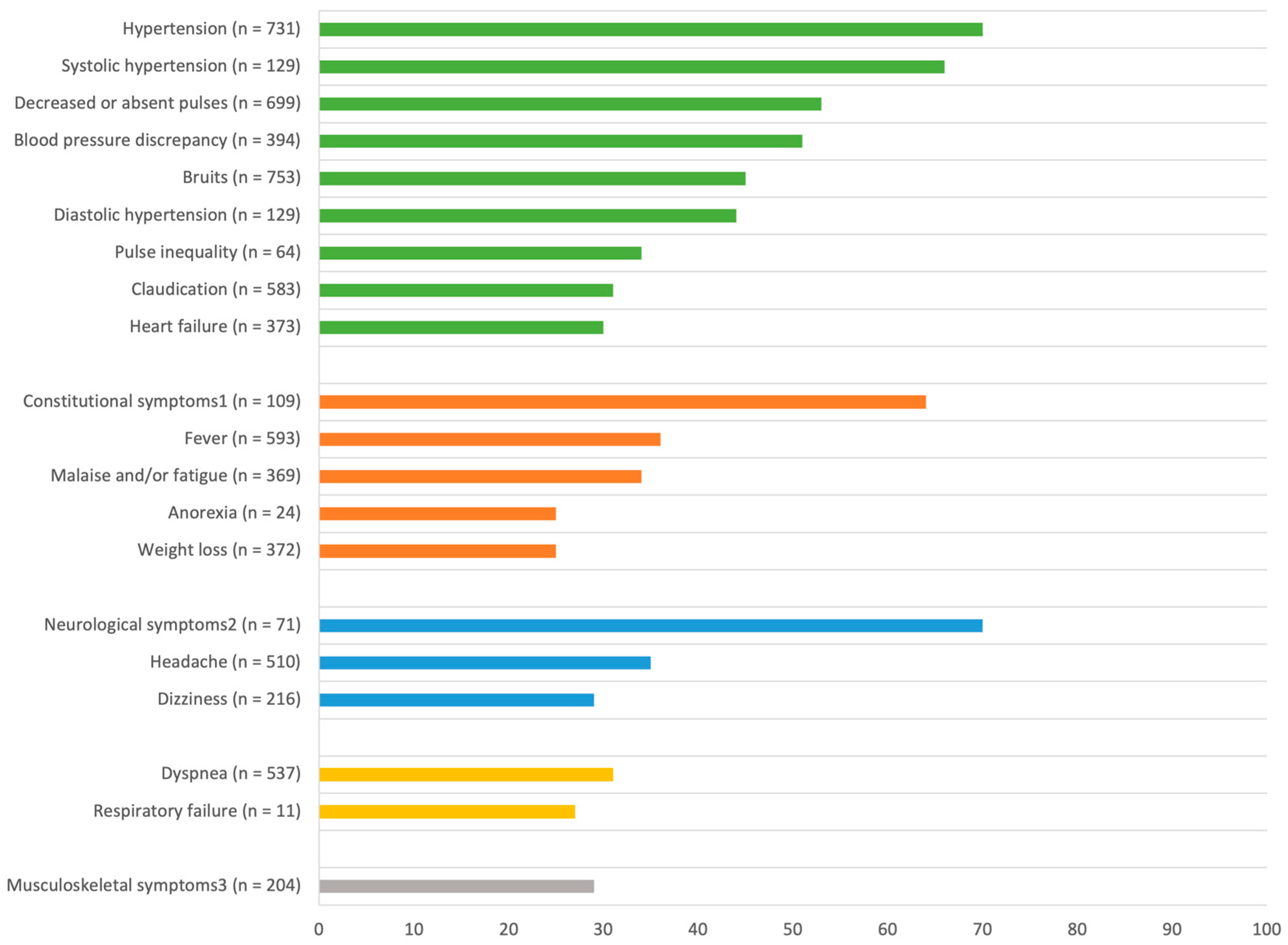
3.3. Clinical Features
3.3.1. General Symptoms [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,35,37,38,39,40,41,42,43,44,45,46,47,48,51,53,54]
3.3.2. Cardiovascular Symptoms [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,51,52,53,54,55]
3.3.3. Neurological Symptoms [14,16,17,18,20,21,22,23,24,25,27,28,29,32,35,37,38,39,40,41,44,45,46,47,48,51,52,53,55]
3.3.4. Musculoskeletal Symptoms [14,16,17,18,20,21,22,23,25,27,28,32,37,38,39,40,41,43,44,46,47,50,51,52,54,55]
3.3.5. Respiratory Symptoms [14,16,19,20,22,23,24,27,28,36,37,38,40,41,42,46,48,51,55]
3.3.6. Gastro-Intestinal Symptoms [14,22,24,25,27,28,30,32,35,37,39,42,43,44,45,46,48,50,51,52,53]
3.3.7. Other Symptoms [14,16,18,19,20,22,24,25,27,32,35,37,38,39,40,41,44,45,46,47,48,51,52,55]
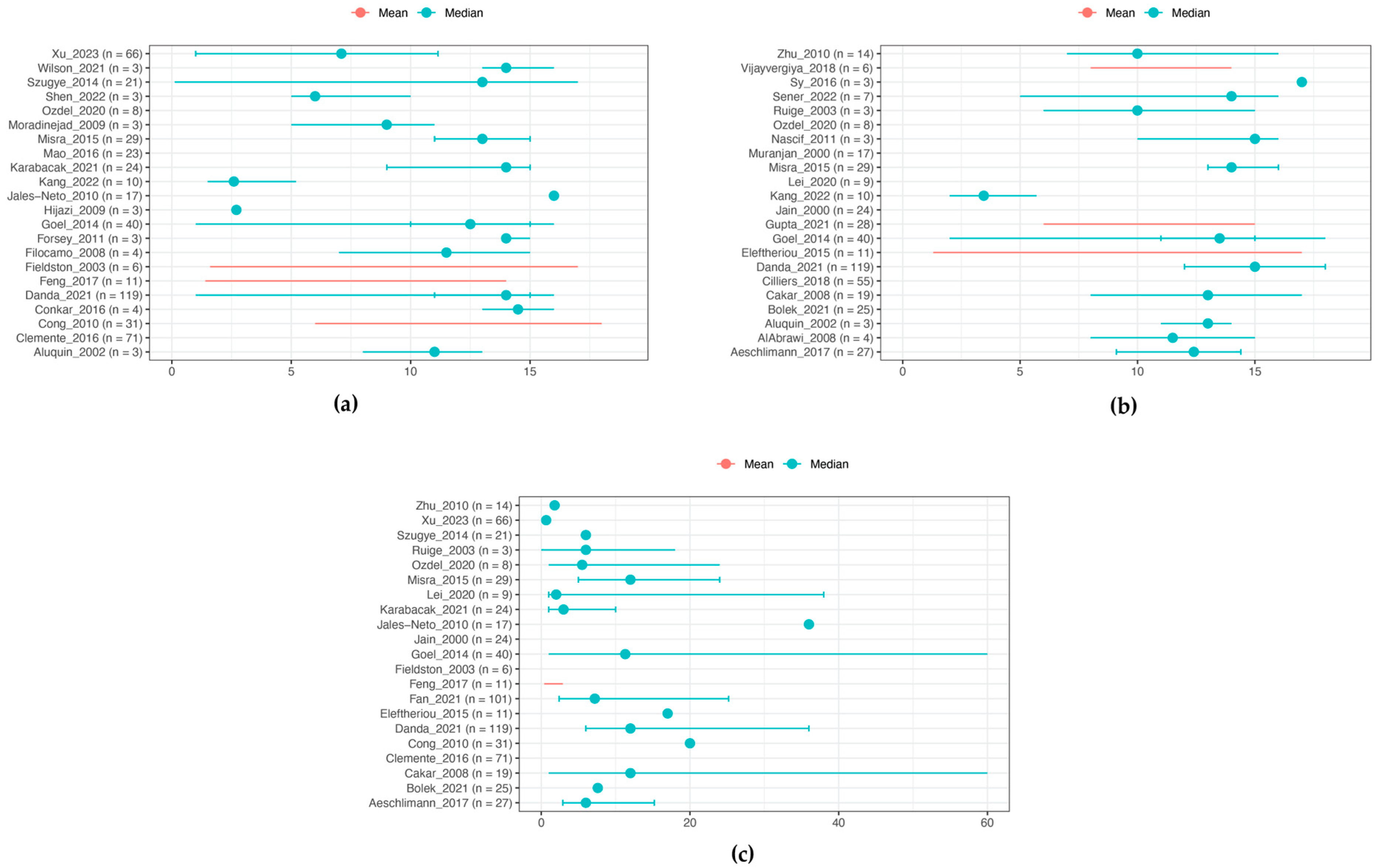
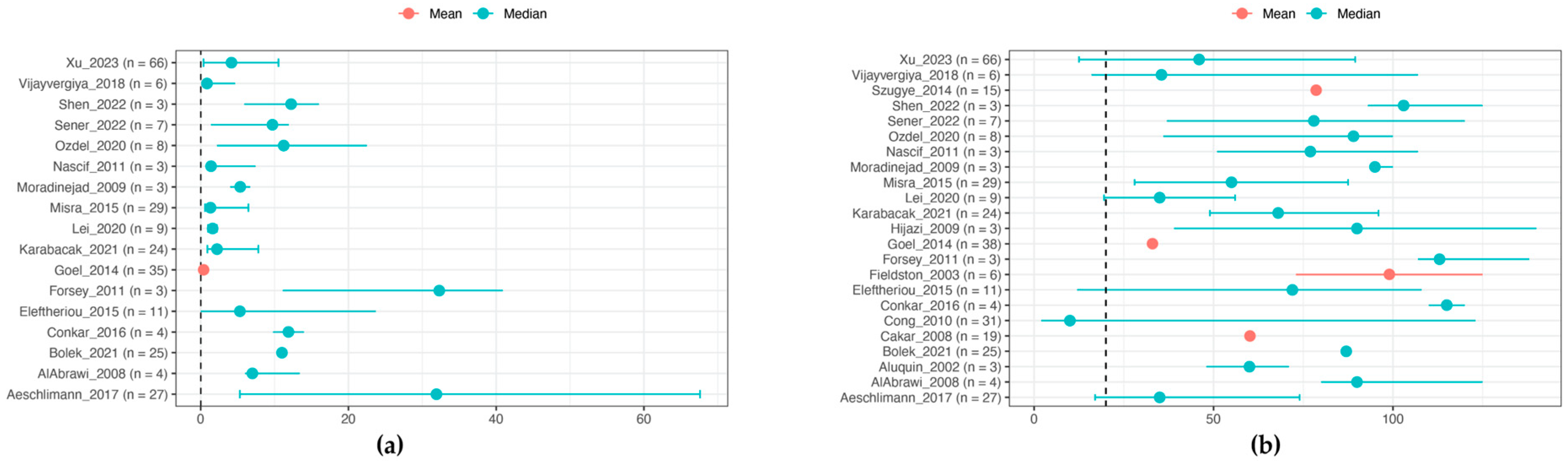
3.4. Laboratory Features
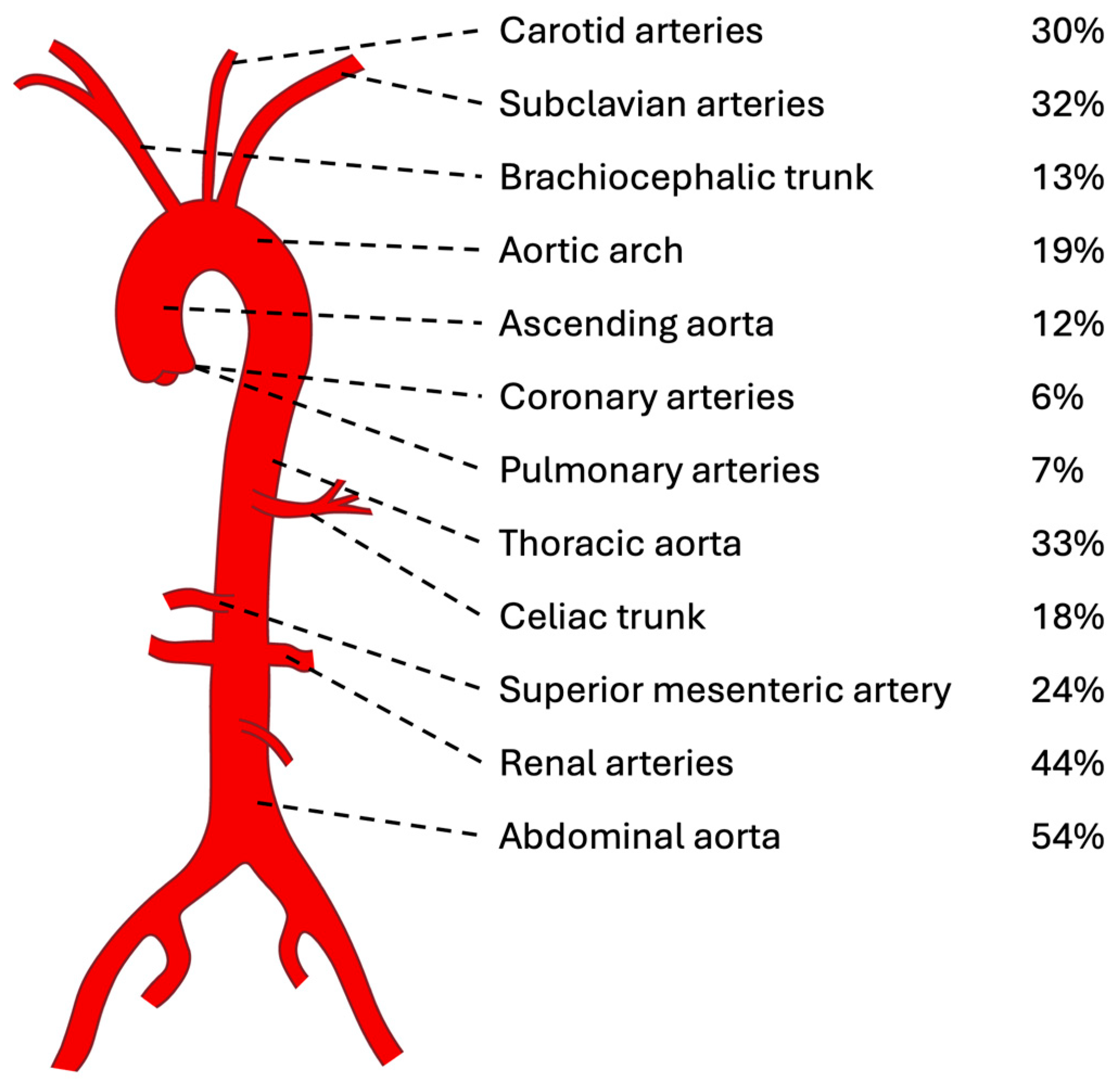
3.5. Imaging Features
3.6. Additional Studies on Imaging Approaches
3.7. Disease Activity Scores
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunner, J.; Feldman, B.M.; Tyrrell, P.N.; Kuemmerle-Deschner, J.B.; Zimmerhackl, L.B.; Gassner, I.; Benseler, S.M. Takayasu Arteritis in Children and Adolescents. Rheumatology 2010, 49, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Aeschlimann, F.A.; Yeung, R.S.M.; Laxer, R.M. An Update on Childhood-Onset Takayasu Arteritis. Front. Pediatr. 