Integrating Genome-Scale Metabolic Models with Patient Plasma Metabolome to Study Endothelial Metabolism In Situ
Abstract
1. Introduction
2. Results
2.1. Assessing RESOS Algorithm
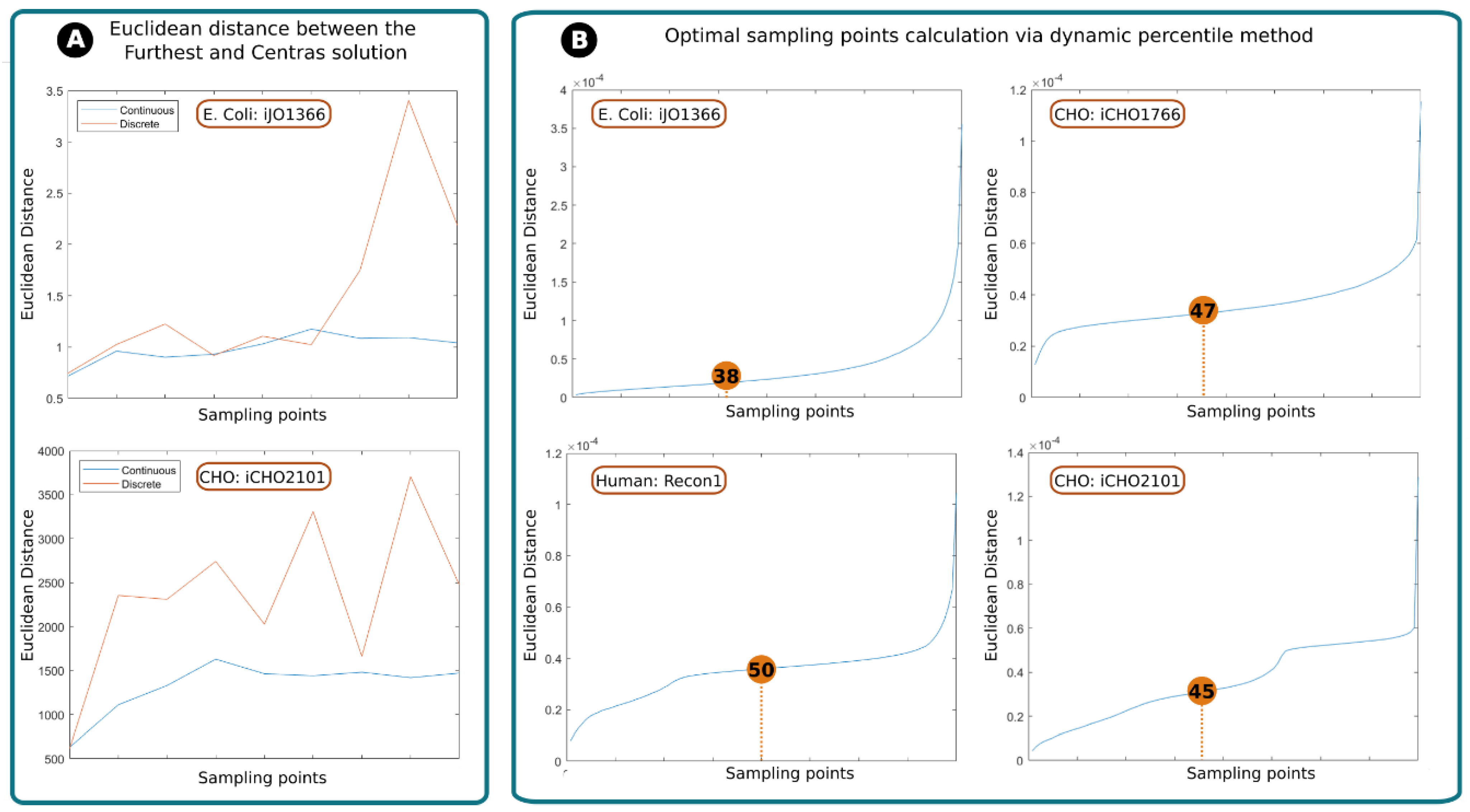
2.1.1. Assessing RESOS Algorithm: Continuous vs. Discrete Approach