2022, 10, 872313. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Pistorio, A.; Iusan, S.M.; Bakkaloglu, A.; Herlin, T.; Brik, R.; Buoncompagni, A.; Lazar, C.; Bilge, I.; Uziel, Y.; et al. EULAR/PRINTO/PRES Criteria for Henoch-Schönlein Purpura, Childhood Polyarteritis Nodosa, Childhood Wegener Granulomatosis and Childhood Takayasu Arteritis: Ankara 2008. Part II: Final Classification Criteria. Ann. Rheum. Dis. 2010, 69, 798–806. [Google Scholar] [CrossRef]
- Hoffman, G.S.; Ahmed, A.E. Surrogate Markers of Disease Activity in Patients with Takayasu Arteritis A Preliminary Report from The International Network for the Study of the Systemic Vasculitides (INSSYS). Int. J. Cardiol. 1998, 66, S191–S194. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.S.; Hallahan, C.W.; Giordano, J.; Leavitt, R.Y.; Fauci, A.S.; Rottem, M.; Hoffman, G.S. Takayasu Arteritis. Ann. Intern. Med. 1994, 120, 919–929. [Google Scholar] [CrossRef]
- Mason, J.C. Takayasu Arteritis—Advances in Diagnosis and Management. Nat. Rev. Rheumatol. 2010, 6, 406–415. [Google Scholar] [CrossRef]
- Wen, D.; Feng, L.; Du, X.; Dong, J.Z.; Ma, C.S. Biomarkers in Takayasu Arteritis. Int. J. Cardiol. 2023, 371, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.P.; Jain, N.; Ora, M.; Singh, K.; Agarwal, V.; Sharma, A. Outcome Measures and Biomarkers for Disease Assessment in Takayasu Arteritis. Diagnostics 2022, 12, 2565. [Google Scholar] [CrossRef]
- Barra, L.; Kanji, T.; Malette, J.; Pagnoux, C. Imaging Modalities for the Diagnosis and Disease Activity Assessment of Takayasu’s Arteritis: A Systematic Review and Meta-Analysis. Autoimmun. Rev. 2018, 17, 175–187. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR Recommendations for the Use of Imaging in Large Vessel Vasculitis in Clinical Practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef]
- Aeschlimann, F.A.; Raimondi, F.; Leiner, T.; Aquaro, G.D.; Saadoun, D.; Grotenhuis, H.B. Overview of Imaging in Adult- and Childhood-Onset Takayasu Arteritis. J. Rheumatol. 2022, 49, 346–357. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Howick, J.; Chalmers, I.; Lind, J.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H.; et al. Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence; OCEBM Levels of Evidence Working Group. 2012. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 1 June 2024).