2.1.2. Assessing RESOS Algorithm: Dynamic Threshold and Determining the Optimal Number of Sampling Points
2.1.3. Assessing the Improvement of the Model’s Predictive Capabilities by Applying RESOS Algorithm
2.2. Assessing Sampling Algorithm Performance
2.3. Applying the Protocol to the Trauma Patient Cohort
2.3.1. Patient-Specific metabolic Model Characterization
2.3.2. Validation of Patient-Specific GEMs
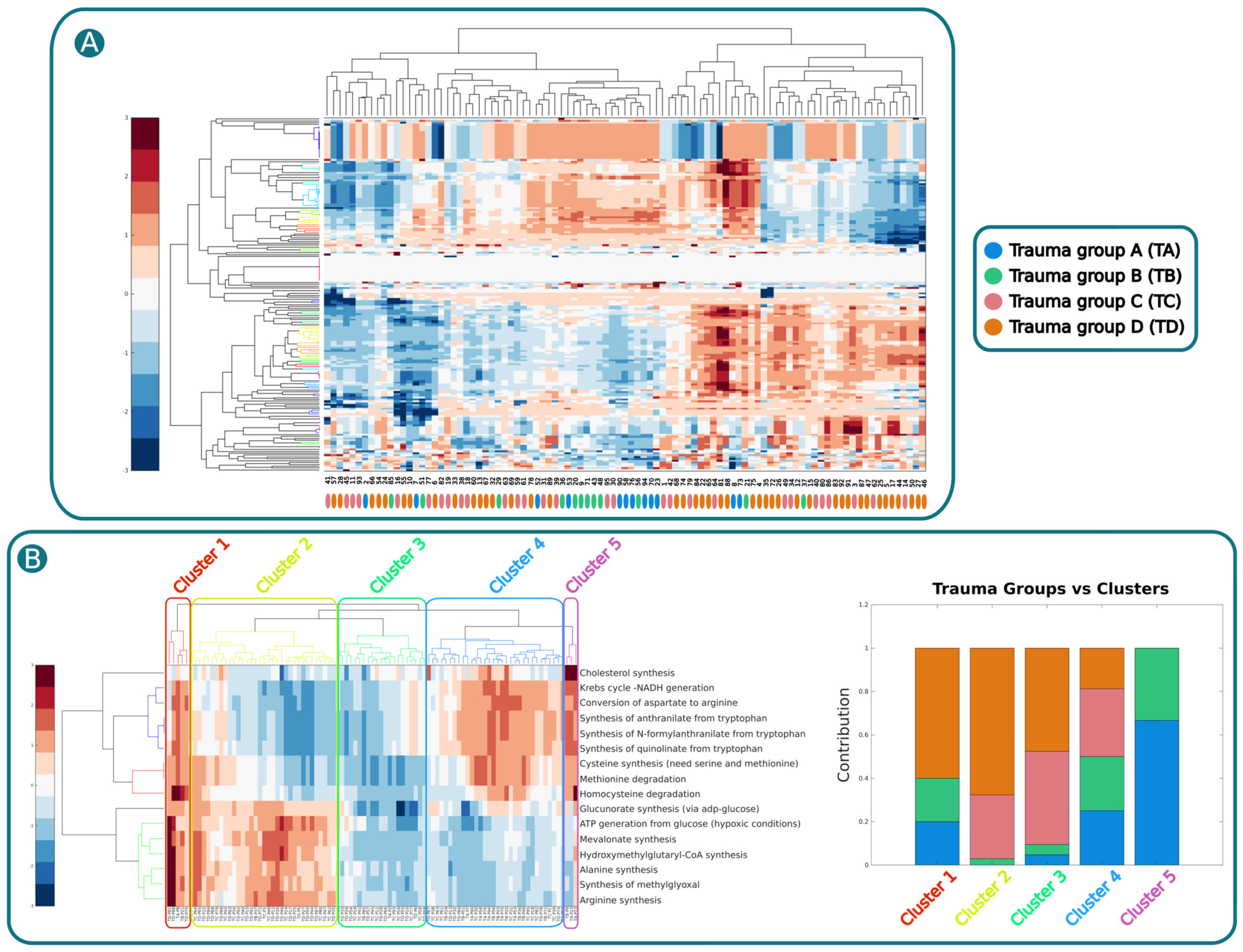
2.4. Identify Common Metabolic Features in Trauma Patients Associated with Metabo-Groups with Different Mortality Rate
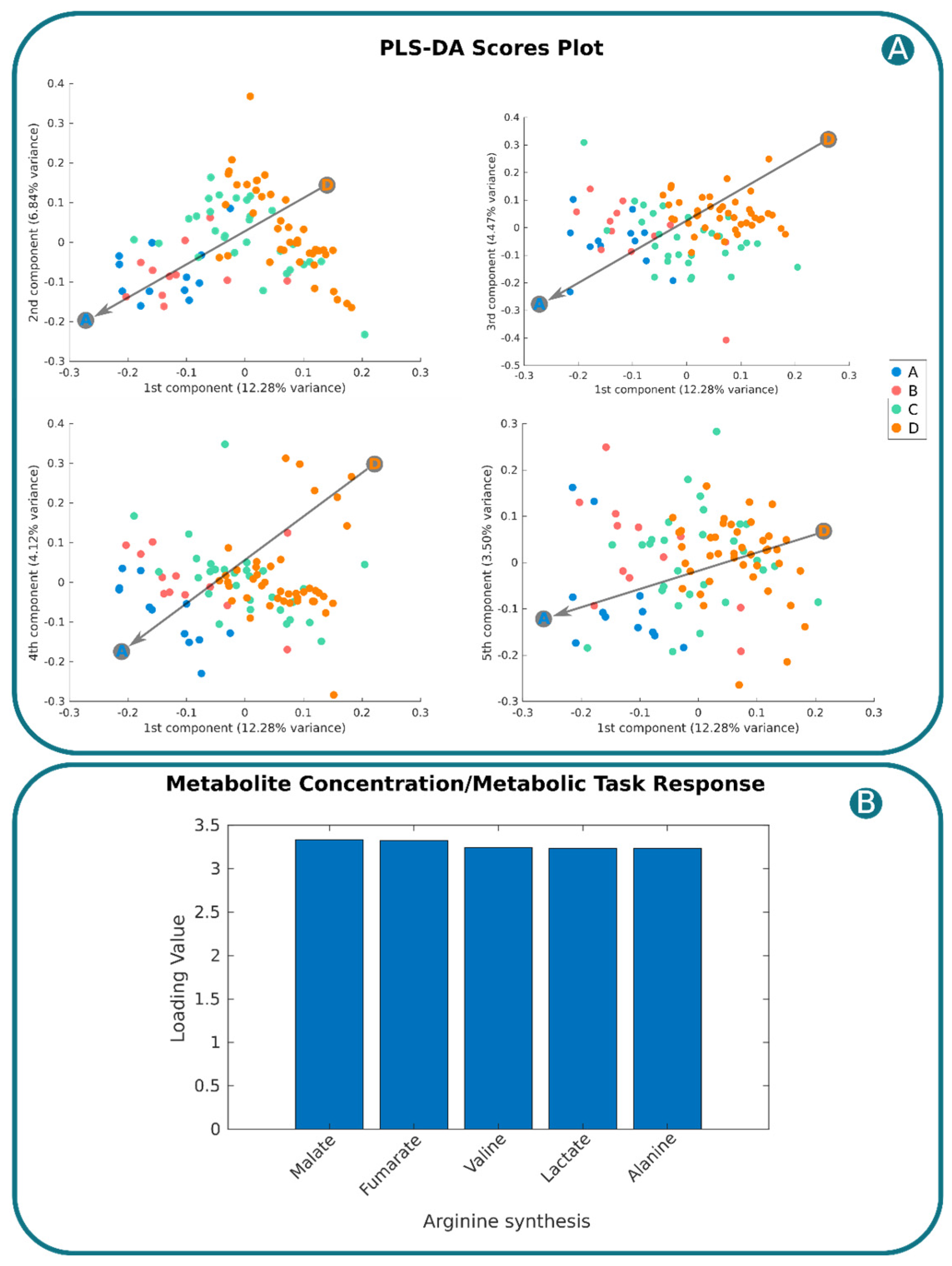
2.5. Linking Plasma Metabolome with Endothelial Cell Metabolic Tasks for Biomarker and Target Discovery