- Zhu, W.H.; Shen, L.G.; Neubauer, H. Clinical Characteristics, Interdisciplinary Treatment and Follow-up of 14 Children with Takayasu Arteritis. World J. Pediatr. 2010, 6, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, L.; Su, G.; Zhu, J.; Kang, M.; Zhang, D.; Lai, J.; Li, X. Clinical Characteristics and Risk Factors of Coronary Artery Lesions in Chinese Pediatric Takayasu Arteritis Patients: A Retrospective Study. Pediatr. Rheumatol. 2023, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.L.; Dai, S.M.; Feng, X.; Wang, Z.W.; Lu, Q.S.; Yuan, L.X.; Zhao, X.X.; Zhao, D.B.; Jing, Z.P. Takayasu’s Arteritis: Clinical Features and Outcomes of 125 Patients in China. Clin. Rheumatol. 2010, 29, 973–981. [Google Scholar] [CrossRef]
- Wilson, L.; Chandran, A.; Fudge, J.C.; Moguillansky, D.; Thatayatikom, A.; Philip, J.; Jacobs, J.P.; Bleiweis, M.; Elder, M.; Gupta, D. Takayasu’s Arteritis Presenting as Acute Myocardial Infarction: Case Series and Review of Literature. Cardiol. Young 2021, 31, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Aluquin, V.P.R.; Albano, S.A.; Chan, F.; Sandborg, C.; Pitlick, P.T. Magnetic Resonance Imaging in the Diagnosis and Follow up of Takayasu’s Arteritis in Children. Ann. Rheum. Dis. 2002, 61, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhao, S.; Wang, B.; Shen, Y.; Xiao, Y.; Xu, Z. Childhood-Onset Takayasu Arteritis Presenting as Pyoderma Gangrenosum-like Vasculitic Ulceration: Three Case Reports and a Literature Review. Australas. J. Dermatol. 2022, 63, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Sener, S.; Basaran, O.; Kaya Akca, U.; Atalay, E.; Kasap Cuceoglu, M.; Balik, Z.; Aliyev, E.; Bayindir, Y.; Batu, E.D.; Hazirolan, T.; et al. Treatment of Childhood-Onset Takayasu Arteritis: Switching between Anti-TNF and Anti-IL-6 Agents. Rheumatology 2022, 61, 4885–4891. [Google Scholar] [CrossRef]
- Ozdel, S.; Baglan, E.; Cakici, E.; Yazilitas, F.; Gungor, T.; Bulbul, M. Childhood-Onset Takayasu Arteritis: A Single-Center Experience. Ann. Clin. Anal. Med. 2020, 11, 229–232. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, N.; Singh, S.; Bali, H.K.; Kumar, L.; Sharma, B.K. Takayasu Arteritis in Children and Young Indians. Int. J. Cardiol. 2000, 75, S153–S157. [Google Scholar] [CrossRef] [PubMed]
- Conkar, S.; Mir, S.; Sozeri, B.; Bulut, I.K.; Cinar, C. Evaluation and Therapy in Four Patients with Takayasu’s Arteritis. Saudi J. Kidney Dis. Transpl. 2016, 27, 164–169. [Google Scholar] [CrossRef]
- Vijayvergiya, R.; Jindal, A.K.; Pilania, R.K.; Suri, D.; Gupta, A.; Sharma, A.; Sinha, S.K.; Singhal, M.; Bahl, A.; Singh, S. Complex Interventions of Abdominal Aorta and Its Branches in Children with Takayasu Arteritis: Clinical Experience from a Tertiary Care Center in North-West India. Int. J. Rheum. Dis. 2019, 22, 140–151. [Google Scholar] [CrossRef]
- Cakar, N.; Yalcinkaya, F.; Duzova, A.; Caliskan, S.; Sirin, A.; Oner, A.; Baskin, E.; Bek, K.; Soylu, A.; Fitoz, S.; et al. Takayasu Arteritis in Children. J. Rheumatol. 2008, 35, 913–919. [Google Scholar] [PubMed]
- Mao, Y.; Yin, L.; Xia, H.; Huang, H.; Zhou, Z.; Chen, T.; Zhou, W. Incidence and Clinical Features of Paediatric Vasculitis in Eastern China: 14-Year Retrospective Study, 1999–2013. J. Int. Med. Res. 2016, 44, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Muranjan, M.N.; Bavdekar, S.B.; More, V.; Deshmukh, H.; Tripathi, M.; Vaswani, R. Study of Takayasu’s Arteritis in Children: Clinical Profile and Management. J. Postgrad. Med. 2000, 46, 3–8. [Google Scholar]
- Moradinejad, M.H.; Ziaee, V.; Kiani, A.; Karimi, P. Report on Takayasu Arteritis in Three Iranian Children. Iran. J. Pediatr. 2009, 19, 307–312. [Google Scholar]
- Karabacak, M.; Kaymaz-Tahra, S.; Şahin, S.; Yıldız, M.; Adroviç, A.; Barut, K.; Direskeneli, H.; Kasapçopur, Ö.; Alibaz-Öner, F. Childhood-Onset versus Adult-Onset Takayasu Arteritis: A Study of 141 Patients from Turkey. Semin. Arthritis Rheum. 2021, 51, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Lai, J.; Zhang, D.; Xu, Y.; Zhu, J.; Li, M. Clinical Observations on Infliximab Treatment of Infantile Onset Takayasu Arteritis. Pediatr. Rheumatol. 2022, 20, 61. [Google Scholar] [CrossRef]