3. Discussion
4. Materials and Methods
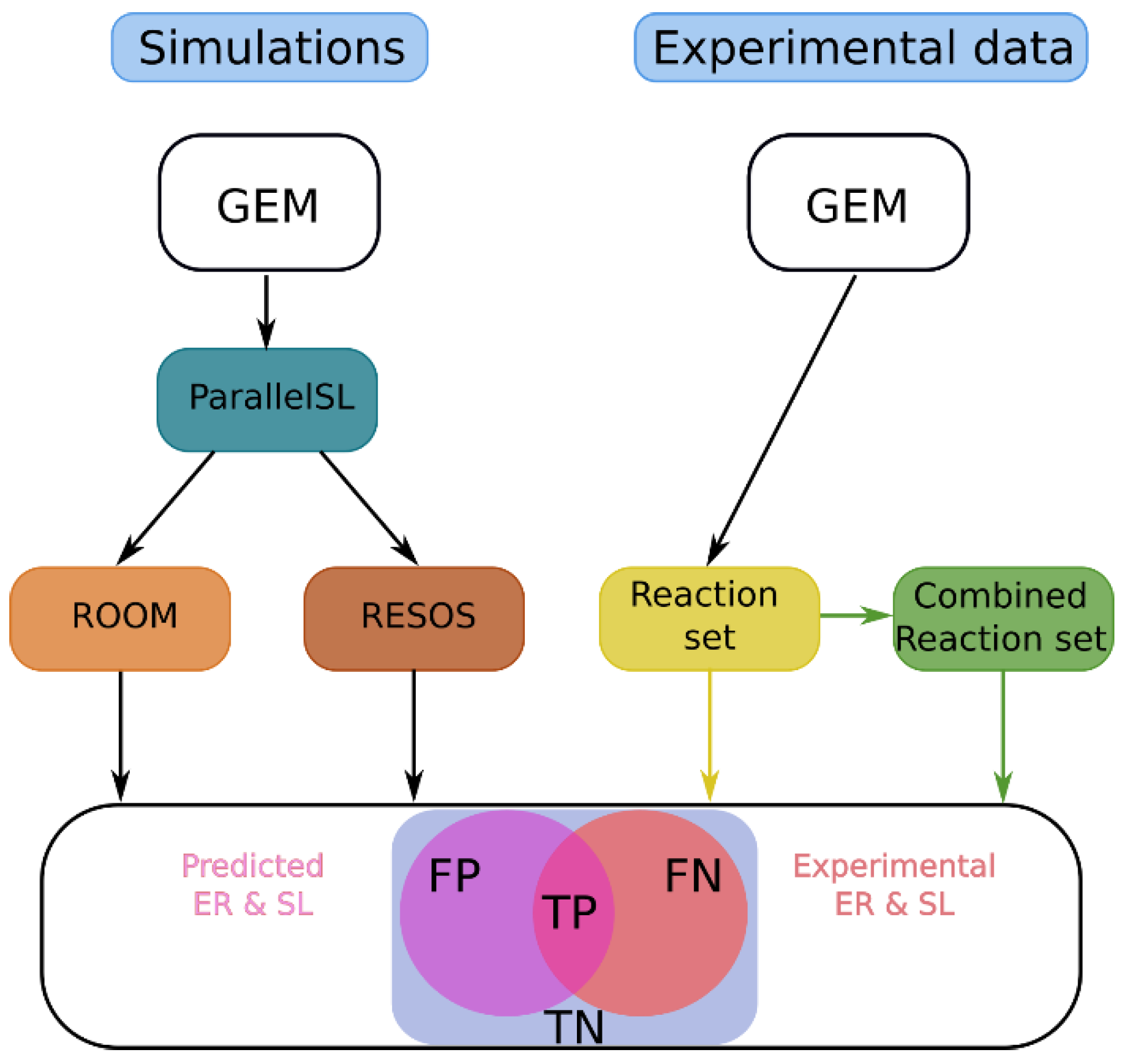
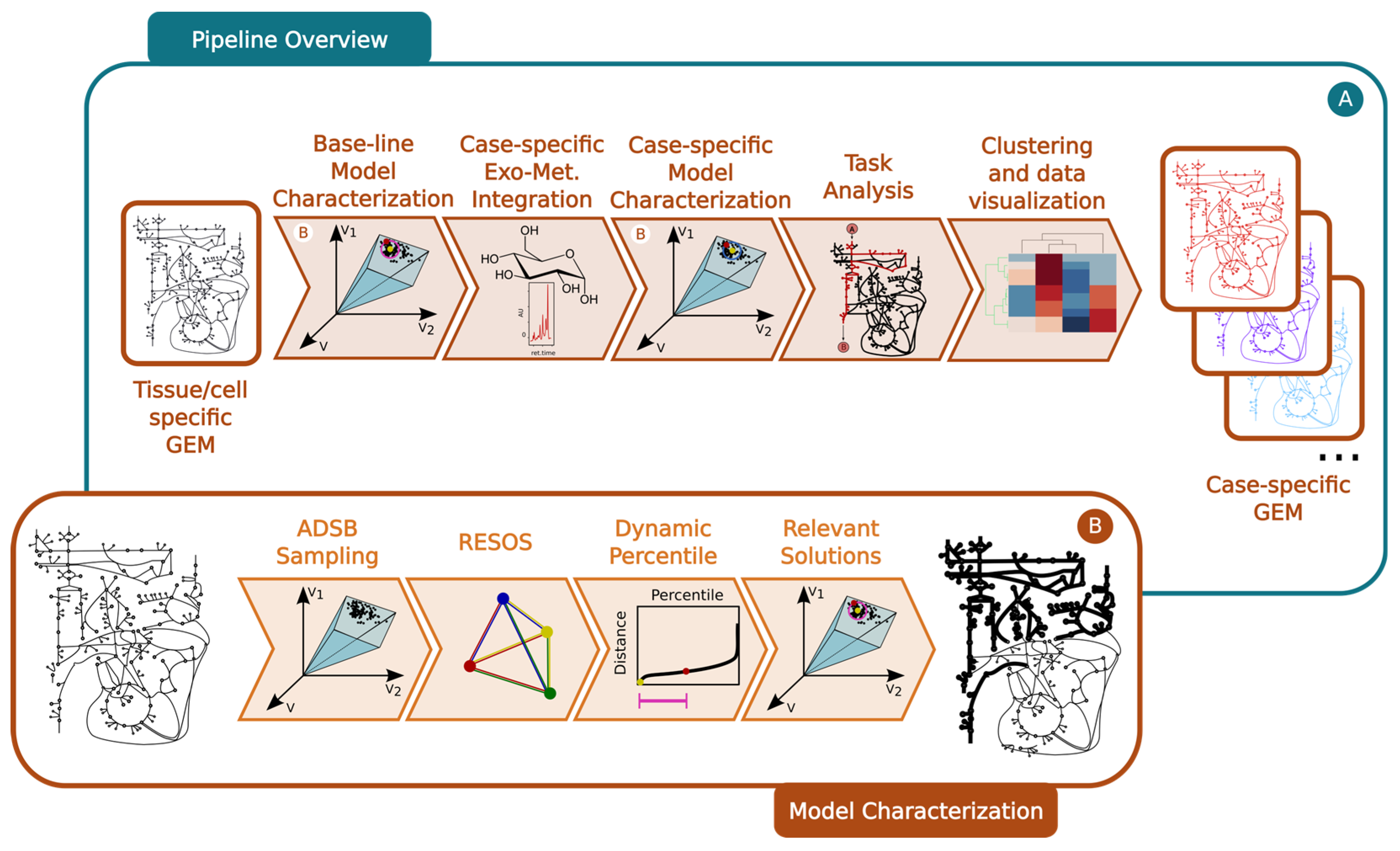
4.1. Protocol Overview
- Omics integration: The cell/organism physiological information is embedded into the baseline GEM using literature-based data, which sets boundaries on the exchange reactions (Figure 6A).
- Exploration of the space of feasible flux solutions using the RESOS algorithm: An in-house-developed algorithm is applied to analyze the sampled solutions and define the maximum and minimum flux allowed for each metabolic reaction. This algorithm identifies relevant regions and characterizes the metabolic flux spectrum without imposing arbitrary boundaries. Furthermore, the algorithm determines both the size and the number of solutions within the solution space to accurately profile the metabolic flux of a given network. These sampling parameters will be utilized in subsequent analyses, effectively reducing computational time.
- Outcome: The final outcome of this step is a characterization of the baseline GEM that includes the maximum and minimum flux allowed for all metabolic reactions (Figure 6B).
- Here, the protocol integrates case-specific metabolomic data into the baseline model (Figure 6A). The following steps are involved:
- Omics integration: Relative exometabolomic data are integrated by calculating the ratio TMi/CMi, where TMi represents the concentration of the ith metabolite in the Tth condition (e.g., disease group, treated group, or individual patient) and CMi represents the concentration of the same metabolite in the control group. To accommodate control group heterogeneity, three models are reconstructed for each condition, using either the minimum, maximum, or mean value of the ith metabolite in the control group (CMi) to calculate the relative metabolomic data (Figure 6A).
- Sampling: The same sampling algorithm employed in the baseline model is applied, using the sampling parameters previously determined by the RESOS algorithm in step 1 (Figure 6B).
- RESOS: The same algorithm used in the baseline model is applied for the case-specific analysis (Figure 6B). Outcome: As a result, three distinct GEMs are reconstructed for each case, and the case is characterized by combining the sampled solutions from the three models. This comprehensive approach provides a robust representation of the metabolic landscape for each specific case.
- Generate a consensus case-specific GEM: First, the three previously reconstructed GEMs (minimum, mean, and maximum) are combined into a single case-specific GEM. This is achieved by adjusting the boundaries of the new GEM using the RESOS algorithm’s output, applied to the fusion of solutions from the three individual case-specific GEMs. The reliability of model reconstruction is ultimately assessed by cross-validating the exo-metabolomic experimental data with the model predictions [15].
4.2. Main Case of Study
4.3. Experimental Data
- Exometabolomics: Blood samples from a cohort of 95 trauma patients [17] and a control group of 20 healthy individuals were used for exometabolomic analysis. These data were integrated into the EC GEM iEC3006 to generate trauma patient-specific GEMs.
4.4. Genome-Scale Metabolic Network Models
- EC GEM iEC3006 (latest reconstruction of EC metabolism) [19]:
- ○
- To construct trauma patient-specific GEMs.
- ○
- To assess the performance of the ADSB sampling algorithm.
- E. coli EcoliCore [35]:
- ○
- To assess the performance of the ADSB sampling algorithm.
- E. coli K12 iJO1366 [24]:
- ○
- To evaluate the enhancement in the reliability of model predictions when applying the RESOS algorithm.
- ○
- To evaluate the dynamic percentile method implemented in the RESOS algorithm.
- General Human GEM Recon1 [28]:
- ○
- To evaluate the dynamic percentile method implemented in the RESOS algorithm.
- ○
- To evaluate the dynamic percentile method implemented in the RESOS algorithm.
4.5. Exometabolomics Data Integration
4.6. Sampling Algorithm
4.7. Exploring the Space of Feasible Flux Solutions Using RESOS Algorithm
4.8. Identifying the Set of Representative Flux Solutions by Applying a Dynamic Non-Arbitrary Percentile Threhold Method
4.9. Task Analysis
4.10. Clustering Analysis
4.11. Unveiling Potential Therapeutic Targets by Applying Sensitivity Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, C.; Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hua, Q. Applications of Genome-Scale Metabolic Models in Biotechnology and Systems Medicine. Front. Physiol. 2016, 6, 413. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, W.J.; Kim, D.I.; Lee, S.Y. Applications of genome-scale metabolic network model in metabolic engineering. J. Ind. Microbiol. Biotechnol. 2015, 42, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Passi, A.; Tibocha-Bonilla, J.D.; Kumar, M.; Tec-Campos, D.; Zengler, K.; Zuniga, C. Genome-Scale Metabolic Modeling Enables In-Depth Understanding of Big Data. Metabolites 2022, 12, 14. [Google Scholar] [CrossRef]