- Lei, C.; Huang, Y.; Yuan, S.; Chen, W.; Liu, H.; Yang, M.; Shen, Z.; Fang, L.; Fang, Q.; Song, H.; et al. Takayasu Arteritis With Coronary Artery Involvement: Differences Between Pediatric and Adult Patients. Can. J. Cardiol. 2020, 36, 535–542. [Google Scholar] [CrossRef]
- Jales-Neto, L.H.; Levy-Neto, M.; Bonfa, E.; de Carvalho, J.F.; Pereira, R.M.R. Juvenile-Onset Takayasu Arteritis: Peculiar Vascular Involvement and More Refractory Disease. Scand. J. Rheumatol. 2010, 39, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Ruige, J.B.; Van Geet, C.; Nevelsteen, A.; Verhaeghe, R. A 16-Year Survey of Takayasu’s Arteritis in a Tertiary Belgian Center. Int. Angiol. 2003, 22, 414–420. [Google Scholar] [PubMed]
- Zhou, J.; Li, J.; Wang, Y.; Yang, Y.; Zhao, J.; Li, M.; Pang, H.; Wang, T.; Chen, Y.; Tian, X.; et al. Age, Sex and Angiographic Type-Related Phenotypic Differences in Inpatients with Takayasu Arteritis: A 13-Year Retrospective Study at a National Referral Center in China. Front. Cardiovasc. Med. 2023, 10, 1099144. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, R.; Chandar, J.; Nwobi, O.; Muneeruddin, S.; Zilleruelo, G.; Abitbol, C.L. Renal Manifestations in Toddlers with Takayasu’s Arteritis and Malignant Hypertension. Pediatr. Nephrol. 2009, 24, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Hari, P.; Bagga, A.; Srivastava, R.N. Sustained Hypertension in Children. Indian. Pediatr. 2000, 37, 268–274. [Google Scholar] [PubMed]
- Szugye, H.S.; Zeft, A.S.; Spalding, S.J. Takayasu Arteritis in the Pediatric Population: A Contemporary United States-Based Single Center Cohort. Pediatr. Rheumatol. 2014, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Kaur, N.; Saxena, A.; Jagia, P.; Kumar, S.; Gupta, S.K.; Sharma, S.; Kothari, S.S.; Ramakrishnan, S. Non-Specific Aortoarteritis (NSAA) in Children: A Prospective Observational Study. BMJ Paediatr. Open 2021, 5, e001106. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, S.; Zhang, F.; Zhang, C.; Gu, Y.; Guo, L. Long-Term Outcomes of Angioplasty for Pediatric Renovascular Hypertension: A Single-Center Experience. Vascular 2023, 31, 122–130. [Google Scholar] [CrossRef]
- Goel, R.; Kumar, T.S.; Danda, D.; Joseph, G.; Jeyaseelan, V.; Surin, A.K.; Bacon, P. Childhood-Onset Takayasu Arteritis—Experience from a Tertiary Care Center in South India. J. Rheumatol. 2014, 41, 1183–1189. [Google Scholar] [CrossRef]
- Clemente, G.; Hilário, M.O.; Len, C.; Silva, C.A.; Sallum, A.M.; Campos, L.M.; Sacchetti, S.; Dos Santos, M.C.; Alves, A.G.; Ferriani, V.P.; et al. Brazilian Multicenter Study of 71 Patients with Juvenile-Onset Takayasu’s Arteritis: Clinical and Angiographic Features. Rev. Bras. Reumatol. 2016, 56, 145–151. [Google Scholar] [CrossRef]
- Forsey, J.; Dhandayuthapani, G.; Hamilton, M.C.K.; Martin, R.P.; Tizard, E.J.; Ramanan, A. V Takayasu Arteritis: Key Clinical Factors for Early Diagnosis. Arch. Dis. Child. Educ. Pract. Ed. 2011, 96, 176–182. [Google Scholar] [CrossRef]
- Filocamo, G.; Buoncompagni, A.; Viola, S.; Loy, A.; Malattia, C.; Ravelli, A.; Martini, A. Treatment of Takayasu’s Arteritis with Tumor Necrosis Factor Antagonists. J. Pediatr. 2008, 153, 432–434. [Google Scholar] [CrossRef]