- Bi, X.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Construction of Multiscale Genome-Scale Metabolic Models: Frameworks and Challenges. Biomolecules 2022, 12, 721. [Google Scholar] [CrossRef]
- Sarathy, C.; Breuer, M.; Kutmon, M.; Adriaens, M.E.; Evelo, C.T.; Arts, I.C.W. Comparison of metabolic states using genome-scale metabolic models. PLoS Comput. Biol. 2021, 17, e1009522. [Google Scholar] [CrossRef]
- Ebrahim, A.; Brunk, E.; Tan, J.; O’Brien, E.J.; Kim, D.; Szubin, R.; Lerman, J.A.; Lechner, A.; Sastry, A.; Bordbar, A.; et al. Multi-omic data integration enables discovery of hidden biological regularities. Nat. Commun. 2016, 7, 13091. [Google Scholar] [CrossRef]
- Rossi, G.P.; Barton, M.; Dhaun, N.; Rizzoni, D.; Seccia, T.M. Challenges in the evaluation of endothelial cell dysfunction: A statement from the European Society of Hypertension Working Group on Endothelin and Endothelial Factors. J. Hypertens. 2023, 41, 369–379. [Google Scholar] [CrossRef]
- Bouïs, D.; Hospers, G.A.; Meijer, C.; Molema, G.; Mulder, N.H. Endothelium in vitro: A review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 2001, 4, 91–102. [Google Scholar] [CrossRef]
- Comellas, A.P.; Briva, A.; Dada, L.A.; Butti, M.L.; Trejo, H.E.; Yshii, C.; Azzam, Z.S.; Litvan, J.; Chen, J.; Lecuona, E.; et al. Endothelin-1 impairs alveolar epithelial function via endothelial ETB receptor. Am. J. Respir. Crit. Care Med. 2009, 179, 113–122. [Google Scholar] [CrossRef]
- Mannino, R.G.; Qiu, Y.; Lam, W.A. Endothelial cell culture in microfluidic devices for investigating microvascular processes. Biomicrofluidics 2018, 12, 042203. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, J.; Henriksen, H.H.; Marin de Mas, I.; Johansson, P.I. An explorative metabolomic analysis of the endothelium in pulmonary hypertension. Sci. Rep. 2022, 12, 13284. [Google Scholar] [CrossRef] [PubMed]
- Gelbach, P.E.; Cetin, H.; Finley, S.D. Flux sampling in genome-scale metabolic modeling of microbial communities. BMC Bioinform. 2024, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.; Razaghi-Moghadam, Z.; Nikoloski, Z. Maximizing multi-reaction dependencies provides more accurate and precise predictions of intracellular fluxes than the principle of parsimony. PLoS Comput. Biol. 2023, 19, e1011489. [Google Scholar] [CrossRef] [PubMed]
- Arlot, S.; Celisse, A. A survey of cross-validation procedures for model selection. Stat. Surv. 2010, 4, 40–79. [Google Scholar] [CrossRef]
- Richelle, A.; Kellman, B.P.; Wenzel, A.T.; Chiang, A.W.T.; Reagan, T.; Gutierrez, J.M.; Joshi, C.; Li, S.; Liu, J.K.; Masson, H.; et al. Model-based assessment of mammalian cell metabolic functionalities using omics data. Cell Rep. Methods 2021, 1, 100040. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, H.H.; Marín de Mas, I.; Nielsen, L.K.; Krocker, J.; Stensballe, J.; Karvelsson, S.T.; Secher, N.H.; Rolfsson, Ó.; Wade, C.E.; Johansson, P.I. Endothelial Cell Phenotypes Demonstrate Different Metabolic Patterns and Predict Mortality in Trauma Patients. Int. J. Mol. Sci. 2023, 24, 2257. [Google Scholar] [CrossRef] [PubMed]
- Schonlau, M. Visualizing non-hierarchical and hierarchical cluster analyses with clustergrams. Comput. Stat. 2004, 19, 95–111. [Google Scholar] [CrossRef]
- Henriksen, H.H.; Marín de Mas, I.; Herand, H.; Krocker, J.; Wade, C.E.; Johansson, P.I. Metabolic systems analysis identifies a novel mechanism contributing to shock in patients with endotheliopathy of trauma (EoT) involving thromboxane A2 and LTC4. Matrix Biol. Plus 2022, 15, 100115. [Google Scholar] [CrossRef] [PubMed]
- Saa, P.A.; Zapararte, S.; Drovandi, C.C.; Nielsen, L.K. LooplessFluxSampler: An efficient toolbox for sampling the loopless flux solution space of metabolic models. BMC Bioinform. 2024, 25, 3. [Google Scholar] [CrossRef]
- DiMaggio, C.; Ayoung-Chee, P.; Shinseki, M.; Wilson, C.; Marshall, G.; Lee, D.C.; Wall, S.; Maulana, S.; Leon Pachter, H.; Frangos, S. Traumatic injury in the United States: In-patient epidemiology 2000–2011. Injury 2016, 47, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Bunch, C.M.; Chang, E.; Moore, E.E.; Moore, H.B.; Kwaan, H.C.; Miller, J.B.; Al-Fadhl, M.D.; Thomas, A.V.; Zackariya, N.; Patel, S.S.; et al. SHock-INduced Endotheliopathy (SHINE): A mechanistic justification for viscoelastography-guided resuscitation of traumatic and non-traumatic shock. Front. Physiol. 2023, 14, 1094845. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Conrad, T.M.; Na, J.; Lerman, J.A.; Nam, H.; Feist, A.M.; Palsson, B.Ø. A comprehensive genome-scale reconstruction of Escherichia coli metabolism—2011. Mol. Syst. Biol. 2011, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Fouladiha, H.; Marashi, S.A.; Li, S.; Li, Z.; Masson, H.O.; Vaziri, B.; Lewis, N.E. Systematically gap-filling the genome-scale metabolic model of CHO cells. Biotechnol. Lett. 2021, 43, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Shirkhorshidi, A.S.; Aghabozorgi, S.; Wah, T.Y. A Comparison Study on Similarity and Dissimilarity Measures in Clustering Continuous Data. PLoS ONE 2015, 10, e0144059. [Google Scholar] [CrossRef] [PubMed]
- Hefzi, H.; Ang, K.S.; Hanscho, M.; Bordbar, A.; Ruckerbauer, D.; Lakshmanan, M.; Orellana, C.A.; Baycin-Hizal, D.; Huang, Y.; Ley, D.; et al. A consensus genome-scale reconstruction of Chinese hamster ovary cell metabolism. Cell Syst. 2016, 3, 434–443.e8. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.C.; Becker, S.A.; Jamshidi, N.; Thiele, I.; Mo, M.L.; Vo, T.D.; Srivas, R.; Palsson, B.Ø. Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc. Natl. Acad. Sci. USA 2007, 104, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Shlomi, T.; Berkman, O.; Ruppin, E. Regulatory on/off minimization of metabolic flux changes after genetic perturbations. Proc. Natl. Acad. Sci. USA 2005, 102, 7695–7700. [Google Scholar] [CrossRef]
- Baba, T.; Huan, H.C.; Datsenko, K.; Wanner, B.L.; Mori, H. The applications of systematic in-frame, single-gene knockout mutant collection of escherichia coli k-12. Methods Mol. Biol. 2007, 416, 183–194. [Google Scholar]
- Butland, G.; Babu, M.; Díaz-Mejía, J.J.; Bohdana, F.; Phanse, S.; Gold, B.; Yang, W.; Li, J.; Gagarinova, A.G.; Pogoutse, O.; et al. esga: E. coli synthetic genetic array analysis. Nat. Methods 2008, 5, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Baba, T.; Yokoyama, K.; Takeuchi, R.; Nomura, W.; Makishi, K.; Otsuka, Y.; Dose, H.; Wanner, B.L. Identification of essential genes and synthetic lethal gene combinations in escherichia coli k-12. Methods Mol. Biol. 2015, 1279, 45–65. [Google Scholar] [PubMed]
- Apaolaza, I.; José-Eneriz, E.S.; Tobalina, L.; Miranda, E.; Garate, L.; Agirre, X.; Prósper, F.; Planes, F.J. An in-silico approach to predict and exploit synthetic lethality in cancer metabolism. Nat. Commun. 2017, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and analysis of biochemical constraint-based models: The COBRA Toolbox v3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Fleming, R.M.; Palsson, B.Ø. Reconstruction and Use of Microbial Metabolic Networks: The Core Escherichia coli Metabolic Model as an Educational Guide. EcoSal Plus 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Ezugwu, A.E.; Shukla, A.K.; Agbaje, M.B.; Oyelade, O.N.; José-García, A.; Agushaka, J.O. Automatic clustering algorithms: A systematic review and bibliometric analysis of relevant literature. Neural Comput. Applic. 2021, 33, 6247–6306. [Google Scholar] [CrossRef]
- Marin de Mas, I.; Herand, H.; Carrasco, J.; Nielsen, L.K.; Johansson, P.I. A Protocol for the Automatic Construction of Highly Curated Genome-Scale Models of Human Metabolism. Bioengineering 2023, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, B.J.; Pick, C.G.; Hoffer, M.E.; Becker, R.E.; Chiang, Y.H.; Greig, N.H. Repositioning drugs for traumatic brain injury—N-acetyl cysteine and Phenserine. J. Biomed. Sci. 2017, 24, 71. [Google Scholar] [CrossRef] [PubMed]
- Gianola, S.; Bargeri, S.; Biffi, A.; Cimbanassi, S.; D’Angelo, D.; Coclite, D.; Facchinetti, G.; Fauci, A.J.; Ferrara, C.; Di Nitto, M.; et al. Structured approach with primary and secondary survey for major trauma care: An overview of reviews. World J. Emerg. Surg. 2023, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Boero, S.; Musso, A.; Zocchi, M.R. Selective role of mevalonate pathway in regulating perforin but not FasL and TNFalpha release in human Natural Killer cells. PLoS ONE 2013, 8, e62932. [Google Scholar] [CrossRef]
- Ploder, M.; Spittler, A.; Kurz, K.; Neurauter, G.; Pelinka, L.E.; Roth, E.; Fuchs, D. Accelerated Tryptophan Degradation in Trauma and Sepsis Patients is Related to Pro-inflammatory Response and to the Diminished in vitro Response of Monocytes. Pteridines 2009, 20, 54–61. [Google Scholar] [CrossRef]