- Fieldston, E.; Albert, D.; Finkel, T. Hypertension and Elevated ESR as Diagnostic Features of Takayasu Arteritis in Children. J. Clin. Rheumatol. 2003, 9, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tang, X.; Liu, M.; Zhou, J.; Zhao, X.; Li, Q. Clinical Study of Children with Takayasu Arteritis: A Retrospective Study from a Single Center in China. Pediatr. Rheumatol. 2017, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, D.; Varnier, G.; Dolezalova, P.; McMahon, A.M.; Al-Obaidi, M.; Brogan, P.A. Takayasu Arteritis in Childhood: Retrospective Experience from a Tertiary Referral Centre in the United Kingdom. Arthritis Res. Ther. 2015, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.P.; Aggarwal, A.; Lawrence, A.; Agarwal, V.; Misra, R. Pediatric-Onset Takayasu’s Arteritis: Clinical Features and Short-Term Outcome. Rheumatol. Int. 2015, 35, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Danda, D.; Goel, R.; Joseph, G.; Kumar, S.T.; Nair, A.; Ravindran, R.; Jeyaseelan, L.; Merkel, P.A.; Grayson, P.C. Clinical Course of 602 Patients with Takayasu’s Arteritis: Comparison between Childhood-Onset versus Adult Onset Disease. Rheumatology 2021, 60, 2246–2255. [Google Scholar] [CrossRef] [PubMed]
- Bolek, E.C.; Akca, U.K.; Sari, A.; Sag, E.; Demir, S.; Kilic, L.; Sener, Y.Z.; Aykan, H.H.; Kaya, E.B.; Bilginer, Y.; et al. Is Takayasu’s Arteritis More Severe in Children? Clin. Exp. Rheumatol. 2021, 39, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Al Abrawi, S.; Fouillet-Desjonqueres, M.; David, L.; Barral, X.; Cochat, P.; Cimaz, R. Takayasu Arteritis in Children. Pediatr. Rheumatol. 2008, 6, S88–S89. [Google Scholar] [CrossRef]
- Aeschlimann, F.A.; Eng, S.W.M.; Sheikh, S.; Laxer, R.M.; Hebert, D.; Noone, D.; Twilt, M.; Pagnoux, C.; Benseler, S.M.; Yeung, R.S.M. Childhood Takayasu Arteritis: Disease Course and Response to Therapy. Arthritis Res. Ther. 2017, 19, 255. [Google Scholar] [CrossRef]
- Cilliers, A.M.; Adams, P.E.; Ntsinjana, H.; Kala, U. Review of Children with Takayasu’s Arteritis at a Southern African Tertiary Care Centre. Cardiol. Young 2018, 28, 1129–1135. [Google Scholar] [CrossRef]
- Nascif, A.K.S.; Lemos, M.D.; Oliveira, N.S.; Perim, P.C.; Cordeiro, A.C.; Quintino, M. Takayasu’s Arteritis in Children and Adolescents: Report of Three Cases. Rev. Bras. Reumatol. 2011, 51, 524–530. [Google Scholar]
- Sy, A.; Khalidi, N.; Dehghan, N.; Barra, L.; Carette, S.; Cuthbertson, D.; Hoffman, G.S.; Koening, C.L.; Langford, C.A.; McAlear, C.; et al. Vasculitis in Patients with Inflammatory Bowel Diseases: A Study of 32 Patients and Systematic Review of the Literature. Semin. Arthritis Rheum. 2016, 45, 475–482. [Google Scholar] [CrossRef]
- Fan, L.; Yang, L.; Wei, D.; Ma, W.; Lou, Y.; Song, L.; Bian, J.; Zhang, H.; Cai, J. Clinical Scenario and Long-Term Outcome of Childhood Takayasu Arteritis Undergoing 121 Endovascular Interventions: A Large Cohort Over a Fifteen-Year Period. Arthritis Care Res. 2021, 73, 1678–1688. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Li, H.; Li, M.; Gao, Y.; Zhou, Z.; Peng, Y. Application of Deep Learning Image Reconstruction Algorithm to Improve Image Quality in CT Angiography of Children with Takayasu Arteritis. J. X-ray Sci. Technol. 2022, 30, 177–184. [Google Scholar] [CrossRef]
- Visrutaratna, P.; Srisuwan, T.; Sirivanichai, C. Pediatric Renovascular Hypertension in Thailand: CT Angiographic Findings. Pediatr. Radiol. 2009, 39, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, H.; Ogura, M.; Miyasaka, M.; Suzuki, H.; Ishikura, K.; Ishiguro, A.; Ito, S. Ultrasonography as a Diagnostic Support Tool for Childhood Takayasu Arteritis Referred to as Fever of Unknown Origin: Case Series and Literature Review. JMA J. 2021, 4, 358–366. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, M.; Andronikou, S.; Goddard, E.; Sinclair, P.; Lawrenson, J.; Mandelstam, S.; Beningfield, S.J.; Millar, A.J.W. Angiographic Features of 26 Children with Takayasu’s Arteritis. Pediatr. Radiol. 2003, 33, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Grotenhuis, H.B.; Aeschlimann, F.A.; Hui, W.; Slorach, C.; Yeung, R.S.M.; Benseler, S.M.; Bradley, T.J.; Grosse-Wortmann, L. Increased Arterial Stiffness Adversely Affects Left Ventricular Mechanics in Patients with Pediatric Takayasu Arteritis From a Toronto Cohort. J. Clin. Rheumatol. 2019, 25, 171–175. [Google Scholar] [CrossRef]
- Clemente, G.; de Souza, A.W.; Leao Filho, H.; Coelho, F.M.A.; Buchpiguel, C.; Lima, M.; Carneiro, C.; Pereira, R.M.R.; Aikawa, N.; Silva, C.A.; et al. Does [18F]F-FDG-PET/MRI Add Metabolic Information to Magnetic Resonance Image in Childhood-Onset Takayasu’s Arteritis Patients? A Multicenter Case Series. Adv. Rheumatol. 2022, 62, 28. [Google Scholar] [CrossRef]
- Clemente, G.; Pereira, R.M.R.; Aikawa, N.; Silva, C.A.; Campos, L.M.A.; Alves, G.; Buchpiguel, C.; Lima, M.; Carneiro, C.; Filho, H.L.; et al. Is Positron Emission Tomography/Magnetic Resonance Imaging a Reliable Tool for Detecting Vascular Activity in Treated Childhood-Onset Takayasu’s Arteritis? A Multicentre Study. Rheumatology 2022, 61, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Demirkaya, E.; Ozen, S.; Pistorio, A.; Galasso, R.; Ravelli, A.; Hasija, R.; Baskin, E.; Dressler, F.; Fischbach, M.; Garcia Consuegra, J.; et al. Performance of Birmingham Vasculitis Activity Score and Disease Extent Index in Childhood Vasculitides. Clin. Exp. Rheumatol. 2012, 30, S162–S168. [Google Scholar]
- Bayazit, A.K.; Yalcinkaya, F.; Cakar, N.; Duzova, A.; Bircan, Z.; Bakkaloglu, A.; Canpolat, N.; Kara, N.; Sirin, A.; Ekim, M.; et al. Reno-Vascular Hypertension in Childhood: A Nationwide Survey. Pediatr. Nephrol. 2007, 22, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.J.; Bendaly, E.A.; Hurwitz, R.A. Abdominal Coarctation and Associated Comorbidities in Children. Congenit. Heart Dis. 2014, 9, 69–74. [Google Scholar] [CrossRef]
- Arend, W.P.; Michel, B.A.; Bloch, D.A.; Hunder, G.G.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, Y.; Lie, J.T.; Lightfoot, R.W.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Takayasu Arteritis. Arthritis Rheum. 1990, 33, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Ruperto, N.; Dillon, M.J.; Bagga, A.; Barron, K.; Davin, J.C.; Kawasaki, T.; Lindsley, C.; Petty, R.E.; Prieur, A.M.; et al. EULAR/PReS Endorsed Consensus Criteria for the Classification of Childhood Vasculitides. Ann. Rheum. Dis. 2006, 65, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, R.; Noda, M.; Yajima, M.; Sharma, B.K.; Numano, F.; Chandigarh, I. Clinical Manifestations of Takayasu Arteritis in India and Japan—New Classification of Angiographic Findings; Westminster Publications Inc.: Louisville, KY, USA, 1997; Volume 708. [Google Scholar]
- Lupi-Herrera, E.; Marcushamer, J.; Mispireta, J.; Espino Vela, J. Review Takayasu’s Arteritis. Clinical Study of 107 Cases. Am. Heart J. 1977, 93, 94–103. [Google Scholar] [CrossRef]
- Hellmich, B.; Flossmann, O.; Gross, W.L.; Bacon, P.; Cohen-Tervaert, J.W.; Guillevin, L.; Jayne, D.; Mahr, A.; Merkel, P.A.; Raspe, H.; et al. EULAR Recommendations for Conducting Clinical Studies and/or Clinical Trials in Systemic Vasculitis: Focus on Anti-Neutrophil Cytoplasm Antibody-Associated Vasculitis. Ann. Rheum. Dis. 2007, 66, 605–617. [Google Scholar] [CrossRef]
- Luqmani, R.A.; Bacon, P.A.; Moots, R.J.; Janssen, B.A.; Pall, A.; Emery, P.; Savage, C.; Adu, D. Birmingham Vasculitis Activity Score (BVAS) in Systemic Necrotizing Vasculitis. QJM Int. J. Med. 1994, 87, 671–678. [Google Scholar] [CrossRef]
- de Groot, K.; Gross, W.L.; Herlyn, K.; Reinhold-Keller, E. Development and Validation of a Disease Extent Index for Wegener’s Granulomatosis. Clin. Nephrol. 2001, 55, 31–38. [Google Scholar]
- Dolezalova, P.; Price-Kuehne, F.E.; Özen, S.; Benseler, S.M.; Cabral, D.A.; Anton, J.; Brunner, J.; Cimaz, R.; O’neil, K.M.; Wallace, C.A.; et al. Disease Activity Assessment in Childhood Vasculitis: Development and Preliminary Validation of the Paediatric Vasculitis Activity Score (PVAS). Ann. Rheum. Dis. 2013, 72, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.P.; Rathore, U.; Kopp, C.R.; Patro, P.; Agarwal, V.; Sharma, A. Presentation and Clinical Course of Pediatric-Onset versus Adult-Onset Takayasu Arteritis—A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2022, 41, 3601–3613. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (accessed on 18 June 2024).