- Ploder, M.; Spittler, A.; Kurz, K.; Neurauter, G.; Pelinka, L.E.; Roth, E.; Fuchs, D. Accelerated tryptophan degradation predicts poor survival in trauma and sepsis patients. Int. J. Tryptophan Res. 2010, 3, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Todoriki, S.; Hosoda, Y.; Yamamoto, T.; Watanabe, M.; Sekimoto, A.; Sato, H.; Mori, T.; Miyazaki, M.; Takahashi, N.; Sato, E. Methylglyoxal Induces Inflammation, Metabolic Modulation and Oxidative Stress in Myoblast Cells. Toxins 2022, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.P.; Martins, P.; Veríssimo, C.; Simões, M.; Tomé, M.; Grazina, M.; Pimentel, J.; Castro-Sousa, F. Argininemia and plasma arginine bioavailability—Predictive factors of mortality in the severe trauma patients? Nutr. Metab. 2022, 13, 60. [Google Scholar] [CrossRef]
- Mammedova, J.T.; Sokolov, A.V.; Freidlin, I.S.; Starikova, E.A. The Mechanisms of L-Arginine Metabolism Disorder in Endothelial Cells. Biochemistry 2021, 86, 146–155. [Google Scholar] [CrossRef]
- González, M.; Rivas, J.C. L-Arginine/Nitric Oxide Pathway and KCa Channels in Endothelial Cells: A Mini-Review. In Vascular Biology—Selection of Mechanisms and Clinical Applications; IntechOpen: London, UK, 2020; Volume 11. [Google Scholar]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Schreiber, M.A.; Pati, S. Defining and Assessing the Endotheliopathy of Trauma and Its Implications on Trauma-Induced Coagulopathy and Trauma-Related Outcomes. In Trauma Induced Coagulopathy; Moore, H.B., Neal, M.D., Moore, E.E., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Lee, I.; Kim, J.; Kim, K.; Lee, J.; Choi, S.; Kim, W.; Ahn, H.; Kim, J.; Lee, S.; Kim, S.; et al. Malate and Aspartate Increase L-Arginine and Nitric Oxide and Attenuate Hypertension. Cell Rep. 2017, 19, 1631–1639. [Google Scholar]
- Wu, G.; Morris, S.M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Guo, X.; Guo, Y.; Li, J.; Liu, Q.; Wu, H. Arginine Expedites Erastin-Induced Ferroptosis through Fumarate. Int. J. Mol. Sci. 2023, 24, 14595. [Google Scholar] [CrossRef]
- Selamnia, M.; Robert, V.; Mayeur, C.; Duée, P.H.; Blachier, F. Effects of L-valine on growth and polyamine metabolism in human colon carcinoma cells. Biochim. Biophys. Acta 1998, 1379, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Marín de Mas, I.; Marín, S.; Pachón, G.; Rodríguez-Prados, J.C.; Vizán, P.; Centelles, J.J.; Tauler, R.; Azqueta, A.; Selivanov, V.; López de Ceráin, A.; et al. Unveiling the Metabolic Changes on Muscle Cell Metabolism Underlying p-Phenylenediamine Toxicity. Front. Mol. Biosci. 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Cascante, M.; de Atauri, P.; Gomez-Cabrero, D.; Wagner, P.; Centelles, J.J.; Marin, S.; Cano, I.; Velickovski, F.; de Mas, I.M.; Maier, D.; et al. Workforce Preparation: The Biohealth Computing Model for Master and PhD Students. J. Transl. Med. 2014, 12, S11. [Google Scholar] [CrossRef]

| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1 | 0 | |||
| 2 | 0 | |||
| 3 | 0 | |||
| 4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Lance, F.; Montejano-Montelongo, I.; Bautista, E.; Nielsen, L.K.; Johansson, P.I.; Marin de Mas, I. Integrating Genome-Scale Metabolic Models with Patient Plasma Metabolome to Study Endothelial Metabolism In Situ. Int. J. Mol. Sci. 2024, 25, 5406. https://doi.org/10.3390/ijms25105406
Silva-Lance F, Montejano-Montelongo I, Bautista E, Nielsen LK, Johansson PI, Marin de Mas I. Integrating Genome-Scale Metabolic Models with Patient Plasma Metabolome to Study Endothelial Metabolism In Situ. International Journal of Molecular Sciences. 2024; 25(10):5406. https://doi.org/10.3390/ijms25105406
Chicago/Turabian StyleSilva-Lance, Fernando, Isabel Montejano-Montelongo, Eric Bautista, Lars K. Nielsen, Pär I. Johansson, and Igor Marin de Mas. 2024. "Integrating Genome-Scale Metabolic Models with Patient Plasma Metabolome to Study Endothelial Metabolism In Situ" International Journal of Molecular Sciences 25, no. 10: 5406. https://doi.org/10.3390/ijms25105406
APA StyleSilva-Lance, F., Montejano-Montelongo, I., Bautista, E., Nielsen, L. K., Johansson, P. I., & Marin de Mas, I. (2024). Integrating Genome-Scale Metabolic Models with Patient Plasma Metabolome to Study Endothelial Metabolism In Situ. International Journal of Molecular Sciences, 25(10), 5406. https://doi.org/10.3390/ijms25105406