- Goel, R.; Kabeerdoss, J.; Ram, B.; Prakash, J.A.J.; Babji, S.; Nair, A.; Jeyaseelan, L.; Jeyaseelan, V.; Mathew, J.; Balaji, V.; et al. Serum Cytokine Profile in Asian Indian Patients with Takayasu Arteritis and Its Association with Disease Activity. Open Rheumatol. J. 2017, 11, 23–29. [Google Scholar] [CrossRef]
- Arraes, A.E.D.; de Souza, A.W.S.; Mariz, H.A.; Silva, N.P.; Torres, I.C.G.; Pinto, P.N.V.; Lima, E.N.P.; Sato, E.I. 18F-Fluorodeoxyglucose Positron Emission Tomography and Serum Cytokines and Matrix Metalloproteinases in the Assessment of Disease Activity in Takayasu’s Arteritis. Rev. Bras. Reumatol. 2016, 56, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Pulsatelli, L.; Boiardi, L.; Assirelli, E.; Pazzola, G.; Muratore, F.; Addimanda, O.; Dolzani, P.; Versari, A.; Casali, M.; Magnani, L.; et al. Interleukin-6 and Soluble Interleukin-6 Receptor Are Elevated in Large-Vessel Vasculitis: A Cross-Sectional and Longitudinal Study. Clin. Exp. Rheumatol. 2017, 35, S102–S110. [Google Scholar]
- Li, J.; Wang, Y.; Wang, Y.; Wang, Y.; Yang, Y.; Zhao, J.; Li, M.; Tian, X.; Zeng, X. Association between Acute Phase Reactants, Interleukin-6, Tumor Necrosis Factor-α, and Disease Activity in Takayasu’s Arteritis Patients. Arthritis Res. Ther. 2020, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Mahajan, N.; Dhawan, V. Acknowledged Signatures of Matrix Metalloproteinases in Takayasu’s Arteritis. BioMed Res. Int. 2014, 2014, 827105. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, A.; Sakai, N.; Ishigami, M.; Hiraoka, H.; Kashine, S.; Hirata, A.; Nakamura, T.; Yamashita, S.; Matsuzawa, Y. Matrix Metalloproteinases as Novel Disease Markers in Takayasu Arteritis. Circulation 2003, 108, 1469–1473. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, L.; Yan, F.; Liu, H.; Ding, Y.; Hou, J.; Jiang, L. MMP-9 and IL-6 Are Potential Biomarkers for Disease Activity in Takayasu’s Arteritis. Int. J. Cardiol. 2012, 156, 236–238. [Google Scholar] [CrossRef]
- Ishihara, T.; Haraguchi, G.; Tezuka, D.; Kamiishi, T.; Inagaki, H.; Isobe, M. Diagnosis and Assessment of Takayasu Arteritis by Multiple Biomarkers. Circ. J. 2013, 77, 477–483. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, C.; Sun, F.; Li, J.; Yang, Y.; Tian, X.; Zeng, X. Study on the Association of Serum Pentraxin-3 and Lysosomal-Associated Membrane Protein-2 Levels with Disease Activity in Chinese Takayasu’s Arteritis Patients. Clin. Exp. Rheumatol. 2019, 37, S109–S115. [Google Scholar]
- Ramirez, G.A.; Rovere-Querini, P.; Blasi, M.; Sartorelli, S.; Di Chio, M.C.; Baldini, M.; De Lorenzo, R.; Bozzolo, E.P.; Leone, R.; Mantovani, A.; et al. PTX3 Intercepts Vascular Inflammation in Systemic Immune-Mediated Diseases. Front. Immunol. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Lee, G.Y.; Jang, S.Y.; Ko, S.M.; Kim, E.K.; Lee, S.H.; Han, H.; Choi, S.H.; Kim, Y.W.; Choe, Y.H.; Kim, D.K. Cardiovascular Manifestations of Takayasu Arteritis and Their Relationship to the Disease Activity: Analysis of 204 Korean Patients at a Single Center. Int. J. Cardiol. 2012, 159, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dang, A.; Chen, B.; Lv, N.; Wang, X.; Zheng, D. Function of N-Terminal pro-Brain Natriuretic Peptide in Takayasu Arteritis Disease Monitoring. J. Rheumatol. 2014, 41, 1683–1688. [Google Scholar] [CrossRef]
- Cui, X.; Qin, F.; Song, L.; Wang, T.; Geng, B.; Zhang, W.; Jin, L.; Wang, W.; Li, S.; Tian, X.; et al. Novel Biomarkers for the Precisive Diagnosis and Activity Classification of Takayasu Arteritis. Circ. Genom. Precis. Med. 2019, 12, E002080. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peremans, L.; Twilt, M.; Benseler, S.M.; Grisaru, S.; Kirton, A.; Myers, K.A.; Hamiwka, L. Real-World Biomarkers for Pediatric Takayasu Arteritis. Int. J. Mol. Sci. 2024, 25, 7345. https://doi.org/10.3390/ijms25137345
Peremans L, Twilt M, Benseler SM, Grisaru S, Kirton A, Myers KA, Hamiwka L. Real-World Biomarkers for Pediatric Takayasu Arteritis. International Journal of Molecular Sciences. 2024; 25(13):7345. https://doi.org/10.3390/ijms25137345
Chicago/Turabian StylePeremans, Lieselot, Marinka Twilt, Susanne M. Benseler, Silviu Grisaru, Adam Kirton, Kimberly A. Myers, and Lorraine Hamiwka. 2024. "Real-World Biomarkers for Pediatric Takayasu Arteritis" International Journal of Molecular Sciences 25, no. 13: 7345. https://doi.org/10.3390/ijms25137345
APA StylePeremans, L., Twilt, M., Benseler, S. M., Grisaru, S., Kirton, A., Myers, K. A., & Hamiwka, L. (2024). Real-World Biomarkers for Pediatric Takayasu Arteritis. International Journal of Molecular Sciences, 25(13), 7345. https://doi.org/10.3390/ijms25137345





